Abstract
Processing of a protein antigen into fragments is believed to be a prerequisite for its presentation by the antigen-presenting cell to the T cell. This model would predict that, in oligomeric proteins, T cells prepared with specificity for regions that are buried within subunit association surfaces should recognize the respective regions in vitro equally well on the isolated subunit or on the oligomer. Three hemoglobin (Hb) alpha-chain synthetic peptides, corresponding to areas that are situated either completely [alpha-(31-45)] or partially [alpha-(41-45) and alpha-(81-95)] within the interface between the alpha and beta subunits of Hb, and a fourth peptide representing a completely exposed area in tetrameric Hb were used as immunogens in SJL/J (H-2s) mice. Peptide-primed T cells were passaged in vitro with the respective peptide to obtain peptide-specific T-lymphocyte lines. T-cell clones were isolated from these lines by limiting dilution. T-cell lines and clones that were specific for buried regions in the subunit association surfaces recognized the free peptide and the isolated subunit but not the Hb tetramer. On the other hand, T cells with specificity against regions that are not involved in subunit interaction and are completely exposed in the tetramer recognized the peptide, the isolated subunit, and the oligomeric protein equally well. The responses of the T-cell lines and clones were major histocompatibility complex-restricted. Since the same x-irradiated antigen-presenting cells were employed, the results could not be attributed to differences or defects in Hb processing. The findings indicate that in vitro the native (unprocessed and undissociated) oligomeric protein was the trigger of major histocompatibility complex-restricted T-cell responses.
Full text
PDF
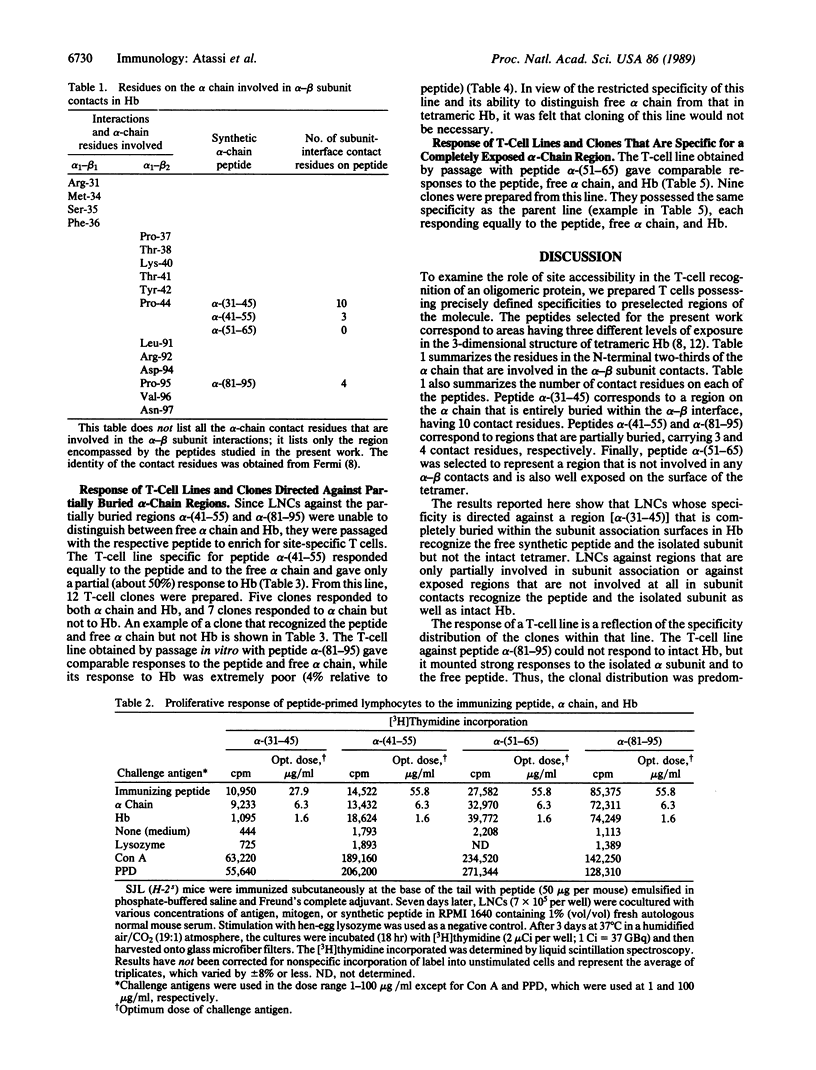


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Beller D. I., Braun J., Unanue E. R. The handling of Listeria monocytogenes by macrophages: the search for an immunogenic molecule in antigen presentation. J Immunol. 1984 Jan;132(1):323–331. [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Antigen processing and presentation at a molecular level. Adv Exp Med Biol. 1987;225:147–154. doi: 10.1007/978-1-4684-5442-0_11. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structures of proteins. Their determination has revealed important aspects of immune recognition and generated strategies for synthetic mimicking of protein binding sites. Eur J Biochem. 1984 Nov 15;145(1):1–20. doi: 10.1111/j.1432-1033.1984.tb08516.x. [DOI] [PubMed] [Google Scholar]
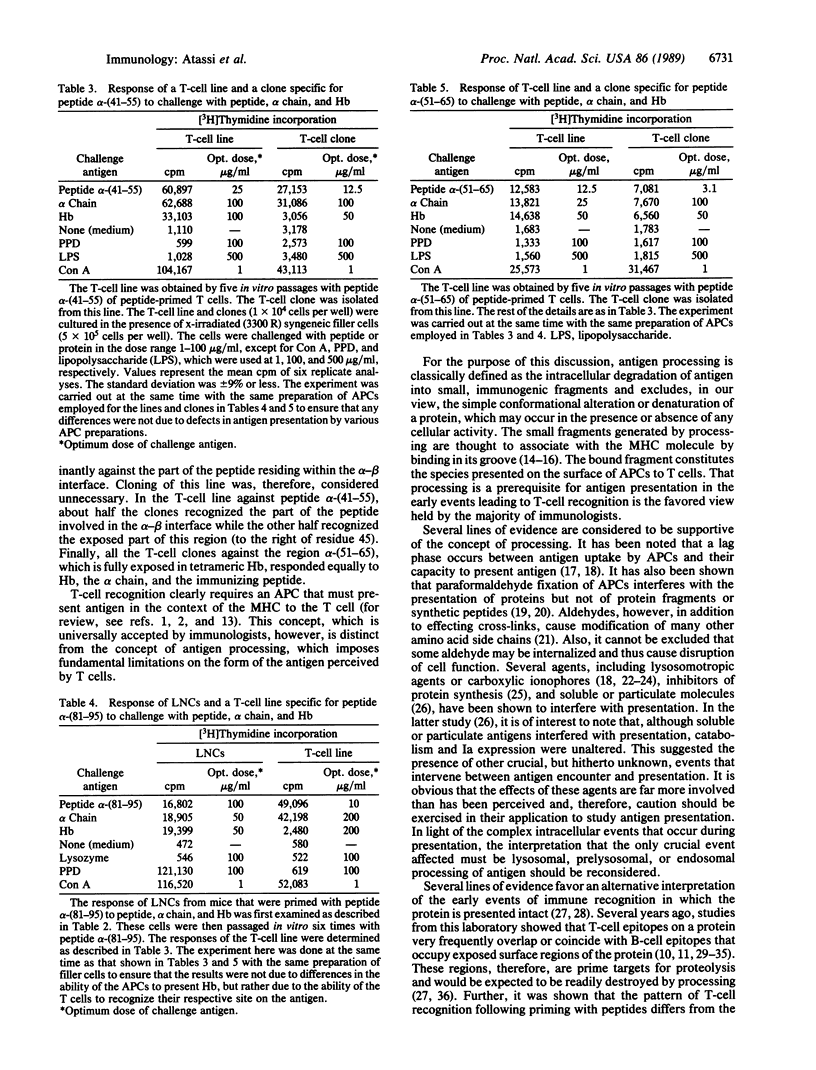
- Atassi M. Z., Bixler G. S., Jr, Yokoi T. Conformation-dependent recognition of a protein by T cells requires presentation without processing. Biochem J. 1989 May 1;259(3):731–735. doi: 10.1042/bj2590731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Chemical studies on haemoglobins A1 and A0. Biochem J. 1964 Oct;93(1):189–197. doi: 10.1042/bj0930189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Precise determination of protein antigenic structures has unravelled the molecular immune recognition of proteins and provided a prototype for synthetic mimicking of other protein binding sites. Mol Cell Biochem. 1980 Aug 29;32(1):21–43. doi: 10.1007/BF00421293. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Precise determination of the entire antigenic structure of lysozyme: molecular features of protein antigenic structures and potential of "surface-simulation" synthesis--a powerful new concept for protein binding sites. Immunochemistry. 1978 Dec;15(12):909–936. doi: 10.1016/0161-5890(78)90126-8. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Yokota S., Twining S. S., Lehmann H., David C. S. Genetic control of the immune response to myoglobin. VI. Inter-site influences in T-lymphocyte proliferative response from analysis of cross-reactions of ten myoglobins in terms of substitutions in the antigenic sites and in environmental residues of the sites. Mol Immunol. 1981 Oct;18(10):945–948. doi: 10.1016/0161-5890(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Atassi M. Z. Antigen presentation of myoglobin: profiles of T cell proliferative responses following priming with synthetic overlapping peptides encompassing the entire molecule. Eur J Immunol. 1985 Sep;15(9):917–922. doi: 10.1002/eji.1830150910. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Atassi M. Z. Molecular localization of the full profile of the continuous regions recognized by myoglobin-primed T-cells using synthetic overlapping peptides encompassing the entire molecule. Immunol Commun. 1983;12(6):593–603. doi: 10.3109/08820138309025440. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Atassi M. Z. T cell recognition of lysozyme. III. Recognition of the 'surface-simulation' synthetic antigenic sites. J Immunogenet. 1984 Jun-Aug;11(3-4):245–250. doi: 10.1111/j.1744-313x.1984.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Atassi M. Z. T cell recognition of myoglobin. Localization of the sites stimulating T cell proliferative responses by synthetic overlapping peptides encompassing the entire molecule. J Immunogenet. 1984 Oct-Dec;11(5-6):339–353. doi: 10.1111/j.1744-313x.1984.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Yoshida T., Atassi M. Z. T cell recognition of lysozyme. IV. Localization and genetic control of the continuous T cell recognition sites by synthetic overlapping peptides representing the entire protein chain. J Immunogenet. 1984 Oct-Dec;11(5-6):327–337. doi: 10.1111/j.1744-313x.1984.tb00819.x. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Yoshida T., Atassi M. Z. T-cell recognition of lysozyme. I. Localization of regions stimulating T-cell proliferative response by synthetic overlapping peptides encompassing the entire molecule. Exp Clin Immunogenet. 1984;1(2):99–111. [PubMed] [Google Scholar]
- Bixler G. S., Yoshida T., Atassi M. Z. Antigen presentation of lysozyme: T-cell recognition of peptide and intact protein after priming with synthetic overlapping peptides comprising the entire protein chain. Immunology. 1985 Sep;56(1):103–112. [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Buchmüller Y., Corradin G. Lymphocyte specificity to protein antigens. V. Conformational dependence of activation of cytochrome c-specific T cells. Eur J Immunol. 1982 May;12(5):412–416. doi: 10.1002/eji.1830120510. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2382–2388. [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Antigen presenting cells and mechanisms of antigen presentation. Crit Rev Immunol. 1985;5(3):263–316. [PubMed] [Google Scholar]
- Cohly H. H., Morrison D. R., Atassi M. Z. Conformation-dependent recognition of a protein by T-lymphocytes: apomyoglobin-specific T-cell clone recognizes conformational changes between apomyoglobin and myoglobin. Immunol Invest. 1988 Jun;17(4):337–342. doi: 10.3109/08820138809041421. [DOI] [PubMed] [Google Scholar]
- Cohly H. H., Morrison D. R., Atassi M. Z. Presentation of antigen to T lymphocytes by non-immune B-cell hybridoma clones: evidence for specific and non-specific presentation. Immunol Invest. 1989 Jun;18(5):651–656. doi: 10.3109/08820138909057752. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Antigen processing and intracellular Ia. Possible roles of endocytosis and protein synthesis in Ia function. J Immunol. 1989 Jan 1;142(1):12–19. [PubMed] [Google Scholar]
- Hawrylowicz C. M., Unanue E. R. Regulation of antigen-presentation-I. IFN-gamma induces antigen-presenting properties on B cells. J Immunol. 1988 Dec 15;141(12):4083–4088. [PubMed] [Google Scholar]
- Jensen P. E. Protein synthesis in antigen processing. J Immunol. 1988 Oct 15;141(8):2545–2550. [PubMed] [Google Scholar]
- Jerne N. K. Idiotypic networks and other preconceived ideas. Immunol Rev. 1984 Jun;79:5–24. doi: 10.1111/j.1600-065x.1984.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. A novel and comprehensive synthetic approach for the elucidation of protein antigenic structures. Determination of the full antigenic profile of the alpha-chain of human haemoglobin. Biochem J. 1980 Oct 1;191(1):261–264. doi: 10.1042/bj1910261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Structurally inherent antigenic sites. Localization of the antigenic sites of the alpha-chain of human haemoglobin in three host species by a comprehensive synthetic approach. Biochem J. 1982 Apr 1;203(1):201–208. doi: 10.1042/bj2030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex and protein recognition by T lymphocytes. Adv Exp Med Biol. 1987;225:1–10. doi: 10.1007/978-1-4684-5442-0_1. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Wong M., Spitzer D. Chloroquine as a probe for antigen processing by accessory cells. Transplantation. 1982 Sep;34(3):150–153. doi: 10.1097/00007890-198209000-00008. [DOI] [PubMed] [Google Scholar]
- Lee P., Matsueda G. R., Allen P. M. T cell recognition of fibrinogen. A determinant on the A alpha-chain does not require processing. J Immunol. 1988 Feb 15;140(4):1063–1068. [PubMed] [Google Scholar]
- Leyva-Cobian F., Unanue E. R. Intracellular interference with antigen presentation. J Immunol. 1988 Sep 1;141(5):1445–1450. [PubMed] [Google Scholar]
- Mills K. H., Skehel J. J., Thomas D. B. Conformational-dependent recognition of influenza virus hemagglutinin by murine T helper clones. Eur J Immunol. 1986 Mar;16(3):276–280. doi: 10.1002/eji.1830160312. [DOI] [PubMed] [Google Scholar]
- Mills K. H., Skehel J. J., Thomas D. B. Extensive diversity in the recognition of influenza virus hemagglutinin by murine T helper clones. J Exp Med. 1986 Jun 1;163(6):1477–1490. doi: 10.1084/jem.163.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Twining S. S., David C. S., Atassi M. Z. Genetic control of immune response to sperm whale myoglobin in mice. II. T lymphocyte proliferative response to the synthetic antigenic sites. J Immunol. 1979 Jul;123(1):182–188. [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden P., Nagy Z. A., Klein J. Antigen presentation by liposomes: inhibition with antibodies. Eur J Immunol. 1986 Jun;16(6):717–720. doi: 10.1002/eji.1830160622. [DOI] [PubMed] [Google Scholar]
- Walden P., Nagy Z. A., Klein J. Induction of regulatory T-lymphocyte responses by liposomes carrying major histocompatibility complex molecules and foreign antigen. Nature. 1985 May 23;315(6017):327–329. doi: 10.1038/315327a0. [DOI] [PubMed] [Google Scholar]
- Walden P., Nagy Z. A., Klein J. Major histocompatibility complex-restricted and unrestricted activation of helper T cell lines by liposome-bound antigens. J Mol Cell Immunol. 1986;2(4):191–197. [PubMed] [Google Scholar]
- Wraith D. C., Vessey A. E. Influenza virus-specific cytotoxic T-cell recognition: stimulation of nucleoprotein-specific clones with intact antigen. Immunology. 1986 Oct;59(2):173–180. [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M., Atassi M. Z. T-cell recognition and antigen presentation of myoglobin. Protein recognition by site-specific T-cell clones is influenced by amino acid substitutions outside the site. Biochem J. 1989 Mar 15;258(3):645–651. doi: 10.1042/bj2580645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M., Bixler G. S., Jr, Atassi M. Z. Preparation of T-lymphocyte lines and clones with specificities to preselected protein sites by in vitro passage with free synthetic peptides: demonstration with myoglobin sites. Mol Immunol. 1983 Oct;20(10):1133–1137. doi: 10.1016/0161-5890(83)90123-2. [DOI] [PubMed] [Google Scholar]
- Young C. R., Atassi M. Z. Dissection of the molecular parameters for T-cell recognition of a myoglobin antigenic site. Adv Exp Med Biol. 1982;150:73–93. doi: 10.1007/978-1-4684-4331-8_5. [DOI] [PubMed] [Google Scholar]
- Young C. R., Atassi M. Z. T-lymphocyte recognition of sperm-whale myoglobin. Responses of T-cells from mouse strains primed with synthetic peptide carrying antigenic site 5 and relation to antigen presentation. J Immunogenet. 1983 Apr;10(2):151–160. doi: 10.1111/j.1744-313x.1983.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]


