Abstract
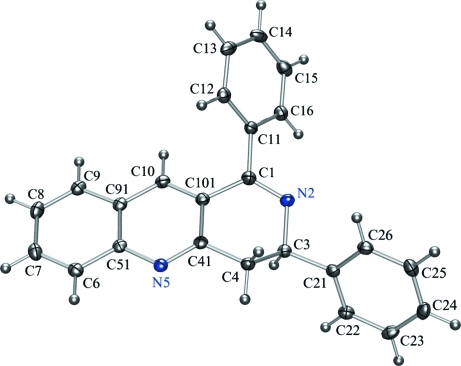
The title compound, C24H18N2, is the first structural example containing the 3,4-dihydrobenzo[b][1,6]naphthyridine fragment. It was synthesized from 2,4,6,8-tetraphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one and was crystallized from a methanol–ethanol solution over two years as a racemate. The C=N double bond [1.2868 (15) Å] is bent significantly out of the plane of the aromatic bicyclic ring system [N—C—C—C = −157.63 (12)°] and out of the plane of the phenyl ring bonded at the 1-position [N—C—C—C = 41.15 (16)°].
Related literature
For the synthesis of 1,3-diphenyl-1,2,3,4-tetrahydrobenzo[b][1,6]naphthyridine, see: Sivakumar (2000 ▶). For the synthesis of 2,4,6,8-tetraphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one, see Ravindran et al. (1991 ▶). For the crystal structures of other naphthyridine derivatives, see: Sivakumar et al. (2003 ▶); Laavanya et al. (2001 ▶).
Experimental
Crystal data
C24H18N2
M r = 334.40
Monoclinic,

a = 10.2658 (4) Å
b = 10.8583 (5) Å
c = 16.1842 (7) Å
β = 107.909 (2)°
V = 1716.63 (13) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 100 K
0.32 × 0.28 × 0.16 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.885, T max = 0.988
20360 measured reflections
3365 independent reflections
3060 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.094
S = 1.02
3365 reflections
241 parameters
Only H-atom displacement parameters refined
Δρmax = 0.40 e Å−3
Δρmin = −0.28 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: modified ORTEP (Johnson, 1965 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810013619/bt5246sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810013619/bt5246Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Comment
Schmidt reaction of 2,4,6,8-tetraphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one leads not to the expected bicyclic amide but to 1,2,3,4-tetrahydrobenzo[b][1,6]naphthyridine. This compound crystallized in form of fine needles from ethanol. Our attempts to yield more compact crystals ended up in a new compound, 1,3-diphenyl-3,4-dihydrobenzo[b][1,6]naphthyridine, which was formed after a lomg term crystallization from a mixture of ethanol and methanol.
Experimental
Synthesis: 2,4,6,8-Tetraphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one (1.34 g, 3.0 mmol) was dissolved in concentrated sulfuric acid (4 ml) under stirring and cooling and by use of an ultrasonic bath. This takes some time and the solution comes up to room temperature during this process. When the substance is completely dissolved, NaN3 (240 mg, 3,7 mmol) was added and the reaction mixture was stirred for 1 h at room temperature. It was quenched with ice water, the yellow solution was extracted 3 times with ether to remove non basic impurities and then alkalized with 2 M NaOH solution. Then it was extracted 5 times with CH2Cl2, the organic layers were combined, washed 3 times with water, dried (Na2SO4), filtered and the solvent removed in vacuo. The residue was dissolved in benzene and filtered and the solvent removed in vacuo. Finally, the residue was dissolved in the minimum amount of hot ethanol and the solution left for crystallization for 2 days. The formed needles were sucked off and dried giving pure 1,3-diphenyl-1,2,3,4-tetrahydro-benzo[b][1,6]naphthyridine (330 mg, 0.98 mmol, 33 % yield). A part of it was dissolved in a mixture of methanol and ethanol and left for crystallization for 2 years. A few crystals of 1,3-diphenyl-3,4-dihydro-benzo[b][1,6]naphthyridine were obtained and subjected to the x-ray structure analysis.
HR—MS data [collected on a GCT-Premier spectrometer, Waters (EI, 70 eV)]: C24H18N2 requires [M]+ 334.1470; Found: 334.1452; C24H17N2 requires [M—H]+ 333.1392; Found: 333.1381.
Figures
Fig. 1.
ORTEP plot (Johnson, 1965) showing the atomic numbering scheme. The probability ellipsoids are drawn at the 50% probability level, the H atoms are drawn with arbitrary radii. Selected distances: C1—N2 1.2868 (15) Å, C1—C101 1.4862 (16) Å, C1—C11 1.4936 (16) Å, N2—C3 1.4769 (14) Å, C3—C21 1.5128 (15) Å, C3—C4 1.5369 (15) Å, C4—C41 1.5073 (15) Å, C41—N5 1.3152 (14) Å, C41—C101 1.4293 (15) Å, N5—C51 1.3764 (14) Å.
Crystal data
| C24H18N2 | F(000) = 704 |
| Mr = 334.40 | Dx = 1.294 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 9913 reflections |
| a = 10.2658 (4) Å | θ = 2.7–26.0° |
| b = 10.8583 (5) Å | µ = 0.08 mm−1 |
| c = 16.1842 (7) Å | T = 100 K |
| β = 107.909 (2)° | Block, yellow |
| V = 1716.63 (13) Å3 | 0.32 × 0.28 × 0.16 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 3365 independent reflections |
| Radiation source: sealed tube | 3060 reflections with I > 2σ(I) |
| graphite | Rint = 0.029 |
| φ and ω scans | θmax = 26.0°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Bruker, 2000) | h = −12→12 |
| Tmin = 0.885, Tmax = 0.988 | k = −13→13 |
| 20360 measured reflections | l = −19→19 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.094 | Only H-atom displacement parameters refined |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0446P)2 + 0.7644P] where P = (Fo2 + 2Fc2)/3 |
| 3365 reflections | (Δ/σ)max < 0.001 |
| 241 parameters | Δρmax = 0.40 e Å−3 |
| 0 restraints | Δρmin = −0.28 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.The non-hydrogen atoms were refined with anisotropic displacement parameters without any constraints. The H atom of the tertiary C—H group was refined with an individual isotropic displacement parameter and all X—C—H angles equal at a C—H distance of 1.00 Å (AFIX 13 of SHELXL-97). The H atoms of the CH2 group were refined with common isotropic displacement parameters and idealized geometry with approximately tetrahedral angles and C—H distances of 0.99 Å (AFIX 23 of SHELXL-97). The H atoms of the phenyl rings as well as the atoma H6, H7, H8, and H9 were put at the external bisector of the C—C—C angle at a C—H distance of 0.95 Å and common isotropic displacement parameters were refined for the H atoms of the same ring (AFIX 43 of SHELXL-97). The H atom H10 was put at the external bisector of the C—C—C angle at a C—H distance of 0.95 Å but the individual isotropic displacement parameter was free to refine (AFIX 43 of SHELXL-97). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.60895 (12) | 0.61918 (11) | 0.71264 (8) | 0.0221 (3) | |
| N2 | 0.67352 (10) | 0.72215 (9) | 0.71977 (6) | 0.0183 (2) | |
| C3 | 0.61969 (11) | 0.81335 (10) | 0.64987 (7) | 0.0162 (2) | |
| H3 | 0.5364 | 0.8516 | 0.6588 | 0.016 (3)* | |
| C4 | 0.57620 (11) | 0.75231 (10) | 0.55983 (7) | 0.0167 (2) | |
| H41 | 0.5341 | 0.8145 | 0.5148 | 0.022 (2)* | |
| H42 | 0.6576 | 0.7180 | 0.5474 | 0.022 (2)* | |
| C41 | 0.47489 (11) | 0.65074 (10) | 0.55714 (7) | 0.0154 (2) | |
| N5 | 0.37687 (9) | 0.62770 (8) | 0.48450 (6) | 0.0160 (2) | |
| C51 | 0.28123 (11) | 0.53968 (10) | 0.48615 (7) | 0.0160 (2) | |
| C6 | 0.17392 (11) | 0.51425 (11) | 0.40906 (7) | 0.0186 (2) | |
| H6 | 0.1692 | 0.5578 | 0.3573 | 0.0233 (17)* | |
| C7 | 0.07669 (12) | 0.42730 (11) | 0.40843 (8) | 0.0216 (3) | |
| H7 | 0.0054 | 0.4109 | 0.3561 | 0.0233 (17)* | |
| C8 | 0.08147 (12) | 0.36181 (11) | 0.48477 (8) | 0.0220 (3) | |
| H8 | 0.0143 | 0.3010 | 0.4833 | 0.0233 (17)* | |
| C9 | 0.18252 (12) | 0.38568 (11) | 0.56076 (8) | 0.0195 (2) | |
| H9 | 0.1839 | 0.3429 | 0.6122 | 0.0233 (17)* | |
| C91 | 0.28508 (11) | 0.47405 (10) | 0.56299 (7) | 0.0168 (2) | |
| C10 | 0.39474 (12) | 0.49926 (10) | 0.63871 (7) | 0.0180 (2) | |
| H10 | 0.4019 | 0.4564 | 0.6911 | 0.022 (3)* | |
| C101 | 0.49068 (11) | 0.58561 (10) | 0.63637 (7) | 0.0173 (2) | |
| C11 | 0.66051 (11) | 0.52633 (10) | 0.78325 (7) | 0.0173 (2) | |
| C12 | 0.67192 (11) | 0.40231 (11) | 0.76360 (7) | 0.0182 (2) | |
| H12 | 0.6417 | 0.3754 | 0.7049 | 0.0262 (16)* | |
| C13 | 0.72750 (11) | 0.31812 (11) | 0.82995 (8) | 0.0217 (3) | |
| H13 | 0.7371 | 0.2341 | 0.8164 | 0.0262 (16)* | |
| C14 | 0.76892 (12) | 0.35674 (12) | 0.91590 (8) | 0.0234 (3) | |
| H14 | 0.8049 | 0.2988 | 0.9611 | 0.0262 (16)* | |
| C15 | 0.75784 (12) | 0.47979 (12) | 0.93583 (8) | 0.0236 (3) | |
| H15 | 0.7867 | 0.5062 | 0.9946 | 0.0262 (16)* | |
| C16 | 0.70445 (12) | 0.56423 (11) | 0.86972 (8) | 0.0208 (3) | |
| H16 | 0.6978 | 0.6486 | 0.8835 | 0.0262 (16)* | |
| C21 | 0.72353 (11) | 0.91467 (10) | 0.65640 (7) | 0.0154 (2) | |
| C22 | 0.68122 (12) | 1.03683 (11) | 0.64243 (7) | 0.0184 (2) | |
| H22 | 0.5871 | 1.0563 | 0.6307 | 0.0257 (16)* | |
| C23 | 0.77463 (13) | 1.13070 (11) | 0.64538 (7) | 0.0210 (3) | |
| H23 | 0.7441 | 1.2136 | 0.6357 | 0.0257 (16)* | |
| C24 | 0.91240 (12) | 1.10332 (11) | 0.66241 (7) | 0.0218 (3) | |
| H24 | 0.9766 | 1.1672 | 0.6646 | 0.0257 (16)* | |
| C25 | 0.95581 (12) | 0.98180 (12) | 0.67630 (8) | 0.0220 (3) | |
| H25 | 1.0500 | 0.9626 | 0.6876 | 0.0257 (16)* | |
| C26 | 0.86247 (12) | 0.88817 (11) | 0.67380 (7) | 0.0190 (2) | |
| H26 | 0.8934 | 0.8055 | 0.6840 | 0.0257 (16)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0239 (6) | 0.0193 (6) | 0.0199 (6) | −0.0017 (5) | 0.0022 (5) | 0.0022 (5) |
| N2 | 0.0227 (5) | 0.0168 (5) | 0.0146 (5) | −0.0015 (4) | 0.0046 (4) | 0.0017 (4) |
| C3 | 0.0175 (5) | 0.0155 (5) | 0.0157 (5) | 0.0009 (4) | 0.0051 (4) | 0.0005 (4) |
| C4 | 0.0197 (5) | 0.0160 (5) | 0.0139 (5) | −0.0009 (4) | 0.0043 (4) | 0.0014 (4) |
| C41 | 0.0166 (5) | 0.0135 (5) | 0.0165 (5) | 0.0029 (4) | 0.0059 (4) | −0.0005 (4) |
| N5 | 0.0173 (5) | 0.0144 (5) | 0.0161 (5) | 0.0017 (4) | 0.0047 (4) | −0.0008 (4) |
| C51 | 0.0170 (5) | 0.0133 (5) | 0.0182 (5) | 0.0029 (4) | 0.0061 (4) | −0.0016 (4) |
| C6 | 0.0196 (6) | 0.0181 (6) | 0.0176 (6) | 0.0033 (4) | 0.0050 (4) | −0.0012 (4) |
| C7 | 0.0176 (6) | 0.0225 (6) | 0.0226 (6) | 0.0008 (5) | 0.0031 (5) | −0.0061 (5) |
| C8 | 0.0181 (6) | 0.0198 (6) | 0.0293 (6) | −0.0038 (4) | 0.0091 (5) | −0.0038 (5) |
| C9 | 0.0205 (6) | 0.0173 (6) | 0.0227 (6) | 0.0005 (4) | 0.0098 (5) | −0.0007 (4) |
| C91 | 0.0178 (5) | 0.0140 (5) | 0.0194 (6) | 0.0022 (4) | 0.0071 (4) | −0.0017 (4) |
| C10 | 0.0218 (6) | 0.0159 (5) | 0.0169 (5) | 0.0014 (4) | 0.0067 (5) | 0.0015 (4) |
| C101 | 0.0191 (6) | 0.0150 (5) | 0.0169 (6) | 0.0014 (4) | 0.0043 (4) | 0.0003 (4) |
| C11 | 0.0151 (5) | 0.0186 (6) | 0.0177 (5) | −0.0011 (4) | 0.0042 (4) | 0.0029 (4) |
| C12 | 0.0164 (5) | 0.0204 (6) | 0.0192 (6) | −0.0019 (4) | 0.0074 (4) | 0.0001 (4) |
| C13 | 0.0171 (5) | 0.0176 (6) | 0.0328 (7) | 0.0015 (4) | 0.0112 (5) | 0.0048 (5) |
| C14 | 0.0165 (6) | 0.0288 (7) | 0.0252 (6) | 0.0029 (5) | 0.0067 (5) | 0.0138 (5) |
| C15 | 0.0206 (6) | 0.0339 (7) | 0.0158 (6) | −0.0013 (5) | 0.0046 (5) | 0.0035 (5) |
| C16 | 0.0204 (6) | 0.0211 (6) | 0.0197 (6) | −0.0004 (5) | 0.0043 (5) | −0.0008 (5) |
| C21 | 0.0191 (5) | 0.0164 (5) | 0.0104 (5) | −0.0003 (4) | 0.0040 (4) | −0.0005 (4) |
| C22 | 0.0199 (6) | 0.0190 (6) | 0.0172 (5) | 0.0031 (4) | 0.0068 (4) | 0.0012 (4) |
| C23 | 0.0318 (6) | 0.0141 (5) | 0.0180 (6) | 0.0006 (5) | 0.0091 (5) | −0.0006 (4) |
| C24 | 0.0265 (6) | 0.0221 (6) | 0.0157 (5) | −0.0096 (5) | 0.0051 (5) | −0.0034 (5) |
| C25 | 0.0169 (6) | 0.0287 (6) | 0.0183 (6) | −0.0010 (5) | 0.0024 (4) | −0.0013 (5) |
| C26 | 0.0207 (6) | 0.0168 (6) | 0.0177 (5) | 0.0031 (4) | 0.0033 (4) | 0.0004 (4) |
Geometric parameters (Å, °)
| C1—N2 | 1.2868 (15) | C10—C101 | 1.3688 (16) |
| C1—C101 | 1.4862 (16) | C10—H10 | 0.95 |
| C1—C11 | 1.4936 (16) | C11—C16 | 1.3938 (16) |
| N2—C3 | 1.4769 (14) | C11—C12 | 1.3969 (16) |
| C3—C21 | 1.5128 (15) | C12—C13 | 1.3920 (16) |
| C3—C4 | 1.5369 (15) | C12—H12 | 0.95 |
| C3—H3 | 1.00 | C13—C14 | 1.3884 (18) |
| C4—C41 | 1.5073 (15) | C13—H13 | 0.95 |
| C4—H41 | 0.99 | C14—C15 | 1.3873 (19) |
| C4—H42 | 0.99 | C14—H14 | 0.95 |
| C41—N5 | 1.3152 (14) | C15—C16 | 1.3875 (17) |
| C41—C101 | 1.4293 (15) | C15—H15 | 0.95 |
| N5—C51 | 1.3764 (14) | C16—H16 | 0.95 |
| C51—C6 | 1.4146 (16) | C21—C22 | 1.3925 (16) |
| C51—C91 | 1.4233 (16) | C21—C26 | 1.3962 (16) |
| C6—C7 | 1.3717 (17) | C22—C23 | 1.3901 (17) |
| C6—H6 | 0.95 | C22—H22 | 0.95 |
| C7—C8 | 1.4132 (17) | C23—C24 | 1.3874 (17) |
| C7—H7 | 0.95 | C23—H23 | 0.95 |
| C8—C9 | 1.3679 (17) | C24—C25 | 1.3887 (18) |
| C8—H8 | 0.95 | C24—H24 | 0.95 |
| C9—C91 | 1.4168 (16) | C25—C26 | 1.3891 (17) |
| C9—H9 | 0.95 | C25—H25 | 0.95 |
| C91—C10 | 1.4126 (16) | C26—H26 | 0.95 |
| N2—C1—C101 | 123.67 (11) | C91—C10—H10 | 120.1 |
| N2—C1—C11 | 117.79 (10) | C10—C101—C41 | 118.67 (10) |
| C101—C1—C11 | 118.47 (10) | C10—C101—C1 | 123.78 (10) |
| C1—N2—C3 | 116.92 (9) | C41—C101—C1 | 117.52 (10) |
| N2—C3—C21 | 110.15 (9) | C16—C11—C12 | 119.29 (10) |
| N2—C3—C4 | 111.61 (9) | C16—C11—C1 | 119.87 (11) |
| C21—C3—C4 | 111.89 (9) | C12—C11—C1 | 120.75 (10) |
| N2—C3—H3 | 107.7 | C13—C12—C11 | 120.04 (11) |
| C21—C3—H3 | 107.7 | C13—C12—H12 | 120.0 |
| C4—C3—H3 | 107.7 | C11—C12—H12 | 120.0 |
| C41—C4—C3 | 109.85 (9) | C14—C13—C12 | 120.07 (11) |
| C41—C4—H41 | 109.7 | C14—C13—H13 | 120.0 |
| C3—C4—H41 | 109.7 | C12—C13—H13 | 120.0 |
| C41—C4—H42 | 109.7 | C15—C14—C13 | 120.13 (11) |
| C3—C4—H42 | 109.7 | C15—C14—H14 | 119.9 |
| H41—C4—H42 | 108.2 | C13—C14—H14 | 119.9 |
| N5—C41—C101 | 123.53 (10) | C14—C15—C16 | 119.89 (11) |
| N5—C41—C4 | 119.76 (10) | C14—C15—H15 | 120.1 |
| C101—C41—C4 | 116.69 (9) | C16—C15—H15 | 120.1 |
| C41—N5—C51 | 117.96 (9) | C15—C16—C11 | 120.55 (11) |
| N5—C51—C6 | 119.02 (10) | C15—C16—H16 | 119.7 |
| N5—C51—C91 | 122.44 (10) | C11—C16—H16 | 119.7 |
| C6—C51—C91 | 118.54 (10) | C22—C21—C26 | 118.52 (10) |
| C7—C6—C51 | 120.56 (11) | C22—C21—C3 | 120.23 (10) |
| C7—C6—H6 | 119.7 | C26—C21—C3 | 121.23 (10) |
| C51—C6—H6 | 119.7 | C23—C22—C21 | 121.02 (11) |
| C6—C7—C8 | 120.68 (11) | C23—C22—H22 | 119.5 |
| C6—C7—H7 | 119.7 | C21—C22—H22 | 119.5 |
| C8—C7—H7 | 119.7 | C24—C23—C22 | 120.00 (11) |
| C9—C8—C7 | 120.24 (11) | C24—C23—H23 | 120.0 |
| C9—C8—H8 | 119.9 | C22—C23—H23 | 120.0 |
| C7—C8—H8 | 119.9 | C23—C24—C25 | 119.51 (11) |
| C8—C9—C91 | 120.22 (11) | C23—C24—H24 | 120.2 |
| C8—C9—H9 | 119.9 | C25—C24—H24 | 120.2 |
| C91—C9—H9 | 119.9 | C24—C25—C26 | 120.43 (11) |
| C10—C91—C9 | 122.76 (11) | C24—C25—H25 | 119.8 |
| C10—C91—C51 | 117.49 (10) | C26—C25—H25 | 119.8 |
| C9—C91—C51 | 119.74 (10) | C25—C26—C21 | 120.52 (11) |
| C101—C10—C91 | 119.80 (10) | C25—C26—H26 | 119.7 |
| C101—C10—H10 | 120.1 | C21—C26—H26 | 119.7 |
| C101—C1—N2—C3 | 3.86 (17) | C4—C41—C101—C1 | −3.06 (15) |
| C11—C1—N2—C3 | −179.16 (10) | N2—C1—C101—C10 | −157.63 (12) |
| C1—N2—C3—C21 | −166.72 (10) | C11—C1—C101—C10 | 25.41 (17) |
| C1—N2—C3—C4 | −41.79 (14) | N2—C1—C101—C41 | 20.27 (17) |
| N2—C3—C4—C41 | 55.03 (12) | C11—C1—C101—C41 | −156.69 (10) |
| C21—C3—C4—C41 | 178.98 (9) | N2—C1—C11—C16 | 41.15 (16) |
| C3—C4—C41—N5 | 146.44 (10) | C101—C1—C11—C16 | −141.71 (11) |
| C3—C4—C41—C101 | −32.39 (13) | N2—C1—C11—C12 | −135.55 (12) |
| C101—C41—N5—C51 | 2.88 (16) | C101—C1—C11—C12 | 41.59 (16) |
| C4—C41—N5—C51 | −175.87 (9) | C16—C11—C12—C13 | −0.41 (16) |
| C41—N5—C51—C6 | 179.30 (10) | C1—C11—C12—C13 | 176.31 (10) |
| C41—N5—C51—C91 | 0.11 (15) | C11—C12—C13—C14 | 1.41 (17) |
| N5—C51—C6—C7 | −179.86 (10) | C12—C13—C14—C15 | −1.41 (17) |
| C91—C51—C6—C7 | −0.64 (16) | C13—C14—C15—C16 | 0.39 (18) |
| C51—C6—C7—C8 | 0.24 (17) | C14—C15—C16—C11 | 0.61 (18) |
| C6—C7—C8—C9 | 0.89 (18) | C12—C11—C16—C15 | −0.60 (17) |
| C7—C8—C9—C91 | −1.58 (17) | C1—C11—C16—C15 | −177.35 (11) |
| C8—C9—C91—C10 | −177.57 (11) | N2—C3—C21—C22 | −139.22 (10) |
| C8—C9—C91—C51 | 1.16 (17) | C4—C3—C21—C22 | 96.01 (12) |
| N5—C51—C91—C10 | −2.06 (16) | N2—C3—C21—C26 | 42.74 (13) |
| C6—C51—C91—C10 | 178.75 (10) | C4—C3—C21—C26 | −82.02 (12) |
| N5—C51—C91—C9 | 179.14 (10) | C26—C21—C22—C23 | 0.20 (16) |
| C6—C51—C91—C9 | −0.05 (16) | C3—C21—C22—C23 | −177.89 (10) |
| C9—C91—C10—C101 | 179.82 (10) | C21—C22—C23—C24 | 0.03 (17) |
| C51—C91—C10—C101 | 1.06 (16) | C22—C23—C24—C25 | 0.09 (17) |
| C91—C10—C101—C41 | 1.65 (16) | C23—C24—C25—C26 | −0.43 (17) |
| C91—C10—C101—C1 | 179.53 (10) | C24—C25—C26—C21 | 0.66 (17) |
| N5—C41—C101—C10 | −3.83 (17) | C22—C21—C26—C25 | −0.54 (16) |
| C4—C41—C101—C10 | 174.95 (10) | C3—C21—C26—C25 | 177.53 (10) |
| N5—C41—C101—C1 | 178.16 (10) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5246).
References
- Bruker (2000). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2004). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Johnson, C. K. (1965). ORTEP Report ORNL-3794. Oak Ridge National Laboratory, Tennessee, USA.
- Laavanya, P., Panchanatheswaran, K., Sivakumar, B., Jeyaraman, R. & Krause Bauer, J. A. (2001). Acta Cryst. E57, o599–o601.
- Ravindran, T., Jeyaraman, R., Murray, R. W. & Singh, M. (1991). J. Org. Chem.56, 4833–4840.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sivakumar, B. (2000). PhD thesis, Bharathidasan University, Tiruchirappalli, India.
- Sivakumar, B., SethuSankar, K., Senthil Kumar, U. P., Jeyaraman, R. & Velmurugan, D. (2003). Acta Cryst. C59, o153–o155. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810013619/bt5246sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810013619/bt5246Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report