Abstract
In the title compound, C33H26Cl3N3OS, the oxadiazole, piperidine and benzothiapezine rings adopt envelope, chair and twist-boat conformations, respectively. In the crystal, the molecular aggregation is characterized by chains of centrosymmetrically related pairs connected through Cl⋯Cl interactions [3.533 (2) Å], extending parallel to (202).
Related literature
For the biological importance of benziothiazepines and oxadiazol derivatives, see: Budriesi et al. (2007 ▶); Sahin et al. (2002 ▶). For ring geometry, see: Boeyens (1978 ▶); Cremer & Pople (1975 ▶). For a related structure, see: Srinivasan et al. (2007 ▶).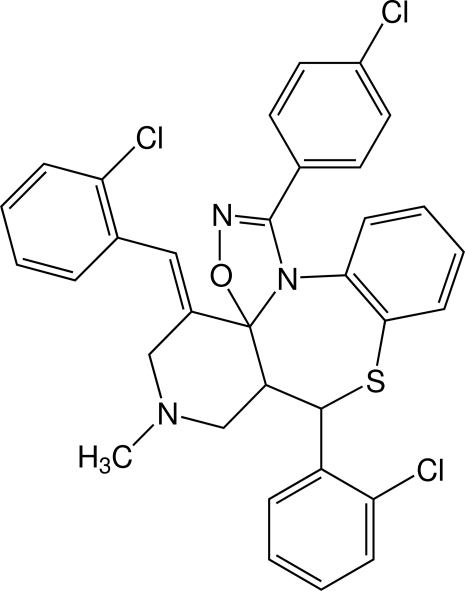
Experimental
Crystal data
C33H26Cl3N3OS
M r = 618.98
Triclinic,

a = 11.015 (3) Å
b = 11.758 (4) Å
c = 11.988 (4) Å
α = 78.87 (2)°
β = 86.89 (3)°
γ = 78.29 (2)°
V = 1491.5 (8) Å3
Z = 2
Mo Kα radiation
μ = 0.41 mm−1
T = 298 K
0.30 × 0.15 × 0.15 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.90, T max = 0.94
42055 measured reflections
11096 independent reflections
7867 reflections with I > 2σ(I)
R int = 0.023
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.146
S = 1.03
11096 reflections
372 parameters
H-atom parameters constrained
Δρmax = 0.64 e Å−3
Δρmin = −0.62 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT-Plus (Bruker, 2004 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810013309/nc2178sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810013309/nc2178Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the Sophisticated Analytical Instrumentation Facility (SAIF), Indian Institute of Technology, Chennai, for the X-ray intensity data collection.
supplementary crystallographic information
Comment
The title compound, C33H26N3OCl3 S, belongs to an important class of heterocycles which exhibit antihypertensive properties. The compound consists of a benzothiazepine, a oxadiazole and a methyl piperidine ring. Benziothiazepines are regarded as a class of calcium channel blockers (Budriesi et al., 2007), oxadiazol derivatives are established as micobicides (Sahin et al., 2002) and piperidines are established as key components of anti-Parkinson's drugs. Accurate description of the molecular geometry of such molecules are indispensable to proceed with the pharmacological investigations which may prove useful in the design of drugs with a wide range of activities. Also, the role of non-conventional hydrogen bonds viz. C—H···X (X= N, O, Cl, F, etc.) in influencing the geometry of the molecular packing can be unambiguously assessed. Recently, an anlogue of the title compound namely, the crystal structure of 1-(4-chlorophenyl)- 8-(4-fluorophenyl)-4-[(E)-(4-fluorophenyl)methylidene]-6-methyl-4,5,6,7,7a, 8-hexahydro[1,2,4]oxadiazolo[5,4-d]pyrido [3,4-c][1,5] benzothiazepine (Srinivasan et al., 2007) was elucidated.
A least-squares plane calculations show that the 2-chlorophenyl attached to thiazepine, 2-chlorophenyl attached to piperidine and 4-chlorophenyl ring of the oxadiazole ring make a dihedral angle of 34.8 (1) °, 51.3 (1) ° and 73.9 (1) °, respectively, with respect to the benzene fused to the thiazepine ring. The torsion angles about the methylidene bond C4—C40—C41—C42 = 39.8 (2) ° and C4—C40—C41—C46 = -141.8 (2) ° indicates a significant twist of the 2-chlorophenyl ring which may be attributed to steric factors. These values that describe the molecular geometry slightly differ from those observed in the 4-fluoro-4-fluoro- 4-chloro analogue (Srinivasan et al., 2007). The oxadiazole, piperidine and benzothiapezine rings adopt the usually expected envelope, chair and twist-boat conformations, respectively. The molecular aggreagation is characterized by linear chains of centrosymmetrically related pairs extending parallel to the (202) plane and connected through Cl···Cl interactions [Cl1···Cl2(-x+1,-y,-z+2) = 3.533 (2) Å. Other Cl···Cl distances observed are Cl2···Cl2(-x+1,-y+1,-z+1) = 3.826 (2) Å and Cl1···Cl3(x,+y-1,+z+1) = 3.952 (2) Å.
Experimental
2-Methyl-11-(2-chlorophenyl)-4-[(E)-(2-chlorophenyl)methylidene]- 1,2,3,4,11,11a-hexahydro-pyrido[3,4-c][1,5]benzothiazepines (1 mmol) and 4-chloro-N-hydroxybenzenecarboximidoyl chloride (1 mmol) were dissolved in benzene (15 ml). Triethylamine (1 mmol) was added to the mixture and refluxed for 20 to 30 min. After completion of the reaction as evident from thin layer chromatography the triethylamine hydrochloride was filtered off, solvent evaporated, product was purified by column chromatography using petroleum ether:ethyl acetate (90:10 v/v) mixture and finally recrystallized from ethyl acetate to obtain pure 1-(4-chlorophenyl)-8-(2-chlorophenyl) -4-[(E)-(2-chlorophenyl)methylidene]-6-methyl-4,5,6,7,7a,8- hexahydro[1,2,4]oxa-diazolo[5,4-d]pyrido[3,4-c][1,5] benzothiazepine as colorless crystals.
Refinement
H atoms were positioned geometrically and refined using a riding model with C—H = 0.95–0.99 Å and with Uiso(H) = 1.2 (1.5 for methyl groups) times Ueq(C).
Figures
Fig. 1.
The molecular structure of (I), with atom labels and 50% probability displacement ellipsoids for non-H atoms. H atoms have been omitted for clarity.
Fig. 2.
A view of the molecular aggregation down the a--axis. H atoms have been omitted and Cl···Cl interactions are indicated by dashed lines.
Crystal data
| C33H26Cl3N3OS | Z = 2 |
| Mr = 618.98 | F(000) = 640 |
| Triclinic, P1 | Dx = 1.378 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 11.015 (3) Å | Cell parameters from 4125 reflections |
| b = 11.758 (4) Å | θ = 2.0–30.0° |
| c = 11.988 (4) Å | µ = 0.41 mm−1 |
| α = 78.87 (2)° | T = 298 K |
| β = 86.89 (3)° | Needle, colourless |
| γ = 78.29 (2)° | 0.30 × 0.15 × 0.15 mm |
| V = 1491.5 (8) Å3 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 11096 independent reflections |
| Radiation source: fine-focus sealed tube | 7867 reflections with I > 2σ(I) |
| graphite | Rint = 0.023 |
| ω and φ scan | θmax = 33.0°, θmin = 1.7° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2004) | h = −16→16 |
| Tmin = 0.90, Tmax = 0.94 | k = −17→17 |
| 42055 measured reflections | l = −18→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.146 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0605P)2 + 0.6302P] where P = (Fo2 + 2Fc2)/3 |
| 11096 reflections | (Δ/σ)max = 0.001 |
| 372 parameters | Δρmax = 0.64 e Å−3 |
| 0 restraints | Δρmin = −0.62 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.57470 (7) | −0.12556 (6) | 1.22882 (7) | 0.0878 (2) | |
| Cl2 | 0.55422 (5) | 0.34566 (6) | 0.58843 (6) | 0.07294 (17) | |
| Cl3 | 0.71901 (7) | 0.77874 (5) | 0.53545 (4) | 0.0769 (2) | |
| C1 | 0.85278 (14) | 0.22540 (11) | 0.96401 (12) | 0.0336 (3) | |
| N2 | 0.97082 (12) | 0.20144 (11) | 0.95229 (12) | 0.0404 (3) | |
| O3 | 1.00715 (9) | 0.30577 (9) | 0.89247 (9) | 0.0378 (2) | |
| C3A | 0.89581 (12) | 0.37735 (11) | 0.83332 (11) | 0.0300 (2) | |
| C4 | 0.89945 (13) | 0.33994 (11) | 0.71870 (12) | 0.0320 (3) | |
| C5 | 1.01522 (14) | 0.36089 (13) | 0.65093 (13) | 0.0368 (3) | |
| H5A | 1.0857 | 0.3030 | 0.6843 | 0.044* | |
| H5B | 1.0069 | 0.3495 | 0.5739 | 0.044* | |
| N6 | 1.03901 (12) | 0.48001 (11) | 0.64726 (10) | 0.0353 (2) | |
| C7 | 1.03493 (13) | 0.51369 (13) | 0.75868 (13) | 0.0355 (3) | |
| H7A | 1.0489 | 0.5938 | 0.7497 | 0.043* | |
| H7B | 1.1005 | 0.4617 | 0.8053 | 0.043* | |
| C7A | 0.90965 (12) | 0.50621 (11) | 0.81809 (12) | 0.0307 (2) | |
| H71A | 0.9104 | 0.5276 | 0.8931 | 0.037* | |
| C8 | 0.80644 (13) | 0.59400 (11) | 0.74674 (12) | 0.0315 (2) | |
| H8 | 0.8252 | 0.5869 | 0.6672 | 0.038* | |
| S9 | 0.65096 (3) | 0.56089 (3) | 0.77852 (3) | 0.03747 (9) | |
| C9A | 0.65984 (13) | 0.52634 (12) | 0.92758 (13) | 0.0333 (3) | |
| C10 | 0.59577 (15) | 0.60462 (13) | 0.99383 (16) | 0.0439 (3) | |
| H10 | 0.5521 | 0.6780 | 0.9589 | 0.053* | |
| C11 | 0.59637 (17) | 0.57444 (16) | 1.11118 (17) | 0.0484 (4) | |
| H11 | 0.5521 | 0.6266 | 1.1549 | 0.058* | |
| C12 | 0.66289 (16) | 0.46670 (16) | 1.16287 (14) | 0.0437 (3) | |
| H12 | 0.6613 | 0.4453 | 1.2417 | 0.052* | |
| C13 | 0.73233 (14) | 0.38968 (13) | 1.09826 (13) | 0.0368 (3) | |
| H13 | 0.7803 | 0.3188 | 1.1340 | 0.044* | |
| C13A | 0.73021 (12) | 0.41847 (11) | 0.98022 (12) | 0.0303 (2) | |
| N14 | 0.79641 (11) | 0.34047 (9) | 0.91010 (10) | 0.0300 (2) | |
| C61 | 1.15951 (16) | 0.48759 (16) | 0.59264 (15) | 0.0462 (4) | |
| H61A | 1.1759 | 0.5652 | 0.5899 | 0.069* | |
| H61B | 1.1591 | 0.4726 | 0.5168 | 0.069* | |
| H61C | 1.2229 | 0.4299 | 0.6354 | 0.069* | |
| C1E | 0.78313 (14) | 0.13884 (11) | 1.02695 (12) | 0.0350 (3) | |
| C2E | 0.65779 (16) | 0.15047 (14) | 1.01121 (16) | 0.0458 (4) | |
| H2E | 0.6168 | 0.2127 | 0.9579 | 0.055* | |
| C3E | 0.59237 (19) | 0.07021 (16) | 1.07412 (19) | 0.0555 (5) | |
| H3E | 0.5074 | 0.0796 | 1.0650 | 0.067* | |
| C4E | 0.6548 (2) | −0.02356 (15) | 1.15020 (17) | 0.0527 (4) | |
| C5E | 0.7800 (2) | −0.03845 (15) | 1.16498 (16) | 0.0529 (4) | |
| H5E | 0.8211 | −0.1032 | 1.2156 | 0.063* | |
| C6E | 0.84464 (17) | 0.04309 (13) | 1.10441 (14) | 0.0443 (3) | |
| H6E | 0.9293 | 0.0341 | 1.1153 | 0.053* | |
| C40 | 0.80948 (15) | 0.29252 (12) | 0.68793 (12) | 0.0364 (3) | |
| H40 | 0.7441 | 0.2867 | 0.7398 | 0.044* | |
| C41 | 0.79968 (17) | 0.24807 (13) | 0.58271 (13) | 0.0419 (3) | |
| C42 | 0.9013 (2) | 0.18426 (17) | 0.53161 (17) | 0.0557 (5) | |
| H42 | 0.9788 | 0.1682 | 0.5647 | 0.067* | |
| C43 | 0.8879 (3) | 0.1446 (2) | 0.4319 (2) | 0.0757 (7) | |
| H43 | 0.9568 | 0.1037 | 0.3980 | 0.091* | |
| C44 | 0.7738 (3) | 0.1654 (2) | 0.38322 (19) | 0.0815 (8) | |
| H44 | 0.7656 | 0.1387 | 0.3164 | 0.098* | |
| C45 | 0.6718 (3) | 0.2253 (2) | 0.43257 (18) | 0.0690 (6) | |
| H45 | 0.5942 | 0.2383 | 0.4002 | 0.083* | |
| C46 | 0.68514 (19) | 0.26646 (16) | 0.53113 (15) | 0.0503 (4) | |
| C81 | 0.80119 (13) | 0.72208 (12) | 0.75331 (12) | 0.0336 (3) | |
| C82 | 0.82787 (18) | 0.75779 (14) | 0.85131 (15) | 0.0460 (4) | |
| H82 | 0.8572 | 0.7006 | 0.9140 | 0.055* | |
| C83 | 0.8122 (2) | 0.87629 (16) | 0.85885 (19) | 0.0585 (5) | |
| H83 | 0.8297 | 0.8974 | 0.9262 | 0.070* | |
| C84 | 0.7708 (2) | 0.96217 (16) | 0.7669 (2) | 0.0635 (5) | |
| H84 | 0.7607 | 1.0416 | 0.7717 | 0.076* | |
| C85 | 0.7445 (2) | 0.93064 (15) | 0.66774 (18) | 0.0598 (5) | |
| H85 | 0.7168 | 0.9885 | 0.6050 | 0.072* | |
| C86 | 0.75923 (17) | 0.81224 (14) | 0.66168 (14) | 0.0436 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.1016 (5) | 0.0606 (3) | 0.0984 (5) | −0.0388 (3) | 0.0198 (4) | 0.0104 (3) |
| Cl2 | 0.0590 (3) | 0.0861 (4) | 0.0774 (4) | −0.0188 (3) | −0.0099 (3) | −0.0169 (3) |
| Cl3 | 0.1281 (5) | 0.0532 (3) | 0.0411 (2) | 0.0047 (3) | −0.0262 (3) | −0.0051 (2) |
| C1 | 0.0396 (7) | 0.0255 (5) | 0.0314 (6) | 0.0003 (5) | 0.0013 (5) | −0.0025 (5) |
| N2 | 0.0403 (6) | 0.0324 (6) | 0.0411 (7) | 0.0011 (5) | 0.0000 (5) | 0.0022 (5) |
| O3 | 0.0318 (5) | 0.0364 (5) | 0.0399 (5) | −0.0018 (4) | −0.0024 (4) | 0.0015 (4) |
| C3A | 0.0312 (6) | 0.0271 (5) | 0.0302 (6) | −0.0029 (4) | −0.0011 (5) | −0.0043 (4) |
| C4 | 0.0377 (7) | 0.0256 (5) | 0.0318 (6) | −0.0046 (5) | 0.0018 (5) | −0.0054 (4) |
| C5 | 0.0399 (7) | 0.0343 (6) | 0.0366 (7) | −0.0070 (5) | 0.0049 (6) | −0.0091 (5) |
| N6 | 0.0375 (6) | 0.0350 (6) | 0.0335 (6) | −0.0097 (5) | 0.0018 (5) | −0.0044 (4) |
| C7 | 0.0357 (7) | 0.0349 (6) | 0.0371 (7) | −0.0096 (5) | −0.0016 (5) | −0.0067 (5) |
| C7A | 0.0334 (6) | 0.0278 (5) | 0.0314 (6) | −0.0065 (5) | −0.0025 (5) | −0.0055 (5) |
| C8 | 0.0366 (6) | 0.0276 (5) | 0.0303 (6) | −0.0060 (5) | −0.0027 (5) | −0.0048 (4) |
| S9 | 0.03504 (17) | 0.03241 (16) | 0.0429 (2) | −0.00581 (13) | −0.00816 (14) | −0.00064 (13) |
| C9A | 0.0302 (6) | 0.0275 (6) | 0.0415 (7) | −0.0045 (5) | 0.0010 (5) | −0.0063 (5) |
| C10 | 0.0401 (8) | 0.0306 (6) | 0.0599 (10) | −0.0017 (6) | 0.0067 (7) | −0.0133 (6) |
| C11 | 0.0483 (9) | 0.0447 (8) | 0.0581 (10) | −0.0099 (7) | 0.0122 (8) | −0.0266 (8) |
| C12 | 0.0472 (8) | 0.0509 (9) | 0.0387 (8) | −0.0146 (7) | 0.0045 (6) | −0.0187 (7) |
| C13 | 0.0387 (7) | 0.0368 (7) | 0.0357 (7) | −0.0069 (5) | −0.0009 (5) | −0.0092 (5) |
| C13A | 0.0294 (6) | 0.0271 (5) | 0.0349 (6) | −0.0046 (4) | 0.0007 (5) | −0.0080 (5) |
| N14 | 0.0330 (5) | 0.0231 (4) | 0.0310 (5) | −0.0016 (4) | 0.0021 (4) | −0.0031 (4) |
| C61 | 0.0456 (8) | 0.0512 (9) | 0.0429 (8) | −0.0179 (7) | 0.0091 (7) | −0.0052 (7) |
| C1E | 0.0426 (7) | 0.0238 (5) | 0.0351 (7) | −0.0006 (5) | 0.0046 (5) | −0.0046 (5) |
| C2E | 0.0457 (8) | 0.0315 (7) | 0.0553 (10) | −0.0037 (6) | −0.0005 (7) | −0.0006 (6) |
| C3E | 0.0514 (10) | 0.0409 (8) | 0.0724 (13) | −0.0125 (7) | 0.0056 (9) | −0.0042 (8) |
| C4E | 0.0684 (12) | 0.0340 (7) | 0.0553 (10) | −0.0164 (7) | 0.0136 (9) | −0.0042 (7) |
| C5E | 0.0704 (12) | 0.0319 (7) | 0.0474 (9) | −0.0029 (7) | 0.0042 (8) | 0.0057 (6) |
| C6E | 0.0503 (9) | 0.0322 (7) | 0.0434 (8) | 0.0006 (6) | 0.0012 (7) | 0.0006 (6) |
| C40 | 0.0456 (8) | 0.0308 (6) | 0.0342 (7) | −0.0119 (5) | 0.0033 (6) | −0.0062 (5) |
| C41 | 0.0631 (10) | 0.0334 (7) | 0.0341 (7) | −0.0221 (7) | 0.0032 (7) | −0.0061 (5) |
| C42 | 0.0753 (13) | 0.0449 (9) | 0.0521 (10) | −0.0158 (9) | 0.0077 (9) | −0.0201 (8) |
| C43 | 0.118 (2) | 0.0592 (12) | 0.0582 (13) | −0.0252 (13) | 0.0204 (14) | −0.0288 (10) |
| C44 | 0.141 (3) | 0.0757 (15) | 0.0429 (11) | −0.0470 (17) | 0.0015 (14) | −0.0220 (10) |
| C45 | 0.1051 (18) | 0.0694 (13) | 0.0436 (10) | −0.0437 (13) | −0.0145 (11) | −0.0061 (9) |
| C46 | 0.0713 (12) | 0.0456 (8) | 0.0402 (8) | −0.0299 (8) | −0.0033 (8) | −0.0028 (7) |
| C81 | 0.0385 (7) | 0.0273 (5) | 0.0346 (7) | −0.0064 (5) | −0.0007 (5) | −0.0051 (5) |
| C82 | 0.0612 (10) | 0.0329 (7) | 0.0447 (8) | −0.0057 (7) | −0.0120 (7) | −0.0100 (6) |
| C83 | 0.0764 (13) | 0.0389 (8) | 0.0653 (12) | −0.0070 (8) | −0.0167 (10) | −0.0223 (8) |
| C84 | 0.0848 (15) | 0.0293 (7) | 0.0776 (14) | −0.0098 (8) | −0.0056 (11) | −0.0131 (8) |
| C85 | 0.0850 (14) | 0.0296 (7) | 0.0580 (11) | −0.0053 (8) | −0.0037 (10) | 0.0032 (7) |
| C86 | 0.0584 (10) | 0.0328 (7) | 0.0365 (7) | −0.0057 (6) | −0.0010 (7) | −0.0022 (5) |
Geometric parameters (Å, °)
| Cl1—C4E | 1.7345 (19) | C13A—N14 | 1.4279 (17) |
| Cl2—C46 | 1.736 (2) | C61—H61A | 0.9600 |
| Cl3—C86 | 1.7364 (19) | C61—H61B | 0.9600 |
| C1—N2 | 1.279 (2) | C61—H61C | 0.9600 |
| C1—N14 | 1.4138 (17) | C1E—C2E | 1.379 (2) |
| C1—C1E | 1.467 (2) | C1E—C6E | 1.394 (2) |
| N2—O3 | 1.4169 (17) | C2E—C3E | 1.386 (3) |
| O3—C3A | 1.4698 (17) | C2E—H2E | 0.9300 |
| C3A—N14 | 1.4708 (18) | C3E—C4E | 1.376 (3) |
| C3A—C4 | 1.518 (2) | C3E—H3E | 0.9300 |
| C3A—C7A | 1.5285 (19) | C4E—C5E | 1.371 (3) |
| C4—C40 | 1.331 (2) | C5E—C6E | 1.381 (2) |
| C4—C5 | 1.511 (2) | C5E—H5E | 0.9300 |
| C5—N6 | 1.4684 (19) | C6E—H6E | 0.9300 |
| C5—H5A | 0.9700 | C40—C41 | 1.472 (2) |
| C5—H5B | 0.9700 | C40—H40 | 0.9300 |
| N6—C61 | 1.459 (2) | C41—C46 | 1.394 (3) |
| N6—C7 | 1.461 (2) | C41—C42 | 1.398 (3) |
| C7—C7A | 1.529 (2) | C42—C43 | 1.389 (3) |
| C7—H7A | 0.9700 | C42—H42 | 0.9300 |
| C7—H7B | 0.9700 | C43—C44 | 1.372 (4) |
| C7A—C8 | 1.5420 (19) | C43—H43 | 0.9300 |
| C7A—H71A | 0.9800 | C44—C45 | 1.370 (4) |
| C8—C81 | 1.5124 (19) | C44—H44 | 0.9300 |
| C8—S9 | 1.8364 (16) | C45—C46 | 1.386 (3) |
| C8—H8 | 0.9800 | C45—H45 | 0.9300 |
| S9—C9A | 1.7580 (17) | C81—C82 | 1.386 (2) |
| C9A—C10 | 1.391 (2) | C81—C86 | 1.397 (2) |
| C9A—C13A | 1.3974 (19) | C82—C83 | 1.389 (2) |
| C10—C11 | 1.383 (3) | C82—H82 | 0.9300 |
| C10—H10 | 0.9300 | C83—C84 | 1.371 (3) |
| C11—C12 | 1.377 (3) | C83—H83 | 0.9300 |
| C11—H11 | 0.9300 | C84—C85 | 1.373 (3) |
| C12—C13 | 1.389 (2) | C84—H84 | 0.9300 |
| C12—H12 | 0.9300 | C85—C86 | 1.384 (2) |
| C13—C13A | 1.390 (2) | C85—H85 | 0.9300 |
| C13—H13 | 0.9300 | ||
| N2—C1—N14 | 114.41 (13) | N6—C61—H61A | 109.5 |
| N2—C1—C1E | 122.05 (12) | N6—C61—H61B | 109.5 |
| N14—C1—C1E | 123.53 (13) | H61A—C61—H61B | 109.5 |
| C1—N2—O3 | 106.95 (11) | N6—C61—H61C | 109.5 |
| N2—O3—C3A | 105.63 (10) | H61A—C61—H61C | 109.5 |
| O3—C3A—N14 | 101.63 (10) | H61B—C61—H61C | 109.5 |
| O3—C3A—C4 | 105.55 (10) | C2E—C1E—C6E | 119.32 (15) |
| N14—C3A—C4 | 113.86 (11) | C2E—C1E—C1 | 121.26 (13) |
| O3—C3A—C7A | 106.44 (11) | C6E—C1E—C1 | 119.43 (15) |
| N14—C3A—C7A | 117.54 (11) | C1E—C2E—C3E | 120.60 (16) |
| C4—C3A—C7A | 110.41 (11) | C1E—C2E—H2E | 119.7 |
| C40—C4—C5 | 126.87 (13) | C3E—C2E—H2E | 119.7 |
| C40—C4—C3A | 121.26 (13) | C4E—C3E—C2E | 119.13 (19) |
| C5—C4—C3A | 111.85 (12) | C4E—C3E—H3E | 120.4 |
| N6—C5—C4 | 112.64 (11) | C2E—C3E—H3E | 120.4 |
| N6—C5—H5A | 109.1 | C5E—C4E—C3E | 121.18 (16) |
| C4—C5—H5A | 109.1 | C5E—C4E—Cl1 | 119.05 (15) |
| N6—C5—H5B | 109.1 | C3E—C4E—Cl1 | 119.77 (17) |
| C4—C5—H5B | 109.1 | C4E—C5E—C6E | 119.72 (16) |
| H5A—C5—H5B | 107.8 | C4E—C5E—H5E | 120.1 |
| C61—N6—C7 | 109.80 (12) | C6E—C5E—H5E | 120.1 |
| C61—N6—C5 | 108.88 (12) | C5E—C6E—C1E | 120.02 (17) |
| C7—N6—C5 | 113.90 (11) | C5E—C6E—H6E | 120.0 |
| N6—C7—C7A | 110.94 (12) | C1E—C6E—H6E | 120.0 |
| N6—C7—H7A | 109.5 | C4—C40—C41 | 128.64 (14) |
| C7A—C7—H7A | 109.5 | C4—C40—H40 | 115.7 |
| N6—C7—H7B | 109.5 | C41—C40—H40 | 115.7 |
| C7A—C7—H7B | 109.5 | C46—C41—C42 | 117.06 (16) |
| H7A—C7—H7B | 108.0 | C46—C41—C40 | 120.18 (16) |
| C3A—C7A—C7 | 106.66 (11) | C42—C41—C40 | 122.74 (17) |
| C3A—C7A—C8 | 113.68 (11) | C43—C42—C41 | 120.8 (2) |
| C7—C7A—C8 | 109.23 (12) | C43—C42—H42 | 119.6 |
| C3A—C7A—H71A | 109.1 | C41—C42—H42 | 119.6 |
| C7—C7A—H71A | 109.1 | C44—C43—C42 | 120.4 (2) |
| C8—C7A—H71A | 109.1 | C44—C43—H43 | 119.8 |
| C81—C8—C7A | 114.25 (11) | C42—C43—H43 | 119.8 |
| C81—C8—S9 | 108.47 (10) | C45—C44—C43 | 120.3 (2) |
| C7A—C8—S9 | 113.89 (10) | C45—C44—H44 | 119.9 |
| C81—C8—H8 | 106.6 | C43—C44—H44 | 119.9 |
| C7A—C8—H8 | 106.6 | C44—C45—C46 | 119.5 (2) |
| S9—C8—H8 | 106.6 | C44—C45—H45 | 120.3 |
| C9A—S9—C8 | 98.49 (6) | C46—C45—H45 | 120.3 |
| C10—C9A—C13A | 119.60 (14) | C45—C46—C41 | 122.0 (2) |
| C10—C9A—S9 | 120.58 (12) | C45—C46—Cl2 | 117.89 (18) |
| C13A—C9A—S9 | 119.82 (11) | C41—C46—Cl2 | 120.09 (14) |
| C11—C10—C9A | 120.63 (15) | C82—C81—C86 | 116.15 (14) |
| C11—C10—H10 | 119.7 | C82—C81—C8 | 122.89 (13) |
| C9A—C10—H10 | 119.7 | C86—C81—C8 | 120.79 (13) |
| C12—C11—C10 | 119.64 (15) | C81—C82—C83 | 122.09 (16) |
| C12—C11—H11 | 120.2 | C81—C82—H82 | 119.0 |
| C10—C11—H11 | 120.2 | C83—C82—H82 | 119.0 |
| C11—C12—C13 | 120.55 (16) | C84—C83—C82 | 119.96 (18) |
| C11—C12—H12 | 119.7 | C84—C83—H83 | 120.0 |
| C13—C12—H12 | 119.7 | C82—C83—H83 | 120.0 |
| C12—C13—C13A | 120.05 (14) | C83—C84—C85 | 119.84 (17) |
| C12—C13—H13 | 120.0 | C83—C84—H84 | 120.1 |
| C13A—C13—H13 | 120.0 | C85—C84—H84 | 120.1 |
| C13—C13A—C9A | 119.41 (13) | C84—C85—C86 | 119.65 (17) |
| C13—C13A—N14 | 122.17 (12) | C84—C85—H85 | 120.2 |
| C9A—C13A—N14 | 118.41 (13) | C86—C85—H85 | 120.2 |
| C1—N14—C13A | 117.65 (11) | C85—C86—C81 | 122.31 (16) |
| C1—N14—C3A | 102.39 (10) | C85—C86—Cl3 | 117.21 (14) |
| C13A—N14—C3A | 119.70 (11) | C81—C86—Cl3 | 120.46 (12) |
| N14—C1—N2—O3 | 3.72 (17) | C9A—C13A—N14—C3A | 63.38 (17) |
| C1E—C1—N2—O3 | −177.20 (13) | O3—C3A—N14—C1 | −26.29 (12) |
| C1—N2—O3—C3A | −21.41 (15) | C4—C3A—N14—C1 | 86.69 (13) |
| N2—O3—C3A—N14 | 29.51 (12) | C7A—C3A—N14—C1 | −141.96 (12) |
| N2—O3—C3A—C4 | −89.57 (12) | O3—C3A—N14—C13A | 105.95 (12) |
| N2—O3—C3A—C7A | 153.08 (11) | C4—C3A—N14—C13A | −141.07 (12) |
| O3—C3A—C4—C40 | 119.11 (14) | C7A—C3A—N14—C13A | −9.72 (17) |
| N14—C3A—C4—C40 | 8.51 (18) | N2—C1—C1E—C2E | −160.17 (16) |
| C7A—C3A—C4—C40 | −126.24 (14) | N14—C1—C1E—C2E | 18.8 (2) |
| O3—C3A—C4—C5 | −59.56 (14) | N2—C1—C1E—C6E | 20.0 (2) |
| N14—C3A—C4—C5 | −170.17 (11) | N14—C1—C1E—C6E | −161.01 (14) |
| C7A—C3A—C4—C5 | 55.08 (14) | C6E—C1E—C2E—C3E | 2.1 (3) |
| C40—C4—C5—N6 | 132.87 (15) | C1—C1E—C2E—C3E | −177.78 (16) |
| C3A—C4—C5—N6 | −48.55 (16) | C1E—C2E—C3E—C4E | −2.0 (3) |
| C4—C5—N6—C61 | 173.08 (13) | C2E—C3E—C4E—C5E | 0.3 (3) |
| C4—C5—N6—C7 | 50.17 (17) | C2E—C3E—C4E—Cl1 | −179.47 (16) |
| C61—N6—C7—C7A | −179.84 (12) | C3E—C4E—C5E—C6E | 1.3 (3) |
| C5—N6—C7—C7A | −57.44 (16) | Cl1—C4E—C5E—C6E | −178.92 (15) |
| O3—C3A—C7A—C7 | 53.94 (13) | C4E—C5E—C6E—C1E | −1.2 (3) |
| N14—C3A—C7A—C7 | 166.95 (11) | C2E—C1E—C6E—C5E | −0.4 (2) |
| C4—C3A—C7A—C7 | −60.14 (14) | C1—C1E—C6E—C5E | 179.41 (15) |
| O3—C3A—C7A—C8 | 174.39 (11) | C5—C4—C40—C41 | 0.6 (3) |
| N14—C3A—C7A—C8 | −72.60 (15) | C3A—C4—C40—C41 | −177.89 (14) |
| C4—C3A—C7A—C8 | 60.30 (15) | C4—C40—C41—C46 | −141.76 (17) |
| N6—C7—C7A—C3A | 61.13 (14) | C4—C40—C41—C42 | 39.8 (2) |
| N6—C7—C7A—C8 | −62.13 (14) | C46—C41—C42—C43 | 1.9 (3) |
| C3A—C7A—C8—C81 | 166.54 (12) | C40—C41—C42—C43 | −179.61 (17) |
| C7—C7A—C8—C81 | −74.47 (14) | C41—C42—C43—C44 | −1.4 (3) |
| C3A—C7A—C8—S9 | 41.12 (14) | C42—C43—C44—C45 | −0.1 (4) |
| C7—C7A—C8—S9 | 160.11 (9) | C43—C44—C45—C46 | 1.0 (4) |
| C81—C8—S9—C9A | −85.63 (10) | C44—C45—C46—C41 | −0.5 (3) |
| C7A—C8—S9—C9A | 42.81 (11) | C44—C45—C46—Cl2 | 178.16 (17) |
| C8—S9—C9A—C10 | 106.47 (13) | C42—C41—C46—C45 | −1.0 (2) |
| C8—S9—C9A—C13A | −74.58 (12) | C40—C41—C46—C45 | −179.52 (16) |
| C13A—C9A—C10—C11 | −3.0 (2) | C42—C41—C46—Cl2 | −179.57 (13) |
| S9—C9A—C10—C11 | 175.92 (13) | C40—C41—C46—Cl2 | 1.9 (2) |
| C9A—C10—C11—C12 | 1.2 (3) | C7A—C8—C81—C82 | −35.1 (2) |
| C10—C11—C12—C13 | 2.0 (3) | S9—C8—C81—C82 | 93.14 (16) |
| C11—C12—C13—C13A | −3.2 (2) | C7A—C8—C81—C86 | 149.96 (14) |
| C12—C13—C13A—C9A | 1.3 (2) | S9—C8—C81—C86 | −81.81 (16) |
| C12—C13—C13A—N14 | −177.72 (13) | C86—C81—C82—C83 | 0.9 (3) |
| C10—C9A—C13A—C13 | 1.8 (2) | C8—C81—C82—C83 | −174.28 (18) |
| S9—C9A—C13A—C13 | −177.20 (11) | C81—C82—C83—C84 | −1.0 (3) |
| C10—C9A—C13A—N14 | −179.17 (13) | C82—C83—C84—C85 | 0.3 (4) |
| S9—C9A—C13A—N14 | 1.87 (17) | C83—C84—C85—C86 | 0.3 (4) |
| N2—C1—N14—C13A | −118.19 (14) | C84—C85—C86—C81 | −0.4 (3) |
| C1E—C1—N14—C13A | 62.74 (18) | C84—C85—C86—Cl3 | 177.80 (18) |
| N2—C1—N14—C3A | 15.27 (16) | C82—C81—C86—C85 | −0.2 (3) |
| C1E—C1—N14—C3A | −163.80 (13) | C8—C81—C86—C85 | 175.07 (17) |
| C13—C13A—N14—C1 | 7.71 (19) | C82—C81—C86—Cl3 | −178.35 (14) |
| C9A—C13A—N14—C1 | −171.33 (12) | C8—C81—C86—Cl3 | −3.1 (2) |
| C13—C13A—N14—C3A | −117.57 (15) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NC2178).
References
- Boeyens, J. C. A. (1978). J. Cryst. Mol. Struct.8, 317-320.
- Bruker (2004). APEX2 and SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
- Budriesi, R., Cosimelli, B., Ioan, P., Carosati, E., Ugenti, M. P. & Spisani, R. (2007). Curr. Med. Chem.14, 279–287. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Sahin, G., Palaska, E., Ekizoglu, M. & Ozalp, M. (2002). Il Farm.57, 539–542. [DOI] [PubMed]
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Srinivasan, N., Sribala, R., Ranjith Kumar, R., Perumal, S. & Krishnakumar, R. V. (2007). Acta Cryst.E63, o4268.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810013309/nc2178sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810013309/nc2178Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




