Abstract
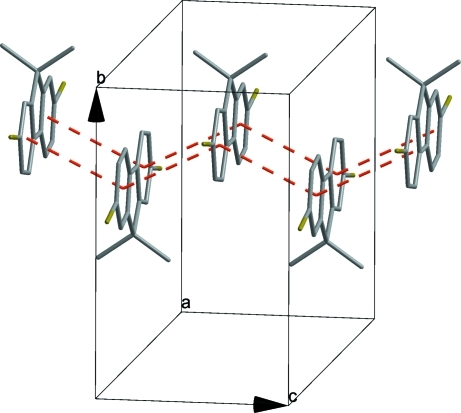
The title molecule, C15H15Br2, has crystallographic m2m site symmetry. As a result, all atoms, except for those of the methyl groups, are exactly coplanar. In the crystal structure, there are weak π–π interactions with a centroid–centroid distance of 3.8409 (15) Å between symmetry-related molecules, which stack along the c axis.
Related literature
For applications of fluorene derivatives, see: Holder et al. (2005 ▶); Kulkarni et al. (2004 ▶); Padmaperuma et al. (2006 ▶); Seneclauze et al. (2007 ▶); Tsuboyama et al. (2003 ▶). For the properties of fluorene-based molecules, see: Scherf & List (2002 ▶). For the synthesis of the title compound, see: Belfield et al. (2000 ▶).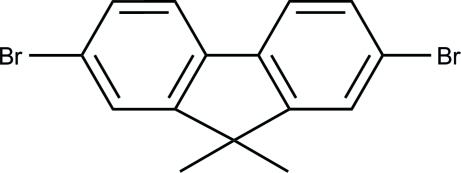
Experimental
Crystal data
C15H12Br2
M r = 352.07
Orthorhombic,
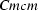
a = 17.097 (4) Å
b = 11.161 (3) Å
c = 6.9120 (17) Å
V = 1319.0 (6) Å3
Z = 4
Mo Kα radiation
μ = 6.12 mm−1
T = 296 K
0.38 × 0.36 × 0.32 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.083, T max = 1.000
3295 measured reflections
662 independent reflections
499 reflections with I > 2σ(I)
R int = 0.047
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.085
S = 1.05
662 reflections
54 parameters
H-atom parameters constrained
Δρmax = 0.42 e Å−3
Δρmin = −0.38 e Å−3
Data collection: SMART-NT (Bruker, 1998 ▶); cell refinement: SAINT-NT (Bruker, 1998 ▶); data reduction: SAINT-NT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg & Berndt, 1999 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810012171/lh5021sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810012171/lh5021Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors acknowledge the Tianjin Binhai Hi-tech Industry Park Management Committee, Huayuan Industrial Park and Haitai Green Industrial Base for support of this work.
supplementary crystallographic information
Comment
Because of their good thermal and chemical stability along with high emission efficiency, fluorene derivatives have shown many applications as electronic materials, especially for organic light emitting diodes (OLEDs) (Holder et al., 2005; Kulkarni et al., 2004; Seneclauze et al., 2007; Padmaperuma et al., 2006; Tsuboyama et al., 2003). In this regard, small molecules, oligomers, or polymers with the 9,9-dialkylfluorene subunit possess interesting emissive properties. The quality and efficiency of such OLEDs have been shown to depend crucially on the stacking mode of the fluorene motif. On the other hand, the selected alkyl groups with different lengths or branched alkyl chains have a deep influence on the property and the packing mode of fluorene-based molecules (Scherf & List, 2002). During our study on such OLEDs crystalline materials, the crystal structure of the title compound has been determined in order to elucidate its molecular conformation and packing mode, which may be useful for further understanding its properties.
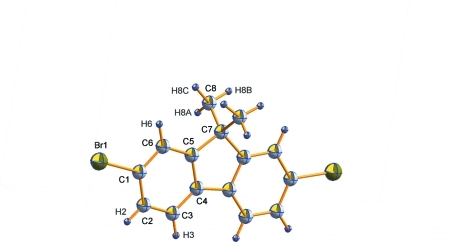
The molecular structure of the title compound is shown in Fig. 1. The complete molecule is generated two mirror planes which intersect each other [ crystallographic m2m site symmetry]. As a result, all the carbon atoms [except for those of the methyl groups] and the bromide atoms are exactly co-planar. In the crystal structure, weak π–π interactions between symmetry related benzene rings [C1-C6] with a centroid to centroid distance of 3.8409 (15) Å and perpendicular distance of 3.456 (1) Å form a one-dimensional chain along the c axis (see Fig. 2).
Experimental
The title compound was prepared according to the literature method (Belfield et al., 2000). Single crystals suitable for X-ray diffraction were obtained by recrystallization of a solution of the title compound in a mixture of ethyl acetate and petroleum ether.
Refinement
H atoms were positioned geometrically and refined as riding atoms, with C—H = 0.96Å and Uiso(H) = 1.5Ueq(C) for methyl H atoms, and C—H = 0.93Å and Uiso(H) = 1.2Ueq(C) for all aromatic H atoms
Figures
Fig. 1.
Molecular structure of title compound with the atom labeling of the asymmetric unit, showing displacement ellipsoids at the 30% probability level.
Fig. 2.
Part of the crystal structure with π–π interactions shown as red dashed lines.
Crystal data
| C15H12Br2 | F(000) = 688 |
| Mr = 352.07 | Dx = 1.773 Mg m−3 |
| Orthorhombic, Cmcm | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2c 2 | Cell parameters from 958 reflections |
| a = 17.097 (4) Å | θ = 2.4–24.1° |
| b = 11.161 (3) Å | µ = 6.12 mm−1 |
| c = 6.9120 (17) Å | T = 296 K |
| V = 1319.0 (6) Å3 | Block, colourless |
| Z = 4 | 0.38 × 0.36 × 0.32 mm |
Data collection
| Bruker SMART CCD diffractometer | 662 independent reflections |
| Radiation source: fine-focus sealed tube | 499 reflections with I > 2σ(I) |
| graphite | Rint = 0.047 |
| φ and ω scans | θmax = 25.0°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −18→20 |
| Tmin = 0.083, Tmax = 1.000 | k = −13→11 |
| 3295 measured reflections | l = −7→8 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.032 | H-atom parameters constrained |
| wR(F2) = 0.085 | w = 1/[σ2(Fo2) + (0.0321P)2 + 2.5078P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max < 0.001 |
| 662 reflections | Δρmax = 0.42 e Å−3 |
| 54 parameters | Δρmin = −0.38 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0097 (9) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Br1 | 0.19165 (3) | 0.13056 (5) | 0.2500 | 0.0696 (4) | |
| C1 | 0.3008 (3) | 0.0990 (4) | 0.2500 | 0.0416 (11) | |
| C2 | 0.3248 (3) | −0.0185 (4) | 0.2500 | 0.0407 (12) | |
| H2 | 0.2883 | −0.0804 | 0.2500 | 0.049* | |
| C3 | 0.4037 (3) | −0.0430 (4) | 0.2500 | 0.0373 (11) | |
| H3 | 0.4210 | −0.1220 | 0.2500 | 0.045* | |
| C4 | 0.4574 (3) | 0.0500 (3) | 0.2500 | 0.0318 (10) | |
| C5 | 0.4314 (3) | 0.1684 (4) | 0.2500 | 0.0332 (10) | |
| C6 | 0.3527 (3) | 0.1946 (4) | 0.2500 | 0.0392 (11) | |
| H6 | 0.3350 | 0.2734 | 0.2500 | 0.047* | |
| C7 | 0.5000 | 0.2560 (5) | 0.2500 | 0.0373 (15) | |
| C8 | 0.5000 | 0.3344 (4) | 0.0682 (8) | 0.0533 (14) | |
| H8A | 0.5000 | 0.2845 | −0.0442 | 0.080* | |
| H8B | 0.5481 | 0.3785 | 0.0613 | 0.080* | 0.50 |
| H8C | 0.4569 | 0.3894 | 0.0736 | 0.080* | 0.50 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0319 (4) | 0.0648 (5) | 0.1121 (6) | 0.0034 (3) | 0.000 | 0.000 |
| C1 | 0.026 (2) | 0.048 (3) | 0.051 (3) | 0.001 (2) | 0.000 | 0.000 |
| C2 | 0.038 (3) | 0.039 (3) | 0.046 (3) | −0.006 (2) | 0.000 | 0.000 |
| C3 | 0.043 (3) | 0.026 (2) | 0.043 (3) | −0.0029 (19) | 0.000 | 0.000 |
| C4 | 0.034 (2) | 0.029 (2) | 0.033 (2) | 0.0008 (18) | 0.000 | 0.000 |
| C5 | 0.036 (3) | 0.028 (2) | 0.036 (2) | −0.0029 (19) | 0.000 | 0.000 |
| C6 | 0.036 (3) | 0.031 (2) | 0.050 (3) | 0.006 (2) | 0.000 | 0.000 |
| C7 | 0.032 (3) | 0.027 (3) | 0.053 (4) | 0.000 | 0.000 | 0.000 |
| C8 | 0.047 (3) | 0.042 (2) | 0.071 (4) | 0.000 | 0.000 | 0.017 (3) |
Geometric parameters (Å, °)
| Br1—C1 | 1.899 (4) | C5—C6 | 1.377 (6) |
| C1—C2 | 1.374 (7) | C5—C7 | 1.528 (6) |
| C1—C6 | 1.388 (7) | C6—H6 | 0.9300 |
| C2—C3 | 1.377 (6) | C7—C5i | 1.528 (6) |
| C2—H2 | 0.9300 | C7—C8ii | 1.531 (6) |
| C3—C4 | 1.386 (6) | C7—C8 | 1.531 (6) |
| C3—H3 | 0.9300 | C8—H8A | 0.9561 |
| C4—C5 | 1.394 (6) | C8—H8B | 0.9600 |
| C4—C4i | 1.457 (9) | C8—H8C | 0.9600 |
| C2—C1—C6 | 122.9 (4) | C5—C6—C1 | 117.5 (4) |
| C2—C1—Br1 | 118.1 (4) | C5—C6—H6 | 121.2 |
| C6—C1—Br1 | 119.1 (4) | C1—C6—H6 | 121.2 |
| C1—C2—C3 | 118.8 (4) | C5—C7—C5i | 100.3 (5) |
| C1—C2—H2 | 120.6 | C5—C7—C8ii | 111.47 (14) |
| C3—C2—H2 | 120.6 | C5i—C7—C8ii | 111.47 (14) |
| C2—C3—C4 | 120.0 (4) | C5—C7—C8 | 111.47 (14) |
| C2—C3—H3 | 120.0 | C5i—C7—C8 | 111.47 (14) |
| C4—C3—H3 | 120.0 | C8ii—C7—C8 | 110.3 (5) |
| C3—C4—C5 | 119.9 (4) | C7—C8—H8A | 109.5 |
| C3—C4—C4i | 131.5 (2) | C7—C8—H8B | 109.5 |
| C5—C4—C4i | 108.6 (3) | H8A—C8—H8B | 104.9 |
| C6—C5—C4 | 120.9 (4) | C7—C8—H8C | 109.5 |
| C6—C5—C7 | 127.9 (4) | H8A—C8—H8C | 113.8 |
| C4—C5—C7 | 111.2 (4) | H8B—C8—H8C | 109.5 |
| C6—C1—C2—C3 | 0.0 | C7—C5—C6—C1 | 180.0 |
| Br1—C1—C2—C3 | 180.0 | C2—C1—C6—C5 | 0.0 |
| C1—C2—C3—C4 | 0.0 | Br1—C1—C6—C5 | 180.0 |
| C2—C3—C4—C5 | 0.0 | C6—C5—C7—C5i | 180.0 |
| C2—C3—C4—C4i | 180.0 | C4—C5—C7—C5i | 0.0 |
| C3—C4—C5—C6 | 0.0 | C6—C5—C7—C8ii | 61.9 (3) |
| C4i—C4—C5—C6 | 180.0 | C4—C5—C7—C8ii | −118.1 (3) |
| C3—C4—C5—C7 | 180.0 | C6—C5—C7—C8 | −61.9 (3) |
| C4i—C4—C5—C7 | 0.0 | C4—C5—C7—C8 | 118.1 (3) |
| C4—C5—C6—C1 | 0.0 |
Symmetry codes: (i) −x+1, y, −z+1/2; (ii) x, y, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5021).
References
- Belfield, K. D., Schafer, K. J., Mourad, W. & Reinhardt, B. A. (2000). J. Org. Chem.65, 4475–4481. [DOI] [PubMed]
- Brandenburg, K. & Berndt, M. (1999). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bruker (1998). SMART-NT and SAINT-NT Bruker AXS Inc., Madison, Wisconsin, USA.
- Holder, E., Langeveld, B. M. W. & Schubert, U. S. (2005). Adv. Mater.17, 1109–1121.
- Kulkarni, A. P., Tonzola, C. J., Babel, A. & Jenekhe, S. A. (2004). Chem. Mater.16, 4556–4573.
- Padmaperuma, A. B., Sapochak, L. S. & Burrows, P. E. (2006). Chem. Mater.18, 2389–2396.
- Scherf, U. & List, E. J. W. (2002). Adv. Mater.14, 447–487.
- Seneclauze, J. B., Retailleau, P. & Ziessel, R. (2007). New J. Chem.31, 1412–1416.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tsuboyama, A., Iwawaki, H., Furugori, M., Mukaide, T., Kamatani, J., Igawa, S., Moriyama, T., Miura, S., Takiguchi, T., Okada, S., Hoshino, M. & Ueno, K. (2003). J. Am. Chem. Soc.125, 12971–12979. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810012171/lh5021sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810012171/lh5021Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report