Abstract
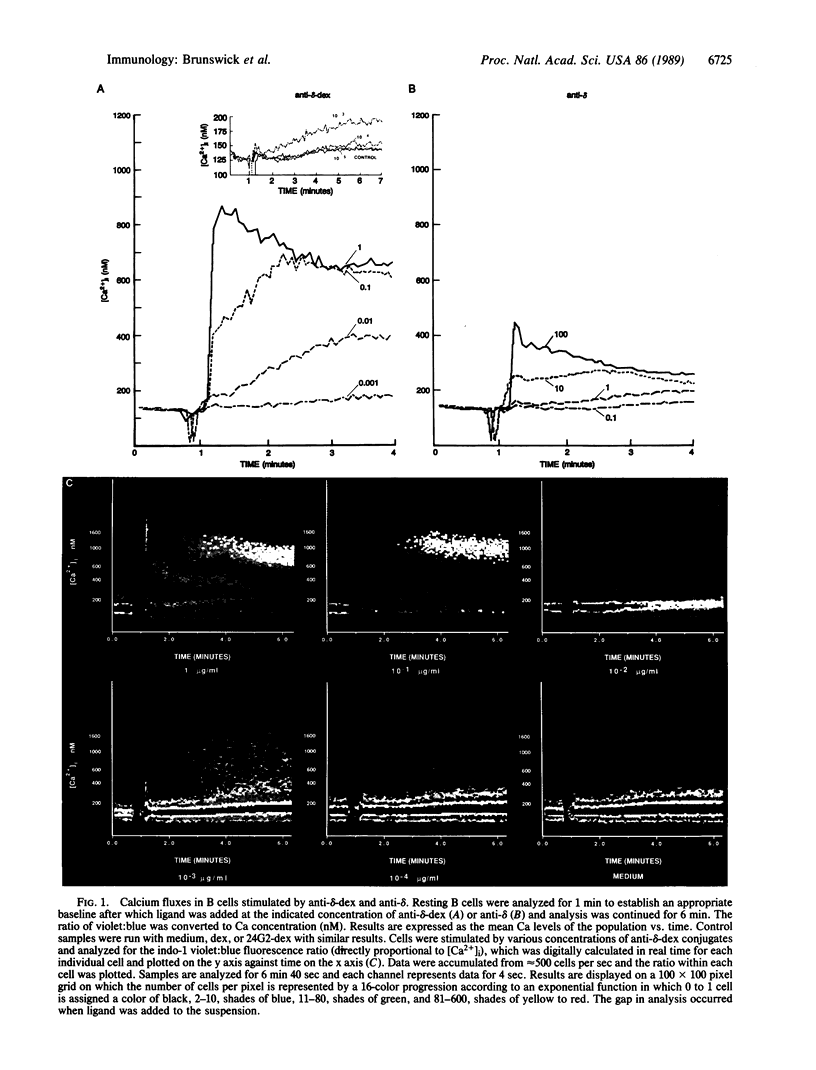
We recently showed that anti-immunoglobulin conjugated to high molecular weight dextran is 1000-fold more mitogenic for B cells than unconjugated anti-immunoglobulin. This system serves as a model for T-cell-independent type 2 antigens such as haptenated Ficoll, dextran, and bacterial polysaccharides, which can also stimulate B-cell proliferation and antibody production at low concentrations. We show here that conjugated anti-immunoglobulin, at concentrations that stimulate significant increases in expression of major histocompatibility complex class II molecules and incorporation of thymidine into DNA, does not induce detectable modulation of surface immunoglobulin. These results indicate that the facilitated T-cell-independent B-cell activation by polysaccharide antigens may result from inability to modulate surface immunoglobulin, possibly resulting in persistent and/or repetitive signaling. Early large increases in Ca2+ and breakdown of inositol phospholipids presently thought to be involved in transduction of the mitogenic signal are not detectable at low concentrations of conjugated anti-immunoglobulin, raising the possibility that these biochemical events may not in fact be central to this signaling pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Klaus G. G. Crosslinking of surface immunoglobulin and Fc receptors on B lymphocytes inhibits stimulation of inositol phospholipid breakdown via the antigen receptors. J Exp Med. 1985 Dec 1;162(6):1825–1836. doi: 10.1084/jem.162.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick M., Finkelman F. D., Highet P. F., Inman J. K., Dintzis H. M., Mond J. J. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J Immunol. 1988 May 15;140(10):3364–3372. [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Dennis G., June C. H., Mizuguchi J., Ohara J., Witherspoon K., Finkelman F. D., McMillan V., Mond J. J. Glucocorticoids suppress calcium mobilization and phospholipid hydrolysis in anti-Ig antibody-stimulated B cells. J Immunol. 1987 Oct 15;139(8):2516–2523. [PubMed] [Google Scholar]
- Fahey K. A., DeFranco A. L. Cross-linking membrane IgM induces production of inositol trisphosphate and inositol tetrakisphosphate in WEHI-231 B lymphoma cells. J Immunol. 1987 Jun 1;138(11):3935–3942. [PubMed] [Google Scholar]
- Goroff D. K., Stall A., Mond J. J., Finkelman F. D. In vitro and in vivo B lymphocyte-activating properties of monoclonal anti-delta antibodies. I. Determinants of B lymphocyte-activating properties. J Immunol. 1986 Apr 1;136(7):2382–2392. [PubMed] [Google Scholar]
- Grupp S. A., Harmony J. A. Increased phosphatidylinositol metabolism is an important but not an obligatory early event in B lymphocyte activation. J Immunol. 1985 Jun;134(6):4087–4094. [PubMed] [Google Scholar]
- Grupp S. A., Snow E. C., Harmony J. A. The phosphatidylinositol response is an early event in the physiologically relevant activation of antigen-specific B lymphocytes. Cell Immunol. 1987 Oct 1;109(1):181–191. doi: 10.1016/0008-8749(87)90303-0. [DOI] [PubMed] [Google Scholar]
- Maino V. C., Hayman M. J., Crumpton M. J. Relationship between enhanced turnover of phosphatidylinositol and lymphocyte activation by mitogens. Biochem J. 1975 Jan;146(1):247–252. doi: 10.1042/bj1460247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi J., Beaven M. A., Ohara J., Paul W. E. BSF-1 action on resting B cells does not require elevation of inositol phospholipid metabolism or increased [Ca2+]i. J Immunol. 1986 Oct 1;137(7):2215–2219. [PubMed] [Google Scholar]
- Mizuguchi J., Ji Y. Y., Nakabayaschi H., Huang K. P., Beaven M. A., Chused T., Paul W. E. Protein kinase C activation blocks anti-IgM-mediated signaling BAL17 B lymphoma cells. J Immunol. 1987 Aug 15;139(4):1054–1059. [PubMed] [Google Scholar]
- Mond J. J., Feuerstein N., Finkelman F. D., Huang F., Huang K. P., Dennis G. B-lymphocyte activation mediated by anti-immunoglobulin antibody in the absence of protein kinase C. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8588–8592. doi: 10.1073/pnas.84.23.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. G., Kass M. J. Molecular events in B cell activation. I. Signals required to stimulate G0 to G1 transition of resting B lymphocytes. J Immunol. 1985 Sep;135(3):1674–1682. [PubMed] [Google Scholar]
- Monroe J. G., Niedel J. E., Cambier J. C. B cell activation. IV. Induction of cell membrane depolarization and hyper-I-A expression by phorbol diesters suggests a role for protein kinase C in murine B lymphocyte activation. J Immunol. 1984 Mar;132(3):1472–1478. [PubMed] [Google Scholar]
- O'Rourke A. M., Mescher M. F. T cell receptor-mediated signaling occurs in the absence of inositol phosphate production. J Biol Chem. 1988 Dec 15;263(35):18594–18597. [PubMed] [Google Scholar]
- Phillips N. E., Parker D. C. Cross-linking of B lymphocyte Fc gamma receptors and membrane immunoglobulin inhibits anti-immunoglobulin-induced blastogenesis. J Immunol. 1984 Feb;132(2):627–632. [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Rothstein T. L., Baeker T. R., Miller R. A., Kolber D. L. Stimulation of murine B cells by the combination of calcium ionophore plus phorbol ester. Cell Immunol. 1986 Oct 15;102(2):364–373. doi: 10.1016/0008-8749(86)90430-2. [DOI] [PubMed] [Google Scholar]
- Smeland E. B., Beiske K., Ohlsson R., Holte H., Ruud E., Blomhoff H. K., Jefferis R., Godal T. Activation of human B cells: alternate options for initial triggering and effects of nonmitogenic concentrations of anti-IgM antibodies on resting and activated cells. J Immunol. 1987 May 15;138(10):3179–3184. [PubMed] [Google Scholar]
- Sussman J. J., Merćep M., Saito T., Germain R. N., Bonvini E., Ashwell J. D. Dissociation of phosphoinositide hydrolysis and Ca2+ fluxes from the biological responses of a T-cell hybridoma. Nature. 1988 Aug 18;334(6183):625–628. doi: 10.1038/334625a0. [DOI] [PubMed] [Google Scholar]
- Valentine M. A., Clark E. A., Shu G. L., Norris N. A., Ledbetter J. A. Antibody to a novel 95-kDa surface glycoprotein on human B cells induces calcium mobilization and B cell activation. J Immunol. 1988 Jun 15;140(12):4071–4078. [PubMed] [Google Scholar]
- Wilson H. A., Greenblatt D., Poenie M., Finkelman F. D., Tsien R. Y. Crosslinkage of B lymphocyte surface immunoglobulin by anti-Ig or antigen induces prolonged oscillation of intracellular ionized calcium. J Exp Med. 1987 Aug 1;166(2):601–606. doi: 10.1084/jem.166.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]