Abstract
The NAD(P)H:menadione oxidoreductase gene (Nmo-1) codes for a quinone reductase (also called DT diaphorase; EC 1.6.99.2) believed to play a central role in protection against oxidative stress. We have studied mice with a radiation-induced chromosomal deletion involving the albino locus (c) on chromosome 7 and found that Nmo-1 mRNA levels and the rate of Nmo-1 gene transcription are markedly increased (greater than 100-fold and greater than 12-fold, respectively) in the untreated c14CoS/c14CoS deletion homozygote, compared with the untreated Cch/Cch wild-type and the Cch/C14CoS heterozygote. These data suggest that a gene located on chromosome 7 encodes a trans-acting regulatory factor that might be a negative effector of the Nmo-1 gene, which we show here is located on chromosome 8 approximately 1.4 centimorgans (about 1000 kilobase pairs) from the Es-2 gene. Conversely, there are no detectable basal levels of cytochrome P1450 (Cyp1a1 gene) or cytochrome P3450 (Cyp1a2 gene) mRNA, indicating that the regulation of basal expression of the Cyp1a1 and Cyp1a2 genes is distinct from that of the Nmo-1 gene. Moreover, the Cyp1a1 and Cyp1a2 genes and the Nmo-1 gene are induced by tetrachlorodibenzo-p-dioxin in the cch/cch, cch/c14CoS, and c14CoS/c14CoS mice. The mechanism of tetrachlorodibenzo-p-dioxin inducibility of the Cyp1a1, Cyp1a2, and Nmo-1 genes is, therefore, independent of the mechanism of Nmo-1 gene activation in untreated c14CoS/c14CoS mice.
Full text
PDF

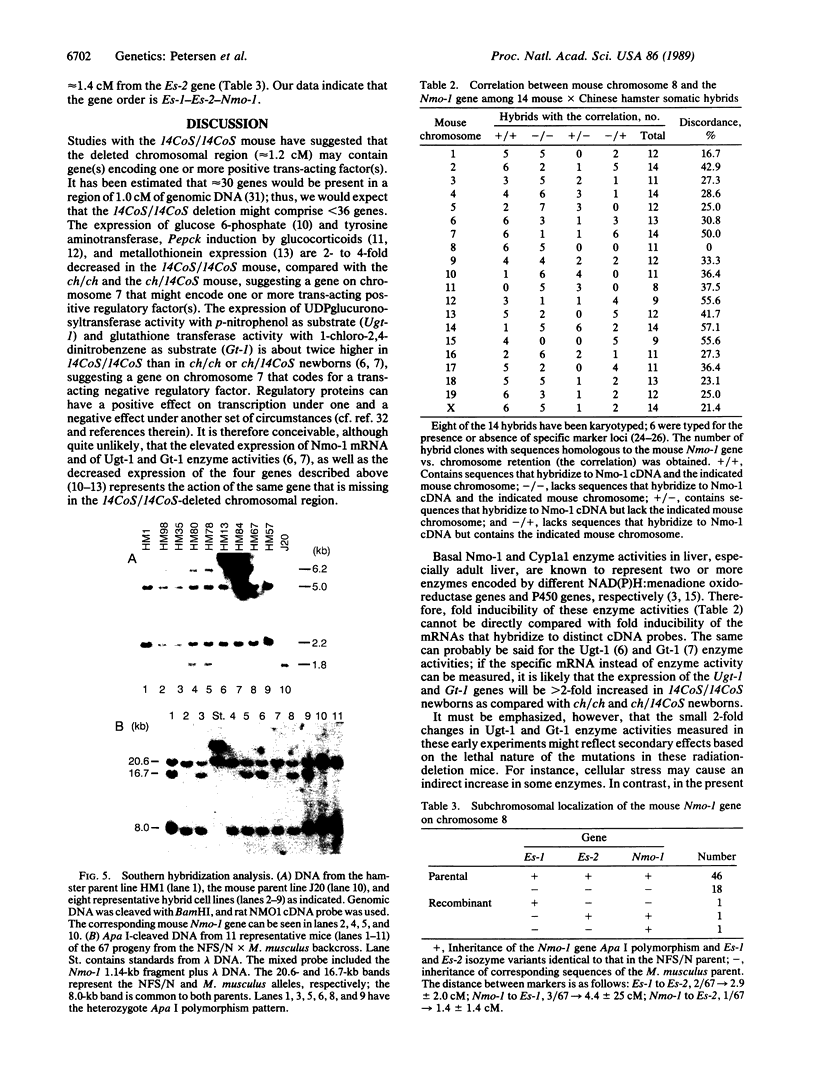

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Waterman M. L., He X., Rosenfeld M. G. Steroid receptor-mediated inhibition of rat prolactin gene expression does not require the receptor DNA-binding domain. Cell. 1988 Mar 11;52(5):685–695. doi: 10.1016/0092-8674(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Chesis P. L., Levin D. E., Smith M. T., Ernster L., Ames B. N. Mutagenicity of quinones: pathways of metabolic activation and detoxification. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1696–1700. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb R. R., Stoming T. A., Whitney J. B., 3rd The aryl hydrocarbon hydroxylase (Ah) locus and a novel restriction-fragment length polymorphism (RFLP) are located on mouse chromosome 12. Biochem Genet. 1987 Jun;25(5-6):401–413. doi: 10.1007/BF00554549. [DOI] [PubMed] [Google Scholar]
- De Flora S., Morelli A., Basso C., Romano M., Serra D., De Flora A. Prominent role of DT-diaphorase as a cellular mechanism reducing chromium(VI) and reverting its mutagenicity. Cancer Res. 1985 Jul;45(7):3188–3196. [PubMed] [Google Scholar]
- DeFranco D., Morris S. M., Jr, Leonard C. M., Gluecksohn-Waelsch S. Metallothionein mRNA expression in mice homozygous for chromosomal deletions around the albino locus. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1161–1164. doi: 10.1073/pnas.85.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gatmaitan Z., Lewis S., Turchin H., Arias I. M. Premature development of ligandin (GSH transferase B) in mice with an inherited defect in endoplasmic reticulum-Golgi structure and function. Biochem Biophys Res Commun. 1977 Mar 21;75(2):337–341. doi: 10.1016/0006-291x(77)91047-6. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Tukey R. H., Nebert D. W. Structural gene products of the Ah locus. Transcriptional regulation of cytochrome P1-450 and P3-450 mRNA levels by 3-methylcholanthrene. Mol Pharmacol. 1984 Jul;26(1):117–121. [PubMed] [Google Scholar]
- Henderson N. S. Intracellular location and genetic control of isozymes of NADP-dependent isocitrate dehydrogenase and malate dehydrogenase. Ann N Y Acad Sci. 1968 Jun 14;151(1):429–440. doi: 10.1111/j.1749-6632.1968.tb11906.x. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Gonzalez F. J., Kozak C. A., Nebert D. W. Regional linkage analysis of the dioxin-inducible P-450 gene family on mouse chromosome 9. Biochem Biophys Res Commun. 1985 Jul 16;130(1):396–406. doi: 10.1016/0006-291x(85)90430-9. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., McBride O. W., Adesnik M., Nebert D. W. Human dioxin-inducible cytosolic NAD(P)H:menadione oxidoreductase. cDNA sequence and localization of gene to chromosome 16. J Biol Chem. 1988 Sep 25;263(27):13572–13578. [PubMed] [Google Scholar]
- Kimura S., Donovan J. C., Nebert D. W. Expression of the mouse P(1)450 gene during differentiation without foreign chemical stimulation. J Exp Pathol. 1987 Winter;3(1):61–74. [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic murine leukemia virus-inducing locus of BALB/c mouse to chromosome 5. Science. 1979 Apr 6;204(4388):69–71. doi: 10.1126/science.219475. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic virus-inducing locus Akv-2 of the AKR mouse. J Exp Med. 1980 Nov 1;152(5):1419–1423. doi: 10.1084/jem.152.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C., Nichols E., Ruddle F. H. Gene linkage analysis in the mouse by somatic cell hybridization: assignment of adenine phosphoribosyltransferase to chromosome 8 and alpha-galactosidase to the X chromosome. Somatic Cell Genet. 1975 Oct;1(4):371–382. doi: 10.1007/BF01538668. [DOI] [PubMed] [Google Scholar]
- Landegren U., Kaiser R., Caskey C. T., Hood L. DNA diagnostics--molecular techniques and automation. Science. 1988 Oct 14;242(4876):229–237. doi: 10.1126/science.3051381. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lind C. Formation of benzo[a]pyrene-3,6-quinol mono- and diglucuronides in rat liver microsomes. Arch Biochem Biophys. 1985 Jul;240(1):226–235. doi: 10.1016/0003-9861(85)90027-x. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Shaw P. A., Krauter K. S., Robinson C., Englard S., Hanson R. W., Gluecksohn-Waelsch S. Trans regulation of the phosphoenolpyruvate carboxykinase (GTP) gene, identified by deletions in chromosome 7 of the mouse. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5184–5188. doi: 10.1073/pnas.83.14.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Nadeau J. H., Kömpf J., Siebert G., Taylor B. A. Linkage of Pgm-3 in the house mouse and homologies of three phosphoglucomutase loci in mouse and man. Biochem Genet. 1981 Jun;19(5-6):465–474. doi: 10.1007/BF00484619. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Genetic differences in microsomal electron transport: the Ah locus. Methods Enzymol. 1978;52:226–240. doi: 10.1016/s0076-6879(78)52026-0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jensen N. M. The Ah locus: genetic regulation of the metabolism of carcinogens, drugs, and other environmental chemicals by cytochrome P-450-mediated monooxygenases. CRC Crit Rev Biochem. 1979;6(4):401–437. doi: 10.3109/10409237909105427. [DOI] [PubMed] [Google Scholar]
- Poland A., Glover E., Taylor B. A. The murine Ah locus: a new allele and mapping to chromosome 12. Mol Pharmacol. 1987 Oct;32(4):471–478. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Chen H. C., Nebert D. W. NAD(P)H:menadione oxidoreductase. Novel purification of enzyme cDNA and complete amino acid sequence, and gene regulation. J Biol Chem. 1986 Nov 25;261(33):15794–15799. [PubMed] [Google Scholar]
- Russell L. B., Montgomery C. S., Raymer G. D. Analysis of the albino-locus region of the mouse: IV. Characterization of 34 deficiencies. Genetics. 1982 Mar;100(3):427–453. doi: 10.1093/genetics/100.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Granner D. K. Regulation of phosphoenolpyruvate carboxykinase gene transcription by insulin and cAMP: reciprocal actions on initiation and elongation. Proc Natl Acad Sci U S A. 1988 May;85(9):2954–2958. doi: 10.1073/pnas.85.9.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid W., Müller G., Schütz G., Gluecksohn-Waelsch S. Deletions near the albino locus on chromosome 7 of the mouse affect the level of tyrosine aminotransferase mRNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2866–2869. doi: 10.1073/pnas.82.9.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R. C., Zannoni V. G. DT-diaphorase and peroxidase influence the covalent binding of the metabolites of phenol, the major metabolite of benzene. Mol Pharmacol. 1984 Jul;26(1):105–111. [PubMed] [Google Scholar]
- Thaler M. M., Erickson R. P., Pelger A. Genetically determined abnormalities of microsomal enzymes in liver of mutant newborn mice. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1244–1250. doi: 10.1016/s0006-291x(76)80148-9. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Gonzalez F. J., Nebert D. W. Developmental and tissue-specific differential regulation of the mouse dioxin-inducible P1-450 and P3-450 genes. Dev Biol. 1985 Nov;112(1):177–184. doi: 10.1016/0012-1606(85)90131-9. [DOI] [PubMed] [Google Scholar]
- Winkler H., Adam G., Mattes E., Schanz M., Hartig A., Ruis H. Co-ordinate control of synthesis of mitochondrial and non-mitochondrial hemoproteins: a binding site for the HAP1 (CYP1) protein in the UAS region of the yeast catalase T gene (CTT1). EMBO J. 1988 Jun;7(6):1799–1804. doi: 10.1002/j.1460-2075.1988.tb03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]