Abstract
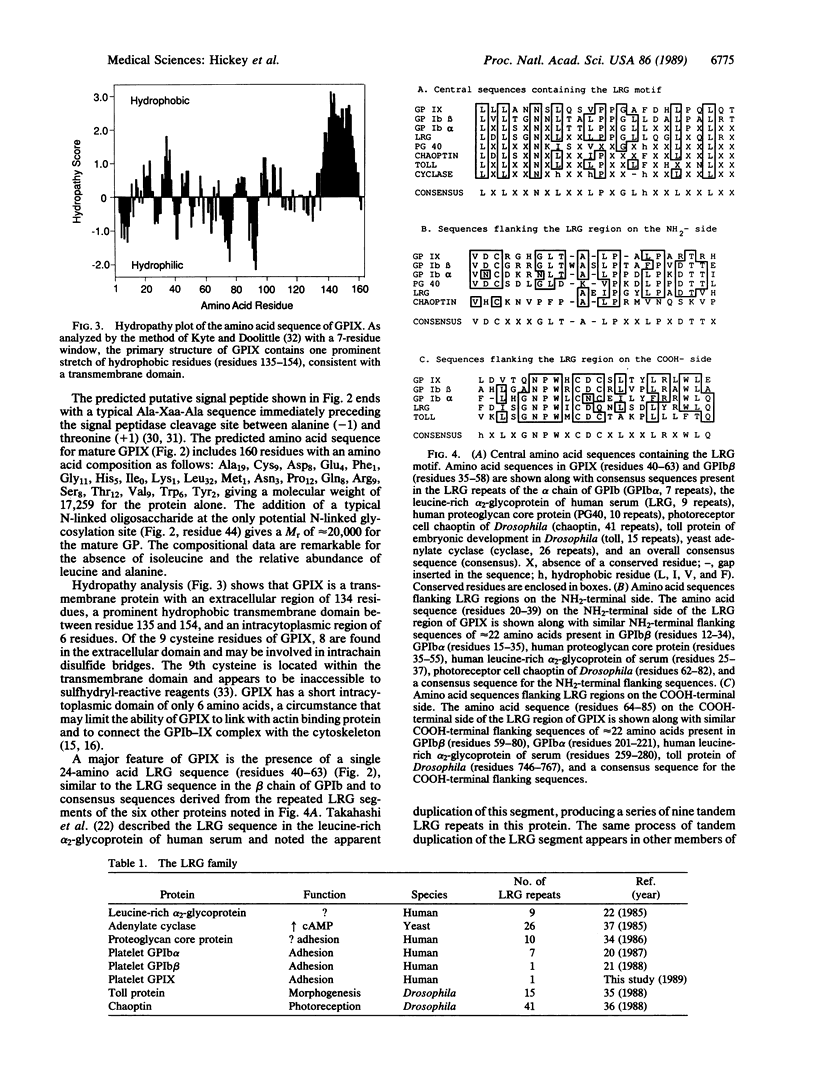
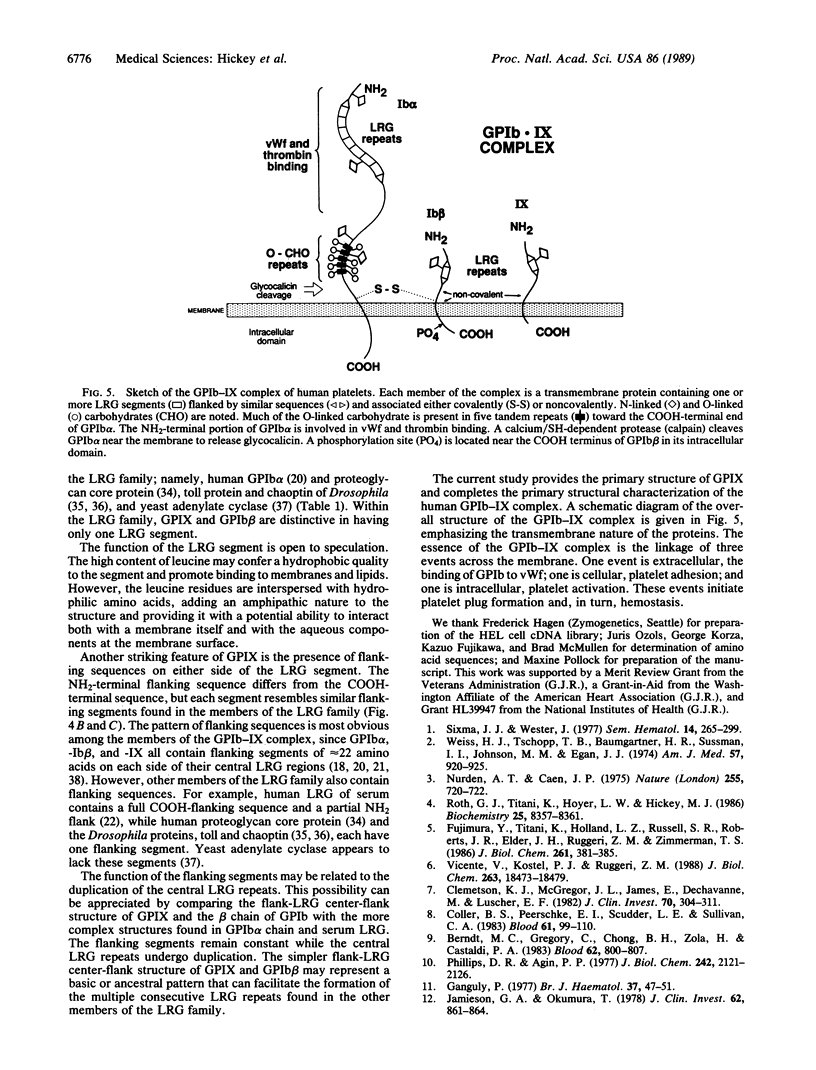
The glycoprotein (GP) Ib-IX complex on the surface of human platelets functions as the von Willebrand factor receptor and mediates von Willebrand factor-dependent platelet adhesion to blood vessels. GPIX is a relatively small (Mr, 17,000) protein that may provide for membrane insertion and orientation of the larger component of the complex, GPIb (Mr, 165,000). Using antibody screening, we cloned a cDNA encoding GPIX from a human erythroleukemia cell cDNA library constructed in phage lambda gt11. Lacking a 5' untranslated region and start codon, the cDNA sequence includes 604 nucleotides, beginning with 495 bases at the 5' end coding for 165 amino acids, followed by a stop codon and 106 noncoding bases at the 3' end. By Northern blot analysis, the GPIX cDNA hybridizes with a single 1.0-kilobase species of platelet poly(A)+ RNA. Translation of the cDNA sequence gives a predicted protein sequence beginning with a truncated putative signal sequence of 5 amino acid followed by a sequence of 17 amino acids matching that determined directly by Edman degradation of intact GPIX. The predicted amino acid sequence of mature GPIX includes an NH2-terminal extracytoplasmic domain of 134 residues, a transmembrane domain of 20 residues, 6 intracytoplasmic residues, and 1 N-linked glycosylation site. GPIX contains a leucine-rich glycoprotein (LRG) sequence of 24 amino acids similar to conserved LRG sequences in GPIb and other proteins from humans, Drosophila, and yeast. "Flanking" sequences of approximately 22 amino acids are present at the NH2 and/or COOH sides of the "central" LRG sequence(s) in GPIX, GPIb, and the other human and Drosophila members of the LRG family. The role of the flank-LRG center-flank structure in the evolution and function of the LRG proteins remains to be defined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berndt M. C., Gregory C., Chong B. H., Zola H., Castaldi P. A. Additional glycoprotein defects in Bernard-Soulier's syndrome: confirmation of genetic basis by parental analysis. Blood. 1983 Oct;62(4):800–807. [PubMed] [Google Scholar]
- Berndt M. C., Gregory C., Kabral A., Zola H., Fournier D., Castaldi P. A. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur J Biochem. 1985 Sep 16;151(3):637–649. doi: 10.1111/j.1432-1033.1985.tb09152.x. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., McGregor J. L., James E., Dechavanne M., Lüscher E. F. Characterization of the platelet membrane glycoprotein abnormalities in Bernard-Soulier syndrome and comparison with normal by surface-labeling techniques and high-resolution two-dimensional gel electrophoresis. J Clin Invest. 1982 Aug;70(2):304–311. doi: 10.1172/JCI110618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Fox J. E. Linkage of a membrane skeleton to integral membrane glycoproteins in human platelets. Identification of one of the glycoproteins as glycoprotein Ib. J Clin Invest. 1985 Oct;76(4):1673–1683. doi: 10.1172/JCI112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Holland L. Z., Russell S. R., Roberts J. R., Elder J. H., Ruggeri Z. M., Zimmerman T. S. von Willebrand factor. A reduced and alkylated 52/48-kDa fragment beginning at amino acid residue 449 contains the domain interacting with platelet glycoprotein Ib. J Biol Chem. 1986 Jan 5;261(1):381–385. [PubMed] [Google Scholar]
- Ganguly P. Binding of thrombin to functionally defective platelets: a hypothesis on the nature of the thrombin receptor. Br J Haematol. 1977 Sep;37(1):47–51. [PubMed] [Google Scholar]
- Hashimoto C., Hudson K. L., Anderson K. V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988 Jan 29;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T. Reduced thrombin binding and aggregation in Bernard-Soulier platelets. J Clin Invest. 1978 Mar;61(3):861–864. doi: 10.1172/JCI109000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalomiris E. L., Coller B. S. Thiol-specific probes indicate that the beta-chain of platelet glycoprotein Ib is a transmembrane protein with a reactive endofacial sulfhydryl group. Biochemistry. 1985 Sep 24;24(20):5430–5436. doi: 10.1021/bi00341a022. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Johnson M. M., Aster R. H. Absence of the platelet receptor for drug-dependent antibodies in the Bernard-Soulier syndrome. J Clin Invest. 1978 Sep;62(3):716–719. doi: 10.1172/JCI109181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Davie E. W., Roth G. J. The alpha and beta chains of human platelet glycoprotein Ib are both transmembrane proteins containing a leucine-rich amino acid sequence. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2135–2139. doi: 10.1073/pnas.85.7.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Papayannopoulou T., Roth G. J. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. Specific roles for platelet surface glycoproteins in platelet function. Nature. 1975 Jun 26;255(5511):720–722. doi: 10.1038/255720a0. [DOI] [PubMed] [Google Scholar]
- Patthy L. Detecting homology of distantly related proteins with consensus sequences. J Mol Biol. 1987 Dec 20;198(4):567–577. doi: 10.1016/0022-2836(87)90200-2. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Reinke R., Krantz D. E., Yen D., Zipursky S. L. Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell. 1988 Jan 29;52(2):291–301. doi: 10.1016/0092-8674(88)90518-1. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Hickey M. J., Chung D. W., Hickstein D. D. Circulating human blood platelets retain appreciable amounts of poly (A)+ RNA. Biochem Biophys Res Commun. 1989 Apr 28;160(2):705–710. doi: 10.1016/0006-291x(89)92490-x. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Ozols J., Nugent D. J., Williams S. A. Isolation and characterization of human platelet glycoprotein IX. Biochem Biophys Res Commun. 1988 Oct 31;156(2):931–939. doi: 10.1016/s0006-291x(88)80933-1. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Titani K., Hoyer L. W., Hickey M. J. Localization of binding sites within human von Willebrand factor for monomeric type III collagen. Biochemistry. 1986 Dec 30;25(26):8357–8361. doi: 10.1021/bi00374a004. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixma J. J., Wester J. The hemostatic plug. Semin Hematol. 1977 Jul;14(3):265–299. [PubMed] [Google Scholar]
- Solum N. O., Olsen T. M., Gogstad G. O., Hagen I., Brosstad F. Demonstration of a new glycoprotein Ib-related component in platelet extracts prepared in the presence of leupeptin. Biochim Biophys Acta. 1983 Mar 23;729(1):53–61. doi: 10.1016/0005-2736(83)90455-8. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1906–1910. doi: 10.1073/pnas.82.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Takio K., Handa M., Ruggeri Z. M. Amino acid sequence of the von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5610–5614. doi: 10.1073/pnas.84.16.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente V., Kostel P. J., Ruggeri Z. M. Isolation and functional characterization of the von Willebrand factor-binding domain located between residues His1-Arg293 of the alpha-chain of glycoprotein Ib. J Biol Chem. 1988 Dec 5;263(34):18473–18479. [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R., Sussman I. I., Johnson M. M., Egan J. J. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am J Med. 1974 Dec;57(6):920–925. doi: 10.1016/0002-9343(74)90170-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B., Kim S. J., McPherson J., Grant R. A. Binding of factor VIII to platelets in the presence of ristocetin. Br J Haematol. 1977 Apr;35(4):535–549. doi: 10.1111/j.1365-2141.1977.tb00619.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]



