Abstract
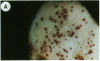
We have employed microprojectiles to deliver genes involved in anthocyanin biosynthesis to cells within intact aleurone and embryo tissues of maize. Clones of the A1 or Bz1 genes were introduced into aleurone tissue that lacked anthocyanins due to mutations of the endogenous A1 or Bz1 gene. Following bombardment, cells within the aleurone developed purple pigmentation, indicating that the mutation in the a1 or bz1 genotypes was corrected by the introduced gene. To analyze the expression of these genes in different genetic backgrounds, chimeric genes containing the 5′ and 3′ regions of the A1 or Bz1 genes fused to a luciferase coding region were constructed. These constructs were introduced into aleurones of genotypes carrying either dominant or recessive alleles of the C1 and R genes, which are known to regulate anthocyanin production. Levels of luciferase activity in permissive backgrounds (C1, R) were 30- to 200-fold greater than those detected in tissue carrying one or both of the recessive alleles (c1, r) of these genes. These results show that genes delivered to intact tissues by microprojectiles are regulated in a manner similar to the endogenous genes. The transfer of genes directly to intact tissues provides a rapid means for analyzing the genetic and tissue-specific regulation of gene expression.
Keywords: gene regulation, gene transfer, particle gun
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callis J., Fromm M., Walbot V. Heat Inducible Expression of a Chimeric Maize hsp70CAT Gene in Maize Protoplasts. Plant Physiol. 1988 Dec;88(4):965–968. doi: 10.1104/pp.88.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987 Dec;1(10):1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- Cone K. C., Burr F. A., Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9631–9635. doi: 10.1073/pnas.83.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K., Nelson O. E. Genetic control of UDPglucose:flavonol 3-O-glucosyltransferase in the endosperm of maize. Biochem Genet. 1977 Jun;15(5-6):509–519. doi: 10.1007/BF00520194. [DOI] [PubMed] [Google Scholar]
- Dooner H. K., Nelson O. E. Interaction among C, R and Vp in the Control of the Bz Glucosyltransferase during Endosperm Development in Maize. Genetics. 1979 Feb;91(2):309–315. doi: 10.1093/genetics/91.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. R., Ha S. B., An G. Identification of an essential upstream element in the nopaline synthase promoter by stable and transient assays. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5745–5749. doi: 10.1073/pnas.84.16.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V., Furtek D. B., Nelson O. E. Cloning of the bronze locus in maize by a simple and generalizable procedure using the transposable controlling element Activator (Ac). Proc Natl Acad Sci U S A. 1984 Jun;81(12):3825–3829. doi: 10.1073/pnas.81.12.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1(5):457–481. [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. S., Clancy M., Paje-Manalo L., Furtek D. B., Hannah L. C., Nelson O. E., Jr The mutation bronze-mutable 4 derivative 6856 in maize is caused by the insertion of a novel 6.7-kilobase pair transposon in the untranslated leader region of the bronze-1 gene. Genetics. 1988 Nov;120(3):779–790. doi: 10.1093/genetics/120.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. M., Harper E. C., Svab Z., Sanford J. C., Fromm M. E., Maliga P. Stable genetic transformation of intact Nicotiana cells by the particle bombardment process. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8502–8505. doi: 10.1073/pnas.85.22.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G., Lau A., Klein J., Golas C., Bologa-Campeanu M., Soldin S., MacLeod S. M., Prober C. Pharmacokinetics and adverse effects of amphotericin B in infants and children. J Pediatr. 1988 Sep;113(3):559–563. doi: 10.1016/s0022-3476(88)80653-x. [DOI] [PubMed] [Google Scholar]
- Larson R. L., Coe E. H., Jr Gene-dependent flavonoid glucosyltransferase in maize. Biochem Genet. 1977 Feb;15(1-2):153–156. doi: 10.1007/BF00484558. [DOI] [PubMed] [Google Scholar]
- McCormick S. Pigment synthesis in maize aleurone from precursors fed to anthocyanin mutants. Biochem Genet. 1978 Aug;16(7-8):777–785. doi: 10.1007/BF00484735. [DOI] [PubMed] [Google Scholar]
- McLaughlin M., Walbot V. Cloning of a mutable bz2 allele of maize by transposon tagging and differential hybridization. Genetics. 1987 Dec;117(4):771–776. doi: 10.1093/genetics/117.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly C., Shepherd N. S., Pereira A., Schwarz-Sommer Z., Bertram I., Robertson D. S., Peterson P. A., Saedler H. Molecular cloning of the a1 locus of Zea mays using the transposable elements En and Mu1. EMBO J. 1985 Apr;4(4):877–882. doi: 10.1002/j.1460-2075.1985.tb03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J., Ghosal D., Wienand U., Peterson P. A., Saedler H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987 Dec 1;6(12):3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J., Wienand U., Peterson P. A., Saedler H. Molecular cloning of the c locus of Zea mays: a locus regulating the anthocyanin pathway. EMBO J. 1986 May;5(5):829–833. doi: 10.1002/j.1460-2075.1986.tb04291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY G. M., COE E. H., Jr Inter-tissue complemention: a simple technique for direct analysis of gene-action sequence. Science. 1962 Oct 12;138(3537):149–150. doi: 10.1126/science.138.3537.149. [DOI] [PubMed] [Google Scholar]
- Ralston E. J., English J. J., Dooner H. K. Sequence of three bronze alleles of maize and correlation with the genetic fine structure. Genetics. 1988 May;119(1):185–197. doi: 10.1093/genetics/119.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J. W., Furtek D. B., Dooner H. K., Nelson O. E., Jr Two mutations in a maize bronze-1 allele caused by transposable elements of the Ac-Ds family alter the quantity and quality of the gene product. Genetics. 1988 Nov;120(3):767–777. doi: 10.1093/genetics/120.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. J., Burr F. A., Burr B. Transposon tagging and molecular analysis of the maize regulatory locus opaque-2. Science. 1987 Nov 13;238(4829):960–963. doi: 10.1126/science.2823388. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Shepherd N., Tacke E., Gierl A., Rohde W., Leclercq L., Mattes M., Berndtgen R., Peterson P. A., Saedler H. Influence of transposable elements on the structure and function of the A1 gene of Zea mays. EMBO J. 1987 Feb;6(2):287–294. doi: 10.1002/j.1460-2075.1987.tb04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Theres N, Scheele T, Starlinger P. Cloning of the Bz2 locus of Zea mays using the transposable element Ds as a gene tag. Mol Gen Genet. 1987 Aug;209(1):193–197. doi: 10.1007/BF00329858. [DOI] [PubMed] [Google Scholar]
- Vernet T., Fleck J., Durr A., Fritsch C., Pinck M., Hirth L. Expression of the gene coding for the small subunit of ribulosebisphosphate carboxylase during differentiation of tobacco plant protoplasts. Eur J Biochem. 1982 Sep 1;126(3):489–494. doi: 10.1111/j.1432-1033.1982.tb06806.x. [DOI] [PubMed] [Google Scholar]
- Walker J. C., Howard E. A., Dennis E. S., Peacock W. J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6624–6628. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]