Abstract
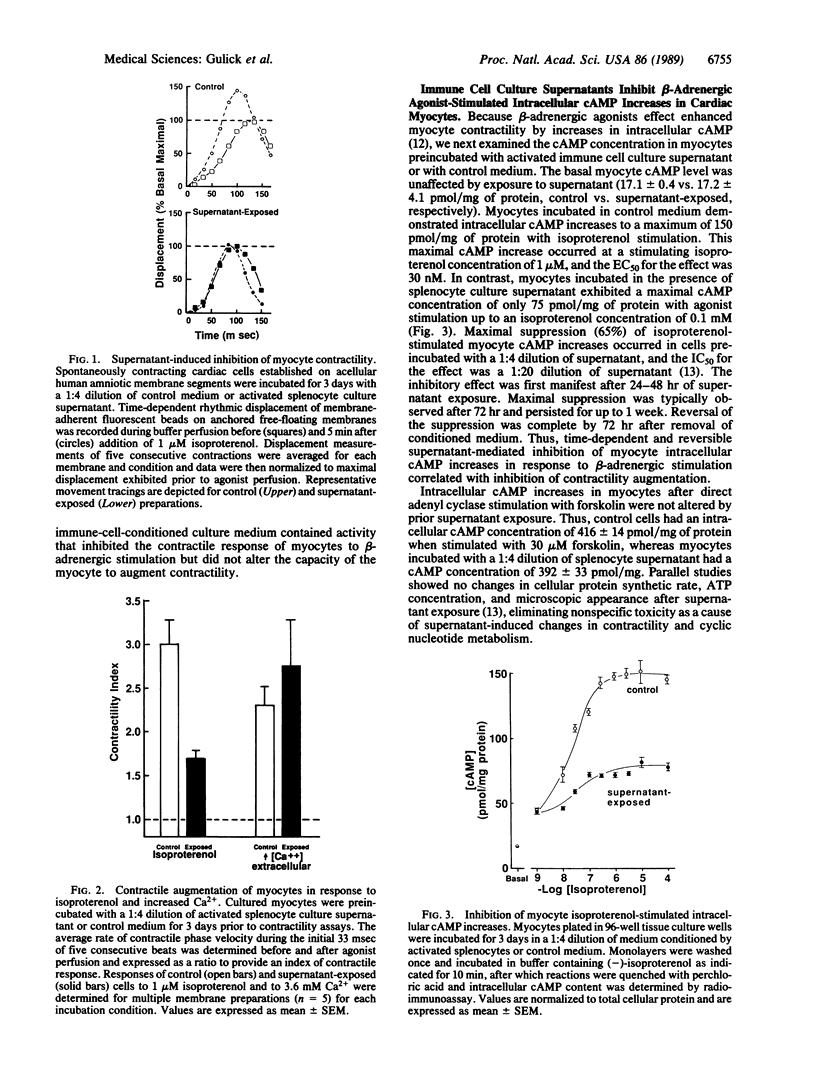
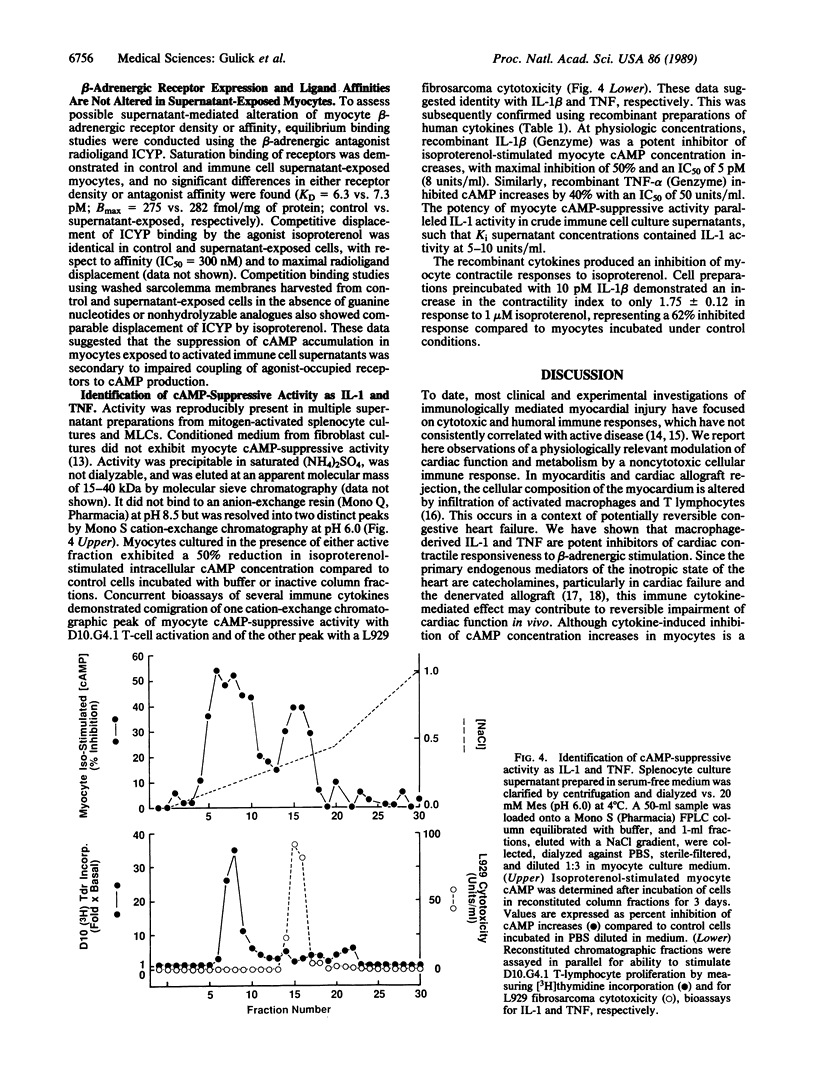
Reversible congestive heart failure can accompany cardiac allograft rejection and inflammatory myocarditis, conditions associated with an immune cell infiltrate of the myocardium. To determine whether immune cell secretory products alter cardiac muscle metabolism without cytotoxicity, we cultured cardiac myocytes in the presence of culture supernatants from activated immune cells. We observed that these culture supernatants inhibit beta-adrenergic agonist-mediated increases in cultured cardiac myocyte contractility and intracellular cAMP accumulation. The myocyte contractile response to increased extracellular Ca2+ concentration is unaltered by prior exposure to these culture supernatants, as is the increase in myocyte intracellular cAMP concentration in response to stimulation with forskolin, a direct adenyl cyclase activator. Inhibition occurs in the absence of alteration in beta-adrenergic receptor density or ligand binding affinity. Suppressive activity is attributable to the macrophage-derived cytokines interleukin 1 and tumor necrosis factor. Thus, these observations describe a role for defined cytokines in regulating the hormonal responsiveness and function of contractile cells. The effects of interleukin 1 and tumor necrosis factor on intracellular cAMP accumulation may be a model for immune modulation of other cellular functions dependent upon cyclic nucleotide metabolism. The uncoupling of agonist-occupied receptors from adenyl cyclase suggests that beta-receptor or guanine nucleotide binding protein function is altered by the direct or indirect action of cytokines on cardiac muscle cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley D., Cohen R. A., Levinsky N. G. Interleukin 1 inhibits contraction of vascular smooth muscle. J Clin Invest. 1989 Jan;83(1):331–335. doi: 10.1172/JCI113879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borow K. M., Neumann A., Arensman F. W., Yacoub M. H. Left ventricular contractility and contractile reserve in humans after cardiac transplantation. Circulation. 1985 May;71(5):866–872. doi: 10.1161/01.cir.71.5.866. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cramer D. V. Cardiac transplantation: immune mechanisms and alloantigens involved in graft rejection. Crit Rev Immunol. 1987;7(1):1–30. [PubMed] [Google Scholar]
- Fenoglio J. J., Jr, Ursell P. C., Kellogg C. F., Drusin R. E., Weiss M. B. Diagnosis and classification of myocarditis by endomyocardial biopsy. N Engl J Med. 1983 Jan 6;308(1):12–18. doi: 10.1056/NEJM198301063080103. [DOI] [PubMed] [Google Scholar]
- Gokel J. M., Reichart B., Struck E. Human cardiac transplantation--evaluation of morphological changes in serial endomyocardial biopsies. Pathol Res Pract. 1985 Jan;179(3):354–364. doi: 10.1016/s0344-0338(85)80144-8. [DOI] [PubMed] [Google Scholar]
- Gulick T., Chung M. K., Pieper S. J., Schreiner G. F., Lange L. G. Immune cytokine inhibition of beta-adrenergic agonist stimulated cyclic AMP generation in cardiac myocytes. Biochem Biophys Res Commun. 1988 Jan 15;150(1):1–9. doi: 10.1016/0006-291x(88)90478-0. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963 Feb;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- Kawai C., Matsumori A., Fujiwara H. Myocarditis and dilated cardiomyopathy. Annu Rev Med. 1987;38:221–239. doi: 10.1146/annurev.me.38.020187.001253. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Lee C. W., Morakis D. J. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980 Dec;11(2):141–152. doi: 10.1016/0304-3835(80)90105-6. [DOI] [PubMed] [Google Scholar]
- Murdock D. K., Collins E. G., Lawless C. E., Molnar Z., Scanlon P. J., Pifarre R. Rejection of the transplanted heart. Heart Lung. 1987 May;16(3):237–245. [PubMed] [Google Scholar]
- Pope S. E., Stinson E. B., Daughters G. T., 2nd, Schroeder J. S., Ingels N. B., Jr, Alderman E. L. Exercise response of the denervated heart in long-term cardiac transplant recipients. Am J Cardiol. 1980 Aug;46(2):213–218. doi: 10.1016/0002-9149(80)90060-0. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Hertel C. [3H]CGP-12177, a beta-adrenergic ligand suitable for measuring cell surface receptors. J Recept Res. 1983;3(1-2):35–43. doi: 10.3109/10799898309041921. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tamayo J., Bellorin-Font E., Sicard G., Anderson C., Martin K. J. Desensitization to parathyroid hormone in the isolated perfused canine kidney: reversal of altered receptor-adenylate cyclase system by guanosine triphosphate in vitro. Endocrinology. 1982 Oct;111(4):1311–1317. doi: 10.1210/endo-111-4-1311. [DOI] [PubMed] [Google Scholar]
- Tazelaar H. D., Billingham M. E. Leukocytic infiltrates in idiopathic dilated cardiomyopathy. A source of confusion with active myocarditis. Am J Surg Pathol. 1986 Jun;10(6):405–412. doi: 10.1097/00000478-198606000-00005. [DOI] [PubMed] [Google Scholar]


