Abstract
The asymmetric unit of the title compound,, C4H4N2O5·0.5C4H8O2, contains one molecule of 5,5-dihydroxybarbituric acid with a nearly planar barbiturate ring and half a molecule of 1,4-dioxane. The geometry of the centrosymmetric dioxane molecule is close to an ideal chair conformation. The crystal structure exhibits a complex three-dimensional hydrogen-bonded network. Barbiturate molecules are connected to one another via N—H⋯O=C, O—H⋯O=C and N—H⋯O(hydroxy) interactions, while the barbituric acid molecule is linked to dioxane by an O—H⋯O contact.
Related literature
For the crystal structure of unsolvated 5,5-dihydroxybarbituric acid, see: Singh (1965 ▶); Harrowfield et al. (1989 ▶). For the related monohydrate, see Lewis & Tocher (2004a
▶). For the related trihydrate, see Mootz & Jeffrey (1965 ▶); Lewis & Tocher (2004b
▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶).
Experimental
Crystal data
C4H4N2O5·0.5C4H8O2
M r = 204.14
Triclinic,
a = 6.0232 (3) Å
b = 8.3954 (4) Å
c = 8.6858 (5) Å
α = 106.007 (4)°
β = 94.459 (3)°
γ = 110.126 (3)°
V = 389.09 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.16 mm−1
T = 120 K
0.10 × 0.10 × 0.10 mm
Data collection
Bruker-Nonius Roper CCD camera on κ-goniostat diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2007 ▶) T min = 0.984, T max = 0.984
5726 measured reflections
1529 independent reflections
1198 reflections with I > 2σ(I)
R int = 0.049
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.131
S = 1.01
1529 reflections
148 parameters
4 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.25 e Å−3
Δρmin = −0.29 e Å−3
Data collection: COLLECT (Hooft, 1998 ▶); cell refinement: DENZO (Otwinowski & Minor, 1997 ▶) and COLLECT; data reduction: DENZO and COLLECT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810015321/jh2150sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810015321/jh2150Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O6i | 0.89 (2) | 2.39 (3) | 3.068 (2) | 134 (3) |
| N1—H1N⋯O7ii | 0.89 (2) | 2.44 (2) | 3.180 (2) | 141 (3) |
| N3—H3N⋯O2iii | 0.88 (2) | 1.93 (2) | 2.810 (2) | 172 (2) |
| O7—H7O⋯O1S | 0.87 (2) | 1.87 (2) | 2.732 (2) | 171 (3) |
| O8—H8O⋯O4iv | 0.83 (2) | 1.95 (2) | 2.751 (2) | 162 (3) |
Symmetry codes: (i) 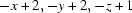 ; (ii)
; (ii) 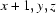 ; (iii)
; (iii) 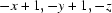 ; (iv)
; (iv) 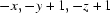 .
.
Acknowledgments
TG acknowledges financial support from the Lise Meitner Program of the Austrian Science Fund (FWF, project LM 1135-N17).
supplementary crystallographic information
Comment
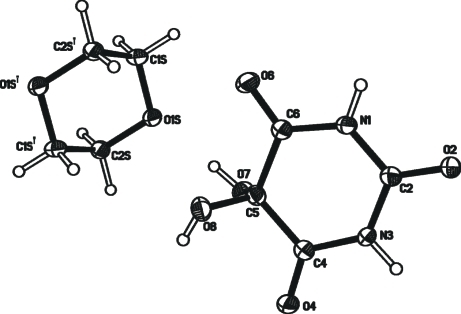
The crystal structures of an unsolvated form (Singh, 1965; Harrowfield et al., 1989), a monohydrate (Lewis & Tocher, 2004a) and a trihydrate (Mootz & Jeffrey, 1965; Lewis & Tocher, 2004b) of 5,5-dihydroxybarbituric acid have been reported previously. The asymmetric unit of the title structure consists of one molecule of the barbituric acid derivative and one half of a dioxane moiety (Fig. 1). The six-membered C4N2 ring of the former is essentially planar, and its bond distances and angles are in agreement with the parameters observed for the unsolvated and hydrate forms.
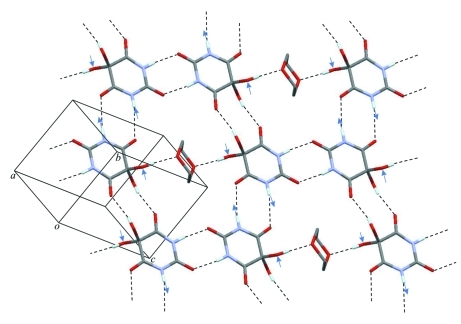
This crystal structure is characterized by extensive hydrogen bonding. Each dihydroxybarbituric acid molecule is linked to two molecules of the same kind via two centrosymmetric N—H···O=C double bridges and a double bridge O—H···O=C connects it to a third molecule. Joining these R22(8) and R22(10) motifs (Bernstein et al., 1995) gives a larger ring of six dihydroxybarbituric acid molecules. Two molecules of each such ring are additionally O—H···O bonded to a dioxane molecule which lies in the centre of the ring. Fig. 2 shows the 2-dimensional H-bonded net parallel to (121) which is obtained from these interactions. Additionally, one NH and one OH group of each dihydroxybarbituric acid molecule are engaged as H-bond donor and acceptor, respectively, in N—H···O(hydroxy) interactions. These particular contacts, indicated by arrows in Fig. 2, connect adjacent H-bonded 2D units of the kind discussed above to one another, and an overall three-dimensional hydrogen bonded network is therefore formed. As expected, the two hydrogen bonds in which the N1—H group is involved exhibit a much less favourable geometry than the single hydrogen bond in which the N3—H group is employed.
Experimental
A solution of 5,5-dibromobarbituric acid (Sigma-Aldrich) in dioxane was filled into an NMR tube for a crystallisation experiment by slow evaporation of the solvent. After four months, an amber-coloured syrup had formed, indicating decomposition of the original compound. This liquid contained a large colourless crystal that prooved to be composed of the title compound.
Refinement
All H atoms were identified in a difference map. H atoms bonded to secondary CH2 (C—H = 0.99 Å) carbon atoms were positioned geometrically, and hydrogen atoms attached to N and O were refined with restrained distances [N—H = 0.88 (2) Å, O—H = 0.82 (2) Å]. The Uiso parameters of all hydrogen atoms were refined freely.
Figures
Fig. 1.
The molecular structures of (I) with displacement ellipsoids drawn at the 50% probability level. Hydrogen atoms are shown as spheres of arbitrary size. Symmetry code: (i) -x+1, -y+2, -z+2.
Fig. 2.
Portion of a hydrogen bonded sheet parallel to (1-21) showing N—H···O=C and O—H···O=C bonds between barbituric acid molecules and O—H···O bonds between barbituric acid and dioxane. N—H···O(hydroxy) interactions linking to two adjacent sheets are indicated by arrows. Dioxane H atoms are omitted for clarity.
Crystal data
| C4H4N2O5·0.5C4H8O2 | Z = 2 |
| Mr = 204.14 | F(000) = 212 |
| Triclinic, P1 | Dx = 1.742 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.0232 (3) Å | Cell parameters from 3190 reflections |
| b = 8.3954 (4) Å | θ = 2.9–26.0° |
| c = 8.6858 (5) Å | µ = 0.16 mm−1 |
| α = 106.007 (4)° | T = 120 K |
| β = 94.459 (3)° | Block, colourless |
| γ = 110.126 (3)° | 0.10 × 0.10 × 0.10 mm |
| V = 389.09 (3) Å3 |
Data collection
| Bruker-Nonius Roper CCD camera on κ-goniostat diffractometer | 1529 independent reflections |
| Radiation source: Bruker–Nonius FR591 rotating anode | 1198 reflections with I > 2σ(I) |
| graphite | Rint = 0.049 |
| Detector resolution: 9.091 pixels mm-1 | θmax = 26.0°, θmin = 3.6° |
| φ & ω scans | h = −7→7 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2007) | k = −10→10 |
| Tmin = 0.984, Tmax = 0.984 | l = −10→10 |
| 5726 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.131 | w = 1/[σ2(Fo2) + (0.0787P)2 + 0.0767P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 1529 reflections | Δρmax = 0.25 e Å−3 |
| 148 parameters | Δρmin = −0.29 e Å−3 |
| 4 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.062 (16) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.7757 (3) | 0.7817 (2) | 0.3822 (2) | 0.0204 (4) | |
| H1N | 0.934 (3) | 0.835 (4) | 0.398 (4) | 0.061 (10)* | |
| N3 | 0.4114 (3) | 0.5803 (2) | 0.2073 (2) | 0.0175 (4) | |
| H3N | 0.342 (4) | 0.506 (3) | 0.107 (2) | 0.029 (6)* | |
| O2 | 0.7627 (3) | 0.65461 (19) | 0.11484 (17) | 0.0242 (4) | |
| O4 | 0.0683 (3) | 0.4836 (2) | 0.29980 (18) | 0.0264 (4) | |
| O6 | 0.8029 (3) | 0.90452 (19) | 0.65175 (17) | 0.0265 (4) | |
| O7 | 0.3029 (2) | 0.83394 (18) | 0.53542 (18) | 0.0212 (4) | |
| H7O | 0.360 (6) | 0.895 (4) | 0.638 (2) | 0.064 (10)* | |
| O8 | 0.3738 (3) | 0.60819 (19) | 0.60882 (17) | 0.0221 (4) | |
| H8O | 0.228 (3) | 0.574 (4) | 0.615 (4) | 0.060 (10)* | |
| C2 | 0.6555 (4) | 0.6706 (3) | 0.2277 (2) | 0.0189 (5) | |
| C4 | 0.2802 (4) | 0.5806 (3) | 0.3280 (2) | 0.0185 (5) | |
| C6 | 0.6801 (3) | 0.8039 (3) | 0.5200 (2) | 0.0186 (5) | |
| C5 | 0.4070 (3) | 0.7064 (3) | 0.5010 (2) | 0.0178 (5) | |
| O1S | 0.4460 (2) | 1.04067 (18) | 0.85656 (16) | 0.0201 (4) | |
| C1S | 0.6766 (4) | 1.1366 (3) | 0.9647 (3) | 0.0218 (5) | |
| H1S1 | 0.6742 | 1.2450 | 1.0457 | 0.030 (6)* | |
| H1S2 | 0.8027 | 1.1755 | 0.9014 | 0.013 (5)* | |
| C2S | 0.2647 (3) | 0.9800 (3) | 0.9481 (2) | 0.0213 (5) | |
| H2S1 | 0.1066 | 0.9113 | 0.8733 | 0.031 (6)* | |
| H2S2 | 0.2530 | 1.0848 | 1.0287 | 0.036 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0145 (9) | 0.0210 (9) | 0.0182 (10) | 0.0021 (8) | 0.0036 (7) | 0.0005 (7) |
| N3 | 0.0161 (9) | 0.0192 (9) | 0.0127 (9) | 0.0042 (7) | 0.0020 (7) | 0.0019 (7) |
| O2 | 0.0192 (8) | 0.0258 (8) | 0.0188 (8) | 0.0030 (6) | 0.0070 (6) | −0.0003 (6) |
| O4 | 0.0159 (8) | 0.0307 (8) | 0.0211 (8) | 0.0004 (7) | 0.0042 (6) | 0.0016 (6) |
| O6 | 0.0191 (8) | 0.0319 (9) | 0.0186 (8) | 0.0056 (7) | 0.0014 (6) | −0.0007 (6) |
| O7 | 0.0190 (8) | 0.0229 (8) | 0.0200 (8) | 0.0092 (6) | 0.0031 (6) | 0.0031 (6) |
| O8 | 0.0214 (8) | 0.0279 (8) | 0.0202 (8) | 0.0096 (7) | 0.0070 (6) | 0.0116 (6) |
| C2 | 0.0179 (10) | 0.0165 (10) | 0.0187 (11) | 0.0036 (8) | 0.0040 (9) | 0.0039 (8) |
| C4 | 0.0170 (10) | 0.0187 (10) | 0.0189 (11) | 0.0056 (8) | 0.0042 (8) | 0.0061 (8) |
| C6 | 0.0166 (10) | 0.0198 (10) | 0.0180 (11) | 0.0060 (8) | 0.0036 (8) | 0.0053 (8) |
| C5 | 0.0162 (10) | 0.0199 (10) | 0.0182 (11) | 0.0069 (8) | 0.0054 (8) | 0.0068 (8) |
| O1S | 0.0158 (7) | 0.0249 (8) | 0.0162 (8) | 0.0054 (6) | 0.0027 (6) | 0.0044 (6) |
| C1S | 0.0152 (10) | 0.0225 (10) | 0.0205 (11) | 0.0019 (8) | −0.0004 (8) | 0.0039 (8) |
| C2S | 0.0138 (10) | 0.0290 (11) | 0.0182 (11) | 0.0059 (9) | 0.0040 (8) | 0.0060 (9) |
Geometric parameters (Å, °)
| N1—C6 | 1.362 (3) | O8—H8O | 0.834 (18) |
| N1—C2 | 1.374 (3) | C4—C5 | 1.532 (3) |
| N1—H1N | 0.886 (18) | C6—C5 | 1.536 (3) |
| N3—C4 | 1.361 (3) | O1S—C2S | 1.435 (2) |
| N3—C2 | 1.373 (3) | O1S—C1S | 1.439 (2) |
| N3—H3N | 0.883 (17) | C1S—C2Si | 1.504 (3) |
| O2—C2 | 1.217 (2) | C1S—H1S1 | 0.9900 |
| O4—C4 | 1.216 (2) | C1S—H1S2 | 0.9900 |
| O6—C6 | 1.214 (2) | C2S—C1Si | 1.504 (3) |
| O7—C5 | 1.394 (2) | C2S—H2S1 | 0.9900 |
| O7—H7O | 0.867 (18) | C2S—H2S2 | 0.9900 |
| O8—C5 | 1.392 (2) | ||
| C6—N1—C2 | 126.66 (17) | O8—C5—C4 | 109.42 (16) |
| C6—N1—H1N | 115 (2) | O7—C5—C4 | 105.53 (15) |
| C2—N1—H1N | 118 (2) | O8—C5—C6 | 106.79 (16) |
| C4—N3—C2 | 125.94 (17) | O7—C5—C6 | 108.58 (15) |
| C4—N3—H3N | 119.4 (16) | C4—C5—C6 | 114.24 (16) |
| C2—N3—H3N | 114.2 (16) | C2S—O1S—C1S | 109.37 (15) |
| C5—O7—H7O | 105 (2) | O1S—C1S—C2Si | 110.63 (16) |
| C5—O8—H8O | 107 (2) | O1S—C1S—H1S1 | 109.5 |
| O2—C2—N3 | 122.08 (19) | C2Si—C1S—H1S1 | 109.5 |
| O2—C2—N1 | 120.85 (18) | O1S—C1S—H1S2 | 109.5 |
| N3—C2—N1 | 117.07 (17) | C2Si—C1S—H1S2 | 109.5 |
| O4—C4—N3 | 121.06 (19) | H1S1—C1S—H1S2 | 108.1 |
| O4—C4—C5 | 120.76 (18) | O1S—C2S—C1Si | 110.95 (16) |
| N3—C4—C5 | 118.18 (17) | O1S—C2S—H2S1 | 109.4 |
| O6—C6—N1 | 121.57 (18) | C1Si—C2S—H2S1 | 109.4 |
| O6—C6—C5 | 120.87 (17) | O1S—C2S—H2S2 | 109.4 |
| N1—C6—C5 | 117.43 (17) | C1Si—C2S—H2S2 | 109.4 |
| O8—C5—O7 | 112.40 (16) | H2S1—C2S—H2S2 | 108.0 |
| C4—N3—C2—O2 | 175.20 (18) | N3—C4—C5—O7 | 112.76 (19) |
| C4—N3—C2—N1 | −5.1 (3) | O4—C4—C5—C6 | 173.36 (17) |
| C6—N1—C2—O2 | −176.10 (18) | N3—C4—C5—C6 | −6.4 (2) |
| C6—N1—C2—N3 | 4.2 (3) | O6—C6—C5—O8 | −57.3 (2) |
| C2—N3—C4—O4 | −173.22 (18) | N1—C6—C5—O8 | 126.73 (18) |
| C2—N3—C4—C5 | 6.6 (3) | O6—C6—C5—O7 | 64.2 (2) |
| C2—N1—C6—O6 | 179.18 (18) | N1—C6—C5—O7 | −111.85 (19) |
| C2—N1—C6—C5 | −4.8 (3) | O6—C6—C5—C4 | −178.39 (17) |
| O4—C4—C5—O8 | 53.7 (2) | N1—C6—C5—C4 | 5.6 (2) |
| N3—C4—C5—O8 | −126.09 (18) | C2S—O1S—C1S—C2Si | 57.5 (2) |
| O4—C4—C5—O7 | −67.4 (2) | C1S—O1S—C2S—C1Si | −57.7 (2) |
Symmetry codes: (i) −x+1, −y+2, −z+2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O6ii | 0.89 (2) | 2.39 (3) | 3.068 (2) | 134 (3) |
| N1—H1N···O7iii | 0.89 (2) | 2.44 (2) | 3.180 (2) | 141 (3) |
| N3—H3N···O2iv | 0.88 (2) | 1.93 (2) | 2.810 (2) | 172 (2) |
| O7—H7O···O1S | 0.87 (2) | 1.87 (2) | 2.732 (2) | 171 (3) |
| O8—H8O···O4v | 0.83 (2) | 1.95 (2) | 2.751 (2) | 162 (3) |
Symmetry codes: (ii) −x+2, −y+2, −z+1; (iii) x+1, y, z; (iv) −x+1, −y+1, −z; (v) −x, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JH2150).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Harrowfield, J. M., Skelton, B. W., Soudi, A. A. & White, A. H. (1989). Aust. J. Chem.42, 1795–1798.
- Hooft, R. W. W. (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Lewis, T. C. & Tocher, D. A. (2004a). Acta Cryst. E60, o1689–o1690.
- Lewis, T. C. & Tocher, D. A. (2004b). Acta Cryst. E60, o1748–o1750.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst.41, 466–470.
- Mootz, D. & Jeffrey, G. A. (1965). Acta Cryst.19, 717–725. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2007). SADABS University of Göttingen,Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Singh, C. (1965). Acta Cryst.19, 759–767.
- Westrip, S. P. (2010). J. Appl. Cryst.43 Submitted.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810015321/jh2150sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810015321/jh2150Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report