Abstract
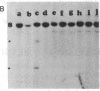
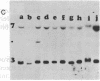
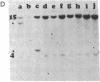
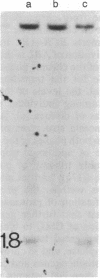
Molecular mechanisms responsible for the clinical progression of chronic myelocytic leukemia to its accelerated phase or to blast crisis have not been defined. We found alterations of the p53 gene (p53 is a 53-kDa nuclear protein) including deletions and rearrangements in 8 of 34 patients in blast crisis and 1 of 4 patients in the accelerated phase, but in only 1 of 38 patients in the chronic phase of chronic myelocytic leukemia. Only two other examples of p53 gene alterations were found among 203 patients with hematologic malignancies and solid tumors. Transcripts of the p53 gene were uniformly found in chronic-phase cells, but gene expression was variable in blast crisis, and transcripts were reduced or undetectable in 10 of 16 patients. Heterogeneous alterations in the structure and expression of the p53 gene appear to be relatively frequent in blast crisis and may be involved in the evolution of disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartram C. R., de Klein A., Hagemeijer A., van Agthoven T., Geurts van Kessel A., Bootsma D., Grosveld G., Ferguson-Smith M. A., Davies T., Stone M. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- Borgström G. H., Vuopio P., de la Chapelle A. Abnormalities of chromosome No. 17 in myeloproliferative disorders. Cancer Genet Cytogenet. 1982 Feb;5(2):123–135. doi: 10.1016/0165-4608(82)90003-6. [DOI] [PubMed] [Google Scholar]
- Bukhman V. L., Ninkina N. N., Chumakov P. M., Khilenkova M. A., Samarina O. P. Strukturnaia organizatsiia gena p53 cheloveka. Soobshchenie I. Molekuliarnoe klonirovanie gena p53 cheloveka. Genetika. 1987 Sep;23(9):1547–1554. [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Harlow E., Williamson N. M., Ralston R., Helfman D. M., Adams T. E. Molecular cloning and in vitro expression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985 Jul;5(7):1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Miller C., Nicolson M. A., Ranyard J., Bosselman R. A. Increased expression of p53 protein in human leukemia cells. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4035–4039. doi: 10.1073/pnas.83.11.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Hjelle B., Bishop J. M. Transforming genes in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1952–1956. doi: 10.1073/pnas.85.6.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Battifora H., Yokota J., Meltzer S., Cline M. J. Specificity of proto-oncogene amplification in human malignant diseases. Mol Biol Med. 1987 Aug;4(4):213–227. [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M., Rassool F. V., Goldman J. M., Graham S. V., Birnie G. D. Genomic alterations involving the c-myc proto-oncogene locus during the evolution of a case of chronic granulocytic leukaemia. Lancet. 1984 Dec 15;2(8416):1362–1365. doi: 10.1016/s0140-6736(84)92058-0. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehleck S. D., McKenna R. W., Arthur D. C., Parkin J. L., Brunning R. D. Transformation of chronic myelogenous leukemia: clinical, morphologic, and cytogenetic features. Am J Clin Pathol. 1984 Jul;82(1):1–14. doi: 10.1093/ajcp/82.1.1. [DOI] [PubMed] [Google Scholar]
- Munroe D. G., Rovinski B., Bernstein A., Benchimol S. Loss of a highly conserved domain on p53 as a result of gene deletion during Friend virus-induced erythroleukemia. Oncogene. 1988 Jun;2(6):621–624. [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rovinski B., Munroe D., Peacock J., Mowat M., Bernstein A., Benchimol S. Deletion of 5'-coding sequences of the cellular p53 gene in mouse erythroleukemia: a novel mechanism of oncogene regulation. Mol Cell Biol. 1987 Feb;7(2):847–853. doi: 10.1128/mcb.7.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Gale R. P., Dreazen O., Berrebi A., Zaizov R., Kubonishi I., Miyoshi I., Canaani E. bcr-abl RNA in patients with chronic myelogenous leukemia. Blood. 1987 Mar;69(3):971–973. [PubMed] [Google Scholar]
- Smith L. J., McCulloch E. A., Benchimol S. Expression of the p53 oncogene in acute myeloblastic leukemia. J Exp Med. 1986 Sep 1;164(3):751–761. doi: 10.1084/jem.164.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Tsunetsugu-Yokota Y., Battifora H., Le Fevre C., Cline M. J. Alterations of myc, myb, and rasHa proto-oncogenes in cancers are frequent and show clinical correlation. Science. 1986 Jan 17;231(4735):261–265. doi: 10.1126/science.3941898. [DOI] [PubMed] [Google Scholar]