Synopsis
Cerebral palsy is the most prevalent physical disability in childhood and includes a group of disorders with varying manifestations and levels of capability in individuals given this diagnosis. This chapter will focus on current and future intervention strategies for improving mobility and participation over the lifespan for ambulatory children with cerebral palsy (CP). The provision and integration of physical therapy, medical and orthopedic surgery management focused primarily on the lower extremities will be discussed here. Some of the newer trends are: more intense and task-related exercise strategies, greater precision in tone identification and management, and a shift towards musculoskeletal surgery that focuses more on promoting dynamic bony alignment and less on releasing or lengthening tendons. Advances in basic and clinical science and technology development are changing existing paradigms and offering renewed hope for improved functioning for children with CP who are currently facing a lifelong disability with unique challenges at each stage in life.
Introduction
Overview on current care of motor disorders in cerebral palsy
Cerebral palsy (CP) is the most prevalent physical disability originating in childhood. The latest figures in the United States indicate that the incidence of CP is 3.6 per 1000 children, with males affected to a greater extent than females (1). Since no cure is yet available or imminent for CP, the motor disability persists throughout the lifespan and interacts with normal developmental and aging processes which alter its presentation over time. The current standard of care for the motor disorder in CP consists of regular physical therapy, followed by multiple, and often concurrent, medical and surgical interventions, most intensively in early childhood through pre-adolescence. While a growing list of treatments have been shown to individually improve motor outcomes, few definitive practice guidelines have been proposed for the management of CP due to limited and fragmented scientific evidence to support multidisciplinary (combined) intervention approaches. Consequently, tremendous variation exists among practitioners, settings and geographical areas in the types of treatments prescribed, the timing and sequencing of interventions, and the range of treatment intensity or frequency. Choice of, and response to, intervention is further complicated by the fact that CP is not a single disease entity with a known causal pathway, but is instead a heterogeneous group of disorders with varying etiologies, brain injury patterns, and associated health conditions.
However, despite these challenges, substantial progress is being made in the understanding and management of CP as the pace of research efforts in this population has accelerated markedly in recent years. The focus of this chapter is on the major clinical and theoretical shifts or trends over the past decade in the medical, surgical and therapeutic approaches to improving mobility, and more specifically ambulatory abilities, in children with cerebral palsy. Some of the more dramatic changes in the field have been conceptual and have fundamentally altered how we perceive, classify, and assess a child with CP and how we gauge success of our interventions. New treatments and intervention strategies have also emerged with varying degrees of evidence to support them. Three-dimensional computer-based analysis of walking as well as other functional motor tasks has continued to expand our knowledge of normal versus disordered motor control and to quantitatively measure outcomes from interventions designed to improve specific motor skills. Brain and musculoskeletal imaging technologies are also advancing rapidly and are providing insights into the neuropathology and pathomechanics of CP that were unattainable prior to their advent.
Major Conceptual changes in goal setting and classification of cerebral palsy
New models of disability
The hallmark of CP is a motor control deficit that differs in distribution, presentation, as well as severity, across individuals. For decades, the direct goal of medical treatment for the motor disability was to alleviate the associated motor impairments such as spasticity and muscle contracture with the assumption that functional improvements would ensue. However, that was not necessarily the case (2), and it was realized that the relationship between impairments and functional activity was neither strong nor linear in many cases (3). Several conceptual models of disability, most prominently and most recently the World Health Organization’s International Classification of Functioning, Disability, and Health (ICF) (4), have shifted the primary focus of treatment to the level of activity and participation of the individual patient. Given family and patient goals in those realms, a treatment plan is proposed and implemented that may still recommend treatments to address specific impairments in body structures and functions, but could also involve alterations in the environment to improve access or suggestions for family lifestyle changes to increase participation in fitness, sports or activity-based recreational activities, among other approaches. Many new evaluative measures reflecting these different domains have emerged and are useful in both clinical practice and research. The development of parent and child report measures has been particularly notable and computer adapted technologies are now being utilized to obtain comprehensive information in a very efficient, user-friendly manner (5). While improving activity and participation should be the major treatment priority, interventions that maintain the status quo or minimize future deformity or disability are also beneficial in this population which may regress in function over time, most dramatically in adulthood (6).
New and expanded classification schemes
Traditional categorization of subtypes of cerebral palsy had been based on the primary type of tone disorder and the distribution of the motor involvement, e.g. spastic quadriplegia, with each diagnostic category including individuals with a broad range of clinical involvement. The advent of functional classification scales in CP has had a profound effect on determining the prognosis for mobility and related goal setting, improving family and professional communication, as well as significantly enhancing research design and interpretation. The Gross Motor Functional Classification Scale (GMFCS) (7), which has been adopted universally, categorizes a child’s functional mobility ranging from Level I which indicates the highest level of mobility with only minor limitations in more challenging tasks and environments to Level V which indicates complete dependence on others for mobility. This chapter will primarily address those in Levels I–III who have the capacity or potential for independent, assisted or unassisted, walking. The even more recent Functional Mobility Scale (FMS) (8) expands the assessment of walking ability by rating performance at three different distances (5, 50 and 500 meters) that correspond to the three major environments that children typically need to navigate: home, school and the community.
Recently, a more global multiaxial classification scheme for cerebral palsy (9) has been proposed that encompasses the primary and secondary tone disorders, anatomical distribution of the neurological involvement, as well as the functional mobility and upper extremity skill classification, associated impairments, and brain imaging results, yielding a far more comprehensive description of the individual patient that should ultimately lead to a more well-focused treatment plan. For example, a child previously categorized as having spastic hemiplegia, could now be described in this new classification scheme as having unilateral motor involvement with the presence of both spasticity and dystonia, Manual Ability Classification System (or MACS) (10) Level II and GMFCS Level I, respectively, with a mild seizure disorder and mild learning deficits, and having MRI evidence of a neonatal stroke.
Factors that influence ambulation in CP and the role of medical care
Promoting, improving or restoring the ability to walk is arguably the most common motor goal in neurorehabilitation medicine. Walking status is clearly related to the type and severity of the neurological deficit but is not necessarily predetermined by those as illustrated by the conceptual framework of the ICF. It can be influenced in a positive or negative direction by personal factors that may include other associated impairments, as well as emotional, behavioral and motivational factors. It can also be affected by an individual’s physical, social, and cultural environments as well as the medical care environment which is the primary focus of this chapter. In the following three sections we will summarize the state-of-the science of medical, surgical and physical therapy interventions aimed at improving ambulatory function in CP. While each of these treatment categories will be discussed in individual sections below, it must be noted that optimal outcomes often depend on the administration of multiple types of interventions administered concurrently or sequentially.
Physical therapy trends for ambulatory children with cerebral palsy
The scientific basis underlying neurological physical therapy has increased exponentially in the past decade. Prior to these advances, therapy was dominated by the use of neurodevelopmental therapy (NDT) approach which has failed to produce consistent clinically significant effects on activity (11) or demonstrate superiority over alternative approaches (12). Evidenced-based therapy approaches that are task-related and more intense in terms of the amount of practice or effort are now advocated (13). The use of external devices such as free weights, weight machines, electrical stimulation units, treadmills, etc. is also increasingly common both in therapy and home programs.
One of the major goals of physical therapy for children with CP in GMFCS levels I–III is to promote independent mobility that includes the ability to ambulate among other forms of mobility. To accomplish this, children must have adequate active range of motion that is not exceedingly impeded by spasticity, dystonia or contracture, sufficient strength to maintain body weight support, in some cases with an assistive device, and motor control abilities to allow them to advance their limbs forward to take steps in an effective and efficient manner (e.g. minimal scissoring or internal rotation at the hips, minimal crouch in stance, sufficient knee flexion and/or ankle dorsiflexion in swing to allow for foot clearance). The speed and energy costs of walking are also major factors in how functional the gait pattern will be for an individual child. Each of the aspects above must be evaluated and addressed by the multidisciplinary team to optimize gait function. Physical therapy can have little if any effect on the control of tone abnormalities which require medical and neurosurgical interventions or on the correction of contracture or bony deformity which require orthopaedic surgery; however, it can have a unique and potentially substantial impact on increasing muscle strength and aerobic conditioning, and in potentially in improving lower extremity coordination and speed, in this population.
Although stretching is still a component of therapy programs, the use of passive stretching alone has not been shown to be effective (14), and the impairment of a body structure level goal, i.e. maintaining muscle length, over time is now more effectively accomplished by interventions such as strength training, dynamic or static orthoses, botulinum toxin injections, or other spasticity reducing medications or surgeries, although still in combination with manual stretching techniques. As noted above, once a contracture develops, orthopaedic surgery to lengthen tendons is often required if the restriction in range is impairing function or positioning. Using muscle ultrasound imaging, Shortland and colleagues (15) demonstrated that the fibers lengths of spastic pennated muscles are not necessarily shorter in CP compared to age-matched peers, but the length of the aponeurosis is shorter because of decreased fiber diameter due to muscle weakness. Their resultant conclusion that strengthening may be a more effective strategy for increasing length in those muscles than stretching runs directly counter to traditional NDT tenets to avoid strengthening or any excessive physical effort so as not to exacerbate spasticity. This long-standing but unsubstantiated belief has been increasingly challenged in the literature and strengthening and other intense activity-based programs are now commonly utilized in pediatric rehabilitation for children with and without spasticity (16). Dodd and coauthors (2002) (17) conducted a systematic review of the strength training literature in CP. At that time, only 10 research studies met the criteria, which included only one randomized controlled trial. Most (8/10) reported significant strength increases as a result of the program. Two studies reported improvements in activity, and one study reported improvement in self-perception. No negative effects, such as reduced range of motion or increased spasticity, were reported. Further studies and literature reviews have corroborated these results as well as those from programs that involve or include aerobic training (18, 19, 20).
Intense task-specific training has been shown to be effective in several neurological populations as well as CP, with the most conclusive findings in CP demonstrated in intense upper limb training paradigms (21). For task-specific training in the lower extremity, locomotor training paradigms are now commonly utilized and reported, with many studies incorporating partial body weight-support systems in addition to motorized treadmills. A recent review of the literature on treadmill training in CP (22) demonstrated fairly consistent positive effects on walking speed and the Standing and Walking, Running & Jumping dimensions of the Gross Motor Function Measure. However, no randomized trials comparing treadmill training to over ground walking of the same intensity have been published to date in CP, even though reviews in stroke and spinal cord suggest that it is the intensity of walking practice, rather than the use of a device, that produces the positive functional outcomes (23,24). Regardless of whether the results are similar with or without an external device, the use of treadmills and other exercise devices have transformed motor rehabilitation by reinforcing the effectiveness of intense practice, enabling individuals unable to support their own weight to practice walking more easily, and by using motors and/or added weight support to push people to the limits of their capabilities (25). Depending on the goal and the exercise protocol designed to accomplish that goal, treadmills can be utilized to increase strength through progressive loading, increase coordination by training spinal circuits, improve aerobic condition through endurance training, and increase gait speed by progressing increasing belt speeds over time (26). Thus far, strength and aerobic training, and treadmill training programs have been evaluated over relatively short time intervals, but it is important to realize that these are short term programs in the context of a lifelong disability and while the effects may be modest, greater functional benefits are likely to accrue with time (27).
The delivery of physical therapy services has changed dramatically over time as health insurance in the US no longer supports the amount of therapy that may be necessary to improve or maintain optimal physical conditioning, with the exception perhaps being therapy following surgery which is often more intense. It has now been clearly demonstrated that ambulatory children with CP are far less active than their peers without CP, with the amount of activity directly proportional to their GMFCS Level (28). Decreased activity can lead to loss of strength and increased muscle stiffness, and ultimately function, over time, as well as to other general health problems in all individuals, with those with motor disabilities at even greater risk (29). Physical activity has additionally been shown to contribute positively to mental and emotional functioning (30, 31). Given the changes in health care policy and the recognition that regular and fairly intense physical activity should be part of a daily routine (32), therapists have been forced to adapt their practices and help families develop other strategies to ensure that their child is as active as possible, in as safe a manner as possible. Identifying activities that are sufficiently physically challenging, enjoyable or feasible enough to be sustainable, and safe for long term use is a critical element and to the extent possible, therapists should have input into the design and implementation of these activities. For example, older children may be able to participate in an exercise program at the local YMCA; however, an understanding of their disability and specific impairments is essential in the design of that program and therapists should be involved in this process to ensure safety and maximum effectiveness. Alternatives to activities that may exacerbate abnormal joint stresses should be considered such as pool exercises, cycles or elliptical trainers in lieu of prolonged treadmill use. For those who are unable to ambulate well outside of the home, adapted and motorized cycles and many over ground mobility devices are now available for recreational or therapeutic use. Beyond the physical and emotional benefits, the importance of activity-based therapies for promoting true neural recovery and restoration in those with brain lesions is just beginning to be realized and is rapidly transforming physical therapy goals and practices (33).
Recent years have also seen the development of multiple intensive therapy or educationally-based programs such as conductive education and Adeli Suit programs, which provide some benefits due to their strong focus on activity and independence, but which also raise some concerns. These include the fact that they have failed to demonstrate superior effectiveness over other approaches despite their significantly greater time intensity (34), they fail to identify the ‘active’ ingredients making it unclear to determine which aspects of the programs are effective and which are not, these often place additional financial and time constraints on families, and their intensity is not sustainable over the long term so children may regress when these programs end. While some of these programs do demonstrate the importance of enhanced training or activity, therapists and the health care system need to ensure that children and families are provided the most effective, time efficient, and preferably community based therapy programs that are well-integrated with other services. To enhance the amount of physical activity as well as participation, children should be strongly encouraged to participate in self-chosen adapted or regular sports and physical recreational activities. As children transition into later adolescence and adulthood, the responsibility increases for them to establish and maintain their own activity-based goals as part of their own healthy lifestyle choices.
Finally, in addition to the delivery or development of exercise programs, therapists perform many roles that have not been well studied or evaluated scientifically, but are likely to be of critical importance to families and patients, and to other members of the multidisciplinary team. As an example, they provide expert advice on how to hold and position a young infant with CP so as to safely promote their motor development, they recommend assistive devices to promote mobility, they may fabricate orthoses or work closely with orthotists and physicians in determining the optimal type of orthosis for an individual patient depending on their abilities and goals. Therapists are often a major source of medical information to the family and because of their regular contact with the child and family they may often be the first to recognize the potential need for other treatments and may work with the family in communicating with or identifying a physician or surgeon who will evaluate the child for other interventions.
Medical management of motor disorders in ambulatory patients with cerebral palsy
Overview of recent changes in the approach to diagnosis and treatment
As noted above CP is a group of disorders of movement and posture with varying causes (35). As our knowledge of specific neurological disorders has expanded we can now potentially identify more of these specific causes of motor and movement disorders in children such as hereditary dystonias and the ever expanding group of mitochondrial disorders (36, 37, 38). Whether these disorders should be grouped with the cerebral palsies remains unclear. In the future identifying specific disorders will be increasingly important as specialized treatments for these conditions become available.
Patients with CP present with both positive and negative features of the upper motor neuron syndrome at the level of body structures and functions in the ICF model (39, 40, 41). These include a variety of tone abnormalities ranging from hypotonia to hyperkinesis. Since patients with CP were traditionally classified by their predominant tone abnormality i.e. spasticity or dyskinesis, the nuances of mixed tone disorders were often missed. Selective, specific therapies (38, 42) increase the importance of correctly identifying mixed patterns of abnormal tone. For example, we now know a patient with mixed spastic dyskinetic CP is not a good candidate for selective dorsal rhizotomy (SDR) but may be a candidate for intrathecal baclofen therapy (ITB). Mixed tone disorders require a more studied approach when considering treatment options (43).
In the past the selection of medical treatment for motor disorders associated with CP often started with what was viewed as less invasive treatment later moving to more invasive interventions. This hierarchical model has largely been replaced by an integrated model where a number of interventions are employed in series, often concurrently, and at various times throughout a patient’s life. In ambulatory patients with CP several treatment modalities may be selected at the same time; for example; botulinum toxin (BoNT) to address focal tone issues such as an equinus foot or flexed knee. BoNT treatment may also be combined with physical therapy to strengthen the antagonist and maximize function, with orthotics to provide additional stretch, and with oral medications at night or during the day to reduce spasms (44, 45). This integrated treatment approach is often provided by an interdisciplinary health care team of physicians and therapists. In the 21st century few clinicians work in isolation when providing care for patients with CP (46). Most pediatric hospitals and clinics provide interdisciplinary care in one form of another although the composition of the team and its functional model vary widely across institutions. Team models range from structured programs to a loose affiliation of clinicians who communicate by email, telephone or written records. Even when located in remote locations many clinicians now have access to the internet and thereby colleagues with whom they may consult. This enhanced communication has fundamentally transformed the relationships between individuals from differing disciplines and the way care is delivered to patients. Enhanced communication between medical, rehabilitation and surgical specialists has increased the knowledge of all practitioners involved in the team with patients as the ultimate beneficiaries of resultant improved care and coordination (47, 48, 49).
Interdisciplinary teams have also moved towards incorporating evidenced based medicine into daily practice, no longer relying solely on past or anecdotal experience to make decisions about care for individuals with CP. Future studies must critically evaluate the short and long term efficacy of treatment, effects on function and quality of life. In the past most studies focused on impairments instead of function or participation. There have now been a number of studies evaluating the functional efficacy of BoNT therapy, SDR and ITB (50, 51). Unfortunately, to date, few oral medication studies have evaluated effects of treatment on function or participation (38, 52). It is critical for future studies of medical management techniques to incorporate functional assessment in outcome data. Key to this evaluation is developing outcome tools that are meaningful and are sensitive to change, as well as prescribing only those interventions that provide clinically important changes in function for the patient.
Rationale for treatment selection
Recent years have seen a greater focus on ‘tone management’, primarily to minimize the development of contractures and facilitate ease of movement. An increasing array of medical treatment options including medications, biological agents and surgical interventions are available for management of abnormal muscle tone in patients with CP (44, 53, 54). A thorough understanding of neurophysiology and pharmacology is required to guide clinicians in selecting the most appropriate medications or agents. Although often requested, there are no medical treatments that ameliorate other impairments associated with CP i.e. weakness, balance and motor control. Other factors affecting patient function and participation include patient motivation, family commitment, social support, access to intervention/treatment and geographic location. Clinicians must consider all of these issues when selecting from available treatment options and interventions.
Medical management of the motor disorders in children with CP is most often provided by one or more physicians including physiatrists, neurologists, developmental pediatricians and orthopedists. One of the first decisions for physicians is to determine whether treatment or intervention is needed. Not all abnormal tone is harmful. There may be benefits to increased tone including preserved skeletal and muscle mass, decreased edema, aiding standing and transfers, prevention of decubiti and reduced DVT risks (40). Treatment should only be initiated if the abnormal tone is symptomatic. The next decision is when to initiate treatment. Medical interventions may be initiated in infancy if deemed necessary. This decision is based on a detailed clinical assessment, review of therapy notes, evaluation of the patient’s impairments, body structures (PROM, AROM) and functional capabilities (55). Physicians must evaluate/observe patients as they perform functional activities to identify problem muscles and work with them to establish treatment goals. The PT or OT treating a patient weekly may identify different problems/goals than the MD who sees a patient quarterly. Input from each member of the team is critical to the decision making process (56).
Once problematic muscle tone has been identified the next step is determining which intervention or interventions to choose among the many now available. Options include oral medications and injectable agents (BoNT, phenol, alcohol). Intrathecal baclofen (ITB) therapy, selective dorsal rhizotomy (SDR) and deep brain stimulation (DBS) are tone reducing treatments that require surgical intervention (44, 45, 54). Once the pump is implanted however, ITB clearly becomes a medical intervention (57). As noted above these and other interventions are often performed in series or in combination during the course of a patient’s life.
Complementary and alternative medicine (CAM) may also be recommended by physicians or chosen by families. CAM options for treating patients with cerebral palsy include acupuncture, massage, homeopathic remedies, herbal treatment and magnet therapy to name a few, each with varying degrees of evidence available. It is important for clinicians to ask about use of these treatments, particularly herbal medications as these may interact with prescribed medications.
Treatment of abnormal tone is frequently initiated to facilitate other interventions such as therapy, casting, and bracing/splinting. Medical treatment is continually revised and continued as needed based on review of clinical and functional goals set by the team, patient and family. Medical therapy is directed towards improving symptoms not only at the impairment level but also at improving function. Functional goals as defined by Mayer et al include: Passive function (functional activities that happen to the patient such as hygiene, dressing etc) or active function (activities the patients perform for themselves) (58). The decisions of when to begin, change and or stop medical therapy are based on short and long term treatment goals and response to treatment.
Many clinicians advocate BoNT therapy as the initial treatment for young ambulatory children with CP and problematic spasticity or dystonia. The goals of early BoNT treatment include preserving PROM, prevention of contractures, facilitating therapy, improving mobility/gait and delaying orthopedic surgery. Other treatment options including oral medications are added as needed based on response to BoNT treatment and treatment goals. A bedtime dose of valium or baclofen may be added to the treatment plan to decrease spasms, pain and increase compliance with nighttime splinting. BoNT therapy is often recommended throughout young childhood. Continued treatment is based on a patient’s response to BoNT therapy and treatment goals. Oral medications are less frequently used during the day due to CNS side effects including sedation, impaired learning and other cognitive side effects. Due to dosing limitations with BoNT treatment, this may be combined with phenol or alcohol blocks (e.g. an obturator nerve block for adductor tone/ scissoring in ambulatory patients) to allow a greater number of muscles to be treated than if BoNT was used alone. (44, 45, 59, 60, 61, 62, 63, 64, 65)
Efficacy of BoNT requires accurate placement of the toxin within the target muscle(s). A variety of approaches are used including anatomic guidance/palpation, EMG and electrical stimulation. Ultrasound has been used for decades to guide biopsies and procedures. Advances in high frequency transducer technology have led to an explosion of ultrasound use in musculoskeletal imaging including guidance for BoNT therapy. Ultrasound allows the painless, accurate identification of target muscles and is therefore frequently preferred by patients over other localization techniques. Other advantages cited are that is offers increased ease of identifying deep muscles or muscles with overlapping anatomy this reducing administration time and it does not require the larger needles as does the EMG guided technique (66, 67, 68, 69).
Although better spasticity management is purportedly diminishing the need for future orthopedic surgery (70) many children who initially benefit from BoNT therapy require orthopedic surgery (71) (see surgical section below), BoNT is often provided prior to muscle lengthening, osteotomy or single event multilevel surgery. This perioperative treatment has been shown to reduce muscle spasm, postoperative pain, length of stay and pain medication use (72). Some clinicians continue to advocate for BoNT therapy throughout a patient’s lifespan. Treatment is often reinitiated during accelerated teen growth to address increased dynamic tone, ROM limitations and changes in gait. BoNT therapy in ambulatory adult patients may also be helpful in preserving ROM, gait, mobility and in reducing pain.
Based on regular re-evaluation of symptoms, impairments and function a patient’s medical treatment is continually modified. In some centers SDR is frequently recommended for ambulatory patients with spastic CP. This is most often performed between 4–8 years of age. Some clinicians advocate performing orthopedic procedures first, then re-evaluating the patient’s function before proceeding with SDR; whereas, others would favor doing the spasticity procedure first, with no guidelines in the literature to support one support over the other. Functional improvements following SDR are highly dependent on patient selection. Comprehensive, interdisciplinary evaluation is mandatory including evaluation of strength, mobility and motor control. In many centers ITB is used most frequently in nonambulatory patients GMFCS level IV–V. Other centers advocate for ITB to improve ambulation in patients with CP. In studies with careful selection criteria improved long-term ambulation is supported by the literature (73, 73, 74, 75, 76, 77, 78).
Review of neuroanatomic and neurophysiologic issues influencing treatment of motor disorders associated with CP
Medical intervention requires a basic understanding of neurophysiology involved in maintenance of normal tone which involves the complex interaction of many structures and pathways within the central nervous system (CNS). Both the pyramidal and extrapyramidal systems are involved in generating positive and inhibitory descending signals to the spinal cord and alpha motor neuron. Lesions at any site along these pathways can disrupt the normal balance between excitation and inhibition. Pharmacologic treatments may be directed at structures within the CNS, the peripheral nerve, neuromuscular junction, or directly at the muscle itself. Medications with CNS mechanisms of action (MOA) include drugs or modalities that modulate/change descending inhibitory signals, reduce the release of excitatory neurotransmitters or modulate spinal cord reflex pathways involved in abnormal tone or spasticity.
A. Definitions of tone disorders
Spasticity is characterized by resistance to stretch and may be either or both velocity and position dependent. It results from CNS pathology leading to loss of descending inhibition, often in the pyramidal tracks. The loss of presynaptic inhibitory signals results in decreased synthesis or transport of GABA to the anterior horn cell. This leads to altered synaptic input and membrane properties and resulting hyperexcitability of the alpha motor neuron. Severe spasticity often masks underlying movement disorders or dystonia. Careful clinical assessment in varied settings, positions and velocities is needed (79, 80, 81, 82)
Dystonia is characterized by sustained muscle contractions or postures, often twisting or repetitive in nature. Classically dystonia presents with co-contraction of agonist and antagonist muscles due to failed reciprocal inhibition. Overflow or spread to proximal and distal muscles and other regions commonly occurs. Some patients may present with dystonic posturing only with activity, in others dystonia may be present at rest. Injury to the brainstem, basal ganglia, striatum and thalamus affects GABA synthesis or transportation leading to dystonia and other movement disorders. Normally the basal ganglia provide the memory for controlled or skilled movements (38, 83, 84). The thalamus creates a zone of inhibition modulating descending signals from the motor cortex. When this area is damaged the modulating effects of the basal ganglia are disrupted leading to abnormal tone and movement patterns. In patients who have cerebral palsy dystonia may not present until a child is nearly 3 years of age or beyond due to continuing brain reorganization and maturation. Inherited forms of dystonia also may present in childhood including Dopa responsive dystonias (DRD). DRD should be considered in any patient presenting with dystonia and cerebral palsy, particularly when there is diurnal variation in symptoms, dystonia is progressive and the lower extremities are preferentially involved (36, 38). Symptoms of DRD vary widely at onset and may mimic motor symptoms of CP. Nygard estimated that 5–10% of patients presenting with pediatric onset dystonia actually have DRD. Given the inherited nature of DRD obtaining a detailed family history is critical. A trial of Dopa may be indicated in most if not all patients presenting with dystonic cerebral palsy to clinically rule out a DRD.
Hyperkinetic disorders include chorea, athetosis, choreoathetosis, ballisms, tics, myoclonus, stereotypies and rigidity. These disorders are less well described than spasticity and dystonia. A pediatric Movement Disorders Taskforce, funded by the NIH, has convened for several years to develop or revise descriptors for each of these disorders to more clearly differentiate them. The final document with these descriptors is not yet published. Presently, treatment options are more limited, but will be listed, as available, in the appendix.
B. Pharmacology of medication management of tone disorders
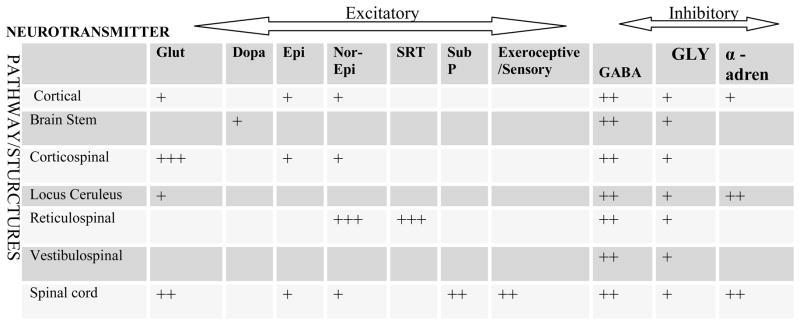
Medications may influence abnormal tone by modulating input to or output from a variety of sites within or outside the CNS including higher cortical centers, the basal ganglia, cerebellum, spinal cord and muscle. The neurons and interneurons influenced by medications utilize a variety of neurotransmitters (NT) including epinephrine, norepinepherine, serotonin, GABA, glutamate, glutamine, dopamine and substance P among others. Descending pathways that may be influenced by medications include are described in more detail in Figure 1 which shows the influence of various pathways on specific neurotransmitters and Table 1 which discusses the primary action or target or each pathway. Medications can act at numerous sites and pathways in the CNS, at the neuromuscular junction and muscle itself. Most centrally acting medications work by altering release of excitatory neurotransmitters or increasing inhibitory neurotransmitter release. Peripheral sites of action include the neuromuscular junction, nerve, and muscle itself. Phenol and alcohol work by demyelinating motor nerves reducing stimulation of the muscle. Neurotoxins work at the neuromuscular junction reducing presynaptic release of acetylcholine thereby decreasing muscle stimulation. There is only one drug that works directly on the muscle. Dantrolene sodium reduces blocks calcium binding in the muscle thereby decreasing excitation/contraction coupling and the force of muscle contraction.
Figure 1.
Known or expected effects of specific neurotrasnmitters on neural structures or pathways. Epi =epinephrine, Nor-Epi= norepinepherine, SERT= serotonin, GABA= Gama-amino butyric Acid, GLUT= glutamate, GLY=glycerine Dopa = dopamine, SubP= substance P, Sensory/exeroceptive= sensory signals from outside CNS
Table 1.
The major function or action of specific brain areas or pathways
| Structure/ Pathway | Brainstem | Pyramidal/ Corticospinal Pathways | Reticulo- Spinal Pathways | Vestibulo- Spinal Pathways | Locus Ceruleus | Spinal Cord | NMJ/ Muscle |
|---|---|---|---|---|---|---|---|
| Function or Action | Modulates: cortical signals/motor Plan. Closed loop contact with cortex. No direct descending output to spinal cord | Discrete distal extremity controlled movements | Trunk and proximal extremity control and posture | Head/trunk control, position in space | Facilitatory drive to spinal cord and spinal pathways | Net balance: facilitation & inhibition leads to increased or decreased signals/drive to α MN | α MN drive leads to release of ACH and muscle contraction |
ACH= Acetylcholine, α MN = alpha motor neuron
Medications for spasticity and dystonia
The MOA of most medications is incompletely understood. Multiple sites of action within the CNS are possible. Variability in efficacy, response, dosing and side effects in individual patients must be considered the norm not the exception. Recognizing this variability is critical in evaluating efficacy, dosing etc. It is estimated that only 30 % of patients have a positive response to oral medications. To date, there is little level 1 evidence to support one medication over another. In addition few studies have evaluated the effect of medications on function. The use of medications is often driven by clinician experience and training, patient age, side effect profile and distribution/severity of the tone disorder.
Selecting Medications and Treatment for a patient
Oral medications and/or ITB are generally used for patients who have generalized and or severe spasticity or dystonia. Injectable medications and agents such as phenol/alcohol and the botulinum toxins are used most often for focal, multifocal and regional tone disorders. Combined treatment with oral medications or ITB and injectable agents is often used in patients with generalized tone disorders. An example of this would be the use of botulinum toxin to address upper extremity spasticity in patients whose tone is incompletely controlled by ITB. Botulinum toxins and oral medications may also be used together to supplement efficacy of the individual agents and prevent side effects of high doses of oral medications. The following is a list of medications that have been used in treated patients with abnormal muscle tone which has expanded in recent years. The use of many of the medications comes from the adult literature and pediatric testing/use is often based on anecdotal experience. Obtaining approval for placebo controlled medication trials in children is complicated by the difficulty in obtaining informed consent in minors. Based on the Best Pharmaceuticals for Children Act which mandates testing for pediatric medications on children, models that would expand pediatric testing are now being explored and implemented. In all future medication trials, incorporating evaluation of functional outcomes is imperative.
The above medications have varying efficacy, mechanism of action and side effect profiles. Prescribing physicians must be familiar with the medications, interactions between medications and drug monitoring. Selection of medications must be based on the patient’s clinical issues, co-morbidities and the medication itself. No one treatment or medication works for a specific tone disorder or group of patients. Selecting medications is an art based on the science of the medication and the motor disability. Included at the end of this article is an appendix of detailed information, as available, of the medications currently in use, MOA, side effects and dosing guidelines if available.
Orthopaedic Management of Motor Disorders in Cerebral Palsy
In the 1800’s William Little, a surgeon from England, began using the technique of percutaneous Achilles tenotomy (transecting the tendon) to treat children and adults with paralytic foot deformities such as those with CP. In fact, the name for cerebral palsy used until even recently was “Little’s Disease.” There was little improvement on the technique of tenotomy or other musculotendinous surgery until the polio epidemic. It was during this time that many different tendon lengthenings and transfers were developed and the field of orthopedics blossomed. With the decline in polio, orthopedic surgeons turned their attention to other neuromuscular diseases such as myelomeningocele and cerebral palsy.
Based on the experience of the polio epidemic, in which only one or a few motor nerve roots were affected, surgeons began operating on one joint at a time. (85,86) For example, a child who was walking on his toes and had a flexion contracture at his hips and knees would have a heel cord lengthening, then the next year, a distal hamstring lengthening, and the next year, a hip flexor lengthening. Mercer Rang called this the “birthday syndrome” in which the child was in the hospital every year of his life getting an operation. This has been shown to be an unwise approach to CP surgery. Currently the best outcomes occur in those children who have single event-multilevel surgery (SEMLS) with physical therapy and appropriate (usually AFO) bracing. (87,88) This involves performing bony osteotomies to correct rotational problems combined with tendon lengthening and transfers at the hip, knee and ankle at the same operative procedure.
The indications to perform orthopedic surgery on children with cerebral palsy are to improve function, prevent deformity, decrease pain from joint dislocation or subluxation, prevent skin pressure areas, improve sitting position, improve cosmesis and hygiene, and to facilitate orthotic management. (89,90) In all cases, the surgeon should work closely with a team which might include physical and occupational therapists, neurologists and physiatrists. There should be consensus on the best treatment plan. For example, the child may be an ambulator who has a combination of spasticity and dystonia, but primarily spasticity. The physiatrist may recommend a course of oral antispasticity medication, perhaps chemodenervation of the muscle or phenol injections in the motor nerves prior to surgery or in conjunction with the surgery. It might be suggested that surgery be delayed for several months to years using physical therapeutic modalities and casting. A critical part of any surgery is the postoperative rehabilitation including appropriate bracing, strengthening and gait training. In those children who are undergoing hip reduction surgery, the wheelchair seating system often does not fit correctly postoperatively and this must be anticipated preoperatively.
The introduction of the GMFCS and the FMS has significantly impacted orthopedic surgery thinking as surgeries designed to improve ambulation are preferred in GMFCS II and III patients, while operations to permit pain-free sitting would be implemented in those in levels GMFCS IV and V. (7,8) It is clear that those patients who have more involvement (IV and V) have more dystonia and mixed motor patterns. Therefore therapies aimed at reducing these movement problems should be implemented concurrently with any planned orthopedic surgery. The GMFCS level can also play a role in deciding whether a child should have a screening radiograph for hip dysplasia. Hagglund et al found that a child with GMFCS I had a zero percent chance of having hip dislocation, while a child with GMFCS V had a 64% chance (91)
Orthopedic surgery does not affect motor control, balance, or improve muscular strength. (92) It may have a short term effect on spasticity as the muscular tension is altered which affects the Golgi tendon apparatus and the muscle spindle. However, as noted in the section on spasticity management, the orthopedic surgeon is a member of a team which includes spasticity management and pre and postoperative rehabilitation. Boyd and Graham suggested a treatment algorithm in which orthopedic surgery is delayed until about age 7–9 years old with a greater focus on physical therapy and spasticity management in the early years. The only exception to that would be if the hip is coming out of the joint secondary to muscle contractures. (93) (Figure 1) This delay has several advantages such as allowing more development of the musculoskeletal system, it permits a “declaration” of the movement disorder since it is sometimes difficult to distinguish between severe spasticity and dystonia in the very young child, and permits studies such as gait lab evaluation to enable single event multilevel surgery. Although the long term effect of this philosophy is still being studied, the hypothesis that fewer orthopedic surgeries and therefore ultimately better long term function is already being observed in patients treated in this manner.
Technology has also had a major impact in the preoperative planning for orthopedic surgery on the lower extremity. In the most advanced centers, three dimensional gait analysis is used to completely evaluate the motion at each of the joints, the forces that cross each joint and when the muscles fire based on dynamic electromyography. Energy utilization is one measure to evaluate the impact of different therapies on the child preoperatively and postoperatively. Although there is controversy as to the clinical utility of gait analysis, there is no question that it is a critical tool to evaluate novel surgeries and the outcomes of therapy. Most experienced orthopedic surgeons who operate on children with CP often rely on these data to plan their surgery.
Computer modeling using cadaveric derived models coupled with gait analysis data from actual patients is an important research tool as well as a method to gain insight into complex biological problems. These models may enable one to determine if a particular surgery would lead to the anticipated results. Delp and his research teams have been leaders in this field and have provided many thought provoking articles in this arena. There have been several excellent studies using computer models to replicate the anatomy and muscle activity about the hip ranging from evaluation of internal rotation gait and its treatment (94), the effect of hip flexion on moment arms (95), to the effect of flexion in crouch gait on hip extension (96) This basic science tool has certainly improved our understanding of the biomechanical forces, but as yet, it has not been utilized to provide an individualized treatment plan for actual patients. This is certainly the hope and promise of this technology.
Orthopedic surgery should be considered in children who have a fixed contracture of their joints; if there is a subluxation or dislocation of a joint, particularly the hip joint; if there are rotational problems which cause walking problems; if there is curvature of the spine which impacts the child’s sitting or might be anticipated to cause pulmonary problems as the child ages; or if there are hygiene or pain problems secondary to any of the above. Pain is usually not present in young children but can be a significant problem as the patients become young adults. Therefore, knowledge of the natural history of cerebral palsy may lead the surgeon to suggest surgery even when there does not appear to be a significant problem such as pes valgus (flat feet), hip subluxation or early scoliosis.
There are basically four major types of orthopedic surgery performed in children with CP:
Musculotendinous or tendon lengthening
Tendon transfers
Osteotomies (cutting bones)
Arthrodesis (fusion)
Peripheral neurectomy may be performed, although the long-term outcome is not predictable and is generally not performed.
1. Musculotendinous lengthening
This is surgery that involves a lengthening of the musculotendinous unit. The tendon can be lengthened in a Z-type fashion (Figure 2) (97) or at the musculotendinous junction or through the fascia as a recession (Figure 3). (97,98) Simple tenotomies (transecting the tendon) often have poor outcomes, although they have a place in hip adductor surgery.
Figure 2.
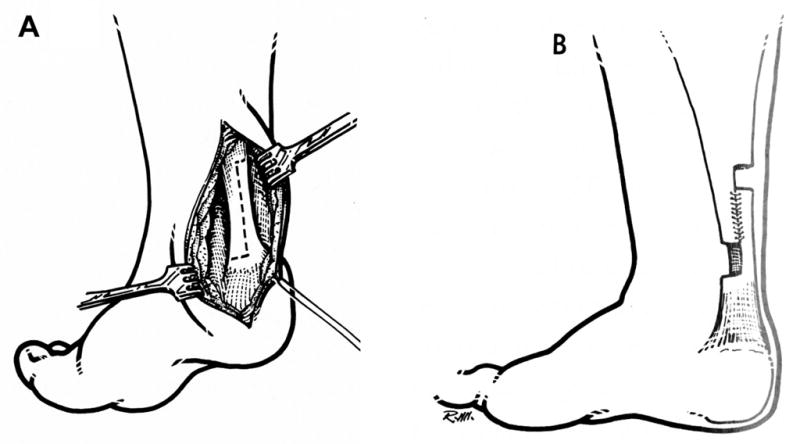
TendoAchilles lengthening procedure showing the intact (A) and resutured (B) tendon
Figure 3.
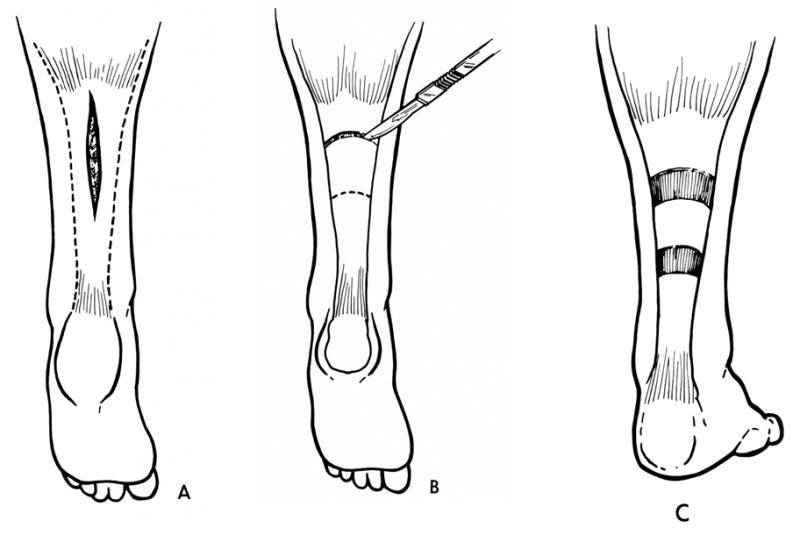
Gastrocsoleus recession procedure which only lengthens the uppermost gastrocnemius portion of the tendon
This procedure is indicated when a contracture is present i.e. the joint that the muscle crosses cannot be moved passively through the full range. There is some decrease in spasticity after tendon lengthening, most likely due an to alteration of the Golgi receptors and muscle spindles in the muscle. Complications include overlengthening of the tendon (usually iatrogenic) and weakness. (99) Recent research suggests that the tendon may actually be long but the muscle fibers are short, so lengthening of the tendon may be deleterious to the muscle (see above).
2. Tendon Transfers
In CP, tendon transfers are used to take a muscle which is spastic and contributing to a deformity and repositioning it (or part of it) to perform another function. The muscle may then be used to balance a joint or even serve as a functional transfer.
In the upper extremity, a common transfer in CP is the Green transfer in which the flexor carpi ulnaris muscle (which serves to palmar flex and ulnarly deviate the wrist) is transferred to the dorsum of the wrist to serve as a wrist extensor and radial deviator. A modification of this technique has been described in which the flexor carpi ulnaris is transferred to the common finger extensors to allow for extension of the fingers and facilitation of release of objects in the hand. (100,101) Other procedures have been devised to improve the position and the function of the hand and fingers. (102)
In the lower extremity, several transfers have been used to improve function. In patients with a stiff knee gait, transfer of the rectus femoris muscle to the hamstrings have been shown to improve the knee range of motion and gait, particularly swing phase clearance of the foot.(103 In patients with spastic hemiplegia and a varus foot deformity, a split transfer of the tibialis anterior or tibialis posterior muscle can correct the position of the foot to neutral. (104)
3. Osteotomies
If left untreated, the action of the spastic muscles can lead to deformity of the bones and alteration of the joint mechanics (subluxation or dislocation). In some ambulatory children, rotational abnormalities (increased femoral anteversion and internal or external tibial torsion) can lead to “lever-arm disease” (105,106,107). All children are born with increased femoral anteversion, but normal ambulation leads to a natural derotation of the femur. This may fail to occur in CP and then must be corrected if the muscles are to work in their normal biomechanical alignment. The use of osteotomies, including shortening osteotomies, has decreased the use of tendon lengthening and is preferred when possible.
In primarily marginal or nonambulatory children, the hip joint can dislocate, causing seating problems and eventually arthritis and pain. In this case, surgery aimed at reducing the hips and preventing further dislocation is performed (107–112) (Figure 4).
Children with cerebral palsy develop multiple deformities at the foot and ankle, which may prevent or limit ambulation and occasionally even the wearing of normal shoes. Multiple osteotomies of the tibia and fibula and all of the bones of the foot have been developed to more correctly align the foot.
4. Arthrodesis
In some cases arthrodesis or fusion of the joints is necessary to place the joint in an optimum position. Although fusion of the hip is rarely warranted because of the difficulty in sitting and/or standing after the fusion, very good results have been obtained in fusion of the thumb and wrist. The best results in fusion in cerebral palsy include arthrodesis of the first metatarsal phalangeal joint of the hallux.(113) So-called extra-articular fusions of the foot for valgus such as Grice or Green procedures are still used to help stabilize the foot, but other newer procedures such as a calcaneal lengthening osteotomy have supplanted this as the preferred treatment of the spastic valgus foot.(114,115) The most common arthrodesis is a spine fusion to treat the scoliosis that children with spastic quadriplegia often develop. (116)
In conclusion, orthopedic surgery has an important role in the management of children and adults with cerebral palsy. The clinician should strive to control the spasticity in conjunction with musculotendinous and bony surgery to maximize each other’s gains. A team approach with physician, nursing, physical, occupational, and speech therapy working together with careful predetermined goals will serve the patient’s needs and aspirations.
Summary
When treating children with cerebral palsy, clinicians need to be constantly asking themselves the question: what can we be doing in childhood, or what should we not be doing, to maximize mobility and independence in adulthood? It is critical that we help them maintain the physical capabilities that they have over time through targeted tone management, joint-friendly exercise strategies, and the judicious use of surgeries that reduce muscle force production or sensory input. More well-informed application of principles of biomechanics, exercise physiology and motor control to this population as well new knowledge of mechanisms underlying neural recovery and restoration are transforming the scientific basis of our intervention strategies. More accurate diagnoses, new developments in prevention of both primary and secondary impairments, and technological advances in brain and body imaging and in rehabilitation device design also have great promise and potential for improving outcomes in terms of health status and most importantly the quality of life for ambulatory children with CP.
Table 2.
The most common (primary) and less common (secondary) medications for the major disorders of tone and movement in cerebral palsy.
| Spasticity Medications | ||
|---|---|---|
| Primary | Secondary | |
| Benzodiazepines | Cyproheptadine | Clorazepate |
| Baclofen | Clonidine | Ketazolam |
| Dantrolene | Lamotrigine | Piracetam |
| Tizanidine | Tiagabine | Progabide |
| Botulinum toxins | Gabapentin | Orphenadrine |
| Phenol/alcohol | Pregabalin | Cannaboids |
| Dystonia Medications | ||
| Primary | Secondary | |
| Dopaminergic therapy | Calcium channel blockers | |
| Levodopa/Carbidopa | Antidopaminergic drugs | |
| Anticholinergics | Tizanadine | |
| Trihexyphenidyl | Clonazepam | |
| Baclofen | Tetrabenazine | |
Contributor Information
Diane L. Damiano, Email: damianod@cc.nih.gov, Chief, Functional & Applied Biomechanics Section, Rehabilitation Medicine Department/ Clinical Center, 10 Center Drive Room 1-1469, National Institutes of Health, Bethesda MD 20892.
Katharine E. Alter, Email: kalter@mail.nih.gov, Senior Clinician, Functional & Applied Biomechanics Section, Rehabilitation Medicine Department/ Clinical Center, 10 Center Drive Room 1-1469, National Institute for Child Health and Human Development, National Institutes of Health, Bethesda MD 20892.
Henry Chambers, Email: hchambers@rchsd.org, David Sutherland Director of Cerebral Palsy Research, Rady Children's Hospital, 3020 Children's Way, San Diego, CA 92123.
References
- 1.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–54. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 2.Sahrmann SA, Norton BJ. The relationship of voluntary movement to spasticity in the upper motor neuron syndrome. Ann Neurol. 1977;2:460–5. doi: 10.1002/ana.410020604. [DOI] [PubMed] [Google Scholar]
- 3.Abel MF, Damiano DL, Blanco JS, Conaway M, Miller F, Dabney K, Sutherland D, Chambers H, Dias L, Sarwark J, Killian J, Doyle S, Root L, LaPlaza J, Widmann R, Snyder B. Relationships among musculoskeletal impairments and functional health status in ambulatory cerebral palsy. J Pediatr Orthop. 2003;23:535–41. [PubMed] [Google Scholar]
- 4.World Health Organization. International classification of functioning, disability, and health. World Health Organization; 2001. [DOI] [PubMed] [Google Scholar]
- 5.Haley SM, Raczek AE, Coster WJ, Dumas HM, Fragala-Pinkham MA. Assessing mobility in children using a computer adaptive testing version of the pediatric evaluation of disability inventory. Arch Phys Med Rehabil. 2005;86:932–9. doi: 10.1016/j.apmr.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Bottos M, Gericke C. Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol. 2003;45:786–90. doi: 10.1017/s0012162203001452. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum PL, Palisano RJ, Bartlett DJ, Galuppi BE, Russell DJ. Development of the Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol. 2008;50:249–53. doi: 10.1111/j.1469-8749.2008.02045.x. [DOI] [PubMed] [Google Scholar]
- 8.Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M. The Functional Mobility Scale (FMS) J Pediatr Orthop. 2004;24:514–20. doi: 10.1097/00004694-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 10.Eliasson AC, Krumlinde-Sundholm L, Rösblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–54. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 11.Knox V, Evans AL. valuation of the functional effects of a course of Bobath therapy in children with cerebral palsy: a preliminary study. Dev Med Child Neurol. 2002;44:447–60. doi: 10.1017/s0012162201002353. [DOI] [PubMed] [Google Scholar]
- 12.Butler C, Darrah J. Effects of neurodevelopmental treatment (NDT) for cerebral palsy: an AACPDM evidence report. Dev Med Child Neurol. 2001;43:778–90. doi: 10.1017/s0012162201001414. [DOI] [PubMed] [Google Scholar]
- 13.Crompton J, Imms C, McCoy AT, Randall M, Eldridge B, Scoullar B, Galea MP. Group-based task-related training for children with cerebral palsy: a pilot study. Phys Occup Ther Pediatr. 2007;27:43–65. [PubMed] [Google Scholar]
- 14.Wiart L, Darrah J, Kembhavi G. Stretching with children with cerebral palsy: what do we know and where are we going? Pediatr Phys Ther. 2008;20:173–8. doi: 10.1097/PEP.0b013e3181728a8c. [DOI] [PubMed] [Google Scholar]
- 15.Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol. 2002;44:158–63. doi: 10.1017/s0012162201001864. [DOI] [PubMed] [Google Scholar]
- 16.Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–40. doi: 10.2522/ptj.20050397. [DOI] [PubMed] [Google Scholar]
- 17.Dodd K, Taylor N, Damiano DL. Systemic Review of Strengthening for Individuals with Cerebral Palsy. Archives of Physical Medicine and Rehabilitation. 2002;83:1157–64. doi: 10.1053/apmr.2002.34286. [DOI] [PubMed] [Google Scholar]
- 18.Mockford M, Caulton JM. Systematic review of progressive strength training in children and adolescents with cerebral palsy who are ambulatory. Pediatr Phys Ther. 2008;20:318–33. doi: 10.1097/PEP.0b013e31818b7ccd. [DOI] [PubMed] [Google Scholar]
- 19.Verschuren O, Ketelaar M, Takken T, Helders PJ, Gorter JW. Exercise programs for children with cerebral palsy: a systematic review of the literature. Am J Phys Med Rehabil. 2008;87:404–17. doi: 10.1097/PHM.0b013e31815b2675. [DOI] [PubMed] [Google Scholar]
- 20.Rogers A, Furler BL, Brinks S, Darrah J. A systematic review of the effectiveness of aerobic exercise interventions for children with cerebral palsy: an AACPDM evidence report. Dev Med Child Neurol. 2008;50:808–14. doi: 10.1111/j.1469-8749.2008.03134.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoare BJ, Wasiak J, Imms C, Carey L. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database Syst Rev. 2007;18:CD004149. doi: 10.1002/14651858.CD004149.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Damiano DL, DeJong SL. A systematic review of the effects of treadmill training and bidy weight support in pediatric rehabilitation. Journal of Neurologic Physical Therapy. 2009 doi: 10.1097/NPT.0b013e31819800e2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Rev Neurother. 2007 Oct;7(10):1417–36. doi: 10.1586/14737175.7.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2008 Apr;16(2):CD006676. doi: 10.1002/14651858.CD006676.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Hesse S. Treadmill training with partial body weight support after stroke: a review. NeuroRehabilitation. 2008;23:55–65. [PubMed] [Google Scholar]
- 26.Cernak K, Stevens V, Price R, Shumway-Cook A. Locomotor training using body-weight support on a treadmill in conjunction with ongoing physical therapy in a child with severe cerebellar ataxia. Phys Ther. 2008;88:88–97. doi: 10.2522/ptj.20070134. [DOI] [PubMed] [Google Scholar]
- 27.Taylor N, Dodd KJ, Damiano DL. Progressive Resistance Exercise in Physical Therapy: A Summary of Systematic Reviews. Physical Therapy. 2005;85:1208–23. [PubMed] [Google Scholar]
- 28.Bjornson KF, Belza B, Kartin D, Logsdon R, McLaughlin JF. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248–57. doi: 10.2522/ptj.20060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durstine JL, Painter P, Franklin BA, Morgan D, Pitetti KH, Roberts SO. Physical activity for the chronically ill and disabled. Sports Med. 2000;30(3):207–19. doi: 10.2165/00007256-200030030-00005. [DOI] [PubMed] [Google Scholar]
- 30.van Uffelen JG, Chin A, Paw MJ, Hopman-Rock M, van Mechelen W. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin J Sport Med. 2008;18:486–500. doi: 10.1097/JSM.0b013e3181845f0b. [DOI] [PubMed] [Google Scholar]
- 31.Ströhle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm. 2009 doi: 10.1007/s00702-008-0092-x. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Morris PJ. Physical activity recommendations for children and adolescents with chronic disease. Curr Sports Med Rep. 2008;7:353–8. doi: 10.1249/JSR.0b013e31818f0795. [DOI] [PubMed] [Google Scholar]
- 33.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86:1406–25. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- 34.Anttila H, Suoranta J, Malmivaara A, Mäkelä M, Autti-Rämö I. Effectiveness of physiotherapy and conductive education interventions in children with cerebral palsy: a focused review. Am J Phys Med Rehabil. 2008;87:478–501. doi: 10.1097/PHM.0b013e318174ebed. [DOI] [PubMed] [Google Scholar]
- 35.O’Shea TM. Diagnosis treatment, and prevention of cerebral palsy. Clin Obstet Gynecol. 2008 Dec;51(4):816–28. doi: 10.1097/GRF.0b013e3181870ba7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nygard TG, Marsden CD, Duvoisin RC. Dopa responsive dystonia. Adv Neurol. 1988;50:377–384. [PubMed] [Google Scholar]
- 37.Bressman SB, Tagliati M, Klein C. Genetics of Dystonia . Dystonia: Etiology, clinical features, and treatment. 2004:11–21. WE MOVE. [Google Scholar]
- 38.Bhidayasiri R, Tarsy D. Treatment of dystonia. Expert Rev Neurotherapeutics. 2006 Jun;6:863–886. doi: 10.1586/14737175.6.6.863. [DOI] [PubMed] [Google Scholar]
- 39.Mayer NH. Spasticity and the stretch reflex. Muscle Nerve. 1997;20(suppl 6):S1–S13. [PubMed] [Google Scholar]
- 40.Mayer NH, Herman RM. Positive Signs and consequences of an upper motor neuron syndrome. Spasticity and other forms of muscle over activity in the upper motor neuron syndrome. :11–26. WE MOVE. [Google Scholar]
- 41.Gracies JM, Nance P, Elovic E. Traditional pharmacological treatment for spasticity part II. Muscle Nerve. 1997:S92–120. [PubMed] [Google Scholar]
- 42.Bhidayasiri R, Tarsy D. Treatment of dystonia. Expert Rev Neurotherapeutics. 2006 Jun;6:863–886. doi: 10.1586/14737175.6.6.863. [DOI] [PubMed] [Google Scholar]
- 43.Adam OR, Jankovic J. Treatment of Dystonia. Parkinsonism and related research. 2007;13:S362–S368. doi: 10.1016/S1353-8020(08)70031-2. [DOI] [PubMed] [Google Scholar]
- 44.Papavasilou AS. Management of the motor problems in cerebral palsy : A critical update for the clinician. Eur J Paediatri Neurol. 2008 Sept 6; doi: 10.1016/j.ejpn.2008.07.009. (epub – in press) [DOI] [PubMed] [Google Scholar]
- 45.Tilton AH. Management of Spasticity in cerebral palsy. Seminars in Pediatric neurology. 2004 March;11(1):58–65. doi: 10.1016/j.spen.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Russman BS, Tilton AH, Gormley ME. Cerebral palsy: A rational approach to a treatment protocol and the roled of botulinum toxin in treatment. Spasticity and other forms of muscle over activity in the upper motor neuron syndrome. :179–192. WE MOVE. [PubMed] [Google Scholar]
- 47.Derstine JB, Shepard PM, Nixon-Cave K, Kinneally M. Rehabil Nurs. 2003 May–June;28(3):92–5. doi: 10.1002/j.2048-7940.2003.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 48.Fedreizzi E. Childs Nerv Syst. 1995;11:21–22. doi: 10.1007/BF00338420. [DOI] [PubMed] [Google Scholar]
- 49.Deepak Sharan. Indina Journal of pediatrics. 2005 Nov;72:989–973. [Google Scholar]
- 50.Gulmans, Vollenbroek-Hutten MMR, Van Gemert-Pijnen JEWC, Van Harten WH. Evaluating quality of patient care communication in integrated care settings: a mixed method approach. Int J Qual Health Care. 2007 Oct;19(5):281–8. doi: 10.1093/intqhc/mzm029. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz MH, Viehweger E, Stout J, Novacheck TF, Gage JR. J Pediatr Orthop. 2004 Jan–Feb;24(1):45–53. [PubMed] [Google Scholar]; Comprehensive treatment of children with cerebral palsy an outcome assessment comprehensive treatment of ambulatory children with cerebral palsy: an outcome assessment. J Pediatr Orthop. 2004 Jan–Feb;24(1):45–53. [PubMed] [Google Scholar]
- 52.Rémy-Néris O, Tiffreau V, Bouilland S, Bussel B, Rémy-Néris O, Tiffreau V, Bouilland S, Bussel B. Arch Phys Med Rehabil. 2003 May;84(5):643–50. doi: 10.1016/s0003-9993(02)04906-7. [DOI] [PubMed] [Google Scholar]
- 53.Oefflinger D, Bagley A, Rogers S, Gorton G, Kryscio R, Abel M, Damiano D, Barnes D, Tylkowski C. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Dev Med Child Neurol. 2008;50:918–25. doi: 10.1111/j.1469-8749.2008.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gracies JM, et al. Traditional pharmacologic treatment for spasticity Part I: Local Treatments. Spasticity and Other Forms of Muscle Overactivity in the Upper Motor Neuron Syndrome. WE MOVE self study guide. [Google Scholar]
- 55.Gracies JM, et al. Traditional pharmacologic treatment for spasticity Part II. Systemic treatment: Spasticity and Other Forms of Muscle Overactivity in the Upper Motor Neuron Syndrome. WE MOVE self study guide. [Google Scholar]
- 56.Tilton AH. Therapeutic Intervention for tone abnormalities in cerebral palsy. NeuroRX. 2006 April;3(2):01–08. doi: 10.1016/j.nurx.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinbok P. Selection of treatment modalities in children with spastic cerebral palsy. Neurosurg Focus. 2006 Aug 15;21(2):e4. doi: 10.3171/foc.2006.21.2.5. [DOI] [PubMed] [Google Scholar]
- 58.Mayer NH, Elovic EP. Neurotoxin Institute, Practical Aspects of Botulinum Neurotoxin Treatment of UMNS [Google Scholar]
- 59.Albright AL. Intrathecal baclofen in cerebral palsy and movement disorders. J Child Neurol. 1996 Nov;:S29–35. doi: 10.1177/0883073896011001S05. review. [DOI] [PubMed] [Google Scholar]
- 60.Bottos M, Benedetti MG, Salucci P, Gasparroni V, Giannini S. Botulinum toxin with and without casting in ambulant children with spastic diplegia: A clinical and functional assessment. Dev Med Child Neurol. 2003 Nov;45(11):758–62. doi: 10.1017/s0012162203001403. [DOI] [PubMed] [Google Scholar]
- 61.Criswell SR, Crowner BE, Racette BA. The use of botulinum toxin therapy for lower extremity spasticity children with cerebral palsy. Neurosurg Focus. 2006 Aug 15;21(2):e1. doi: 10.3171/foc.2006.21.2.2. [DOI] [PubMed] [Google Scholar]
- 62.Gooch JL, Patton CP. Combining botulinum toxin and phenol to manage spasticity in children. Archives Phys Med Rehab. 2004 Jul;85(7):1121–4. doi: 10.1016/j.apmr.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 63.Koman LA, Paterson Smith B, Balkrishnan R. Spasticity associated wit cerebral palsy in children: guidelines for the use of botulinum toxin. A Paediatr Drugs. 2003;5(1):11–23. doi: 10.2165/00128072-200305010-00002. [DOI] [PubMed] [Google Scholar]
- 64.Scholtes VA, Dallmeijer AJ, Becher JG. Can we identify predictors of multilevel botulinum toxin injections in children with cerebral palsy who walk with a flexed knee pattern. J Child Neurol. 2008;23:628–34. doi: 10.1177/0883073807313039. [DOI] [PubMed] [Google Scholar]
- 65.Tilton AH. Injectable neuromuscular blockade in the treatment of spasticity and movement disorders. J Child Neurol. 2003 Sep;18(supp1):S50–66. doi: 10.1177/0883073803018001S0701. [DOI] [PubMed] [Google Scholar]
- 66.Berweck S, Schroeder AS, Gietzek UM, Heinen F. Sonography guided Botulinum toxin in children with cerebral palsy. Neuropediatrics. 2002;33:321–223. doi: 10.1055/s-2002-34500. [DOI] [PubMed] [Google Scholar]
- 67.Berweck S, Schroeder AS, Gietzek UM, Heinen F. Sonography guided Botulinum toxin in children with cerebral palsy. Lancet. 2004 Jan 17;363(9404):249–50. doi: 10.1016/S0140-6736(03)15351-2. [DOI] [PubMed] [Google Scholar]
- 68.Willenborg MJ, Shilt JS, Smith BP, Estrada RL, Castle JA, Koman LA. Technique for iliopsoas ultrasound guided active electromyography directed botulinum A toxin injection in cerebral palsy. J Pediatr Orthop. 2002 Mar–Apr;22(2):165–8. [PubMed] [Google Scholar]
- 69.Westoff B, Seller K, Wil A, Jaeger M, Krauspe R. Ultrasound guided botulinum toxin injection techniques for the iliopsoas muscle. Dev Med Child Neurol. 2003 Dec;45(12):829–32. doi: 10.1017/s0012162203001531. [DOI] [PubMed] [Google Scholar]
- 70.Hagglund F, Andersson S, Duppe J, Pedertsen HL, Nordmark E, Westboom L. Prevention of severe contractures might replace multilevel surgery in cerebral palsy: results of a population based health care programme and new techniques to reduce spasticity. J Pediatr Ortho B. 2005;14:269–73. doi: 10.1097/01202412-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Novachek TF, Gage JR. orthopedic management of spasticity in cerebral palsy. Childs Nerv Syst. 2007 September;23(9):1015–31. doi: 10.1007/s00381-007-0378-6. [DOI] [PubMed] [Google Scholar]
- 72.Brereton K, Low J, Nattrass G, Graham HK. Analgesic effects of botulinum toxin A: a randomized, placebo-controlled clinical trial. Dev Med Child Neurol. 2000 Feb;42(2):116–21. doi: 10.1017/s0012162200000220. [DOI] [PubMed] [Google Scholar]
- 73.Albright AL. selective dorsal rhizotomy and challenge of monitoring its long-term sequelae. J Neurosurg Pediatrics. 2008 March;1(3):178. doi: 10.3171/PED/2008/1/3/178. discussion 178 – 9. [DOI] [PubMed] [Google Scholar]
- 74.Bleyenheuft C, Filipetti P, Caldas C, Lejeune T. Experience with external pump trial prior to implantation for ITB in ambulatory patients with spastic cerebral palsy. Neurophysiol Clin. 2007 Jan–Mar;37(1):23–28. doi: 10.1016/j.neucli.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 75.Gerszten PC, Albright AL, Barry MJ. Effect on ambulation of continuous intrathecal baclofen infusion. Pediatr Neurosurg. 1997 Jul;27(1):40–44. doi: 10.1159/000121223. [DOI] [PubMed] [Google Scholar]
- 76.Langerak NG, Lamberts RP, Fieggen AG, Peter JC, van der Merwe L, Peacock WJ, Vaughan CL. J a perspective gait analysis to be in patients with paraplegic cerebral palsy 20 years after selective dorsal rhizotomy. J Neurosurg Pediatrics. 2008 Mar;1(3):180–86. doi: 10.3171/PED/2008/1/3/180. [DOI] [PubMed] [Google Scholar]
- 77.Sgouros S. Surgical management of spasticity of cerebral origin in children. Acta Neurochir Suppl. 2007;97(prt 1):193–2003. doi: 10.1007/978-3-211-33079-1_27. [DOI] [PubMed] [Google Scholar]
- 78.Abel MF, Damiano DL, Gilgannon M, Carmines D, Kang HG, Bennett BC, Laws ER., Jr JNeurosurg. 2005 Mar;102(2Suppl):157–62. doi: 10.3171/jns.2005.102.2.0157. [DOI] [PubMed] [Google Scholar]
- 79.Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Muscle Nerve Suppl. 1997;6:S92–120. Review. Traditional Pharmacological treatments for spasticity Part. [PubMed] [Google Scholar]
- 80.Gracies JM, Elovic E, McGuire J, Simpson DM. Muscle Nerve Suppl. 1997;6:S61–91. Review. Traditional pharmacological treatments for spasticity part I Muscle Nerve Suppl. 1997. [PubMed] [Google Scholar]
- 81.Gracies JM, Simpson DM. Spastic Dystonia: Etiology, clinical features, and treatment. 2004:195–211. WE MOVE. [Google Scholar]
- 82.Gracies JM. Physiology of Spastic Paresis Par II: Emergence of muscle over activity. Muscle Nerve. 2005 May;31(5):552–71. doi: 10.1002/mus.20285. Review. [DOI] [PubMed] [Google Scholar]
- 83.Brin MF, Comella CL. Pathophysiology of dystonia. Dystonia: Etiology, clinical features, and treatment. 2004:3–10. WE MOVE. [Google Scholar]
- 84.Holton JL, Schneider SA, Ganesharan P, Barreto J, Wood NW, Lees AJ, Bhatia KP. doi: 10.1212/01.wnl.0000302175.76229.f0. [DOI] [PubMed] [Google Scholar]
- 85.Banks HH. Equinus and cerebral palsy--its management. Foot Ankle. 1983;4:149. doi: 10.1177/107110078300400307. [DOI] [PubMed] [Google Scholar]
- 86.Barnett HE. Orthopedic surgery in cerebral palsy. J Am Med Assoc. 1952;150:1396. doi: 10.1001/jama.1952.03680140034008. [DOI] [PubMed] [Google Scholar]
- 87.Graham HK, Baker R, Dobson F, et al. Multilevel orthopaedic surgery in group IV spastic hemiplegia. J Bone Joint Surg Br. 2005;87:548. doi: 10.1302/0301-620X.87B4.15525. [DOI] [PubMed] [Google Scholar]
- 88.Graham HK, Harvey A. Assessment of mobility after multi-level surgery for cerebral palsy. J Bone Joint Surg Br. 2007;89:993. doi: 10.1302/0301-620X.89B8.19446. [DOI] [PubMed] [Google Scholar]
- 89.DeLuca PA. The musculoskeletal management of children with cerebral palsy. Pediatr Clin North Am. 1996;43:1135. doi: 10.1016/s0031-3955(05)70454-5. [DOI] [PubMed] [Google Scholar]
- 90.Renshaw TS, Green NE, Griffin PP, et al. Cerebral palsy: orthopaedic management. Instr Course Lect. 1996;45:475. [PubMed] [Google Scholar]
- 91.Hagglund G, Lauge-Pedersen H, Wagner P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet Disord. 2007;8:101. doi: 10.1186/1471-2474-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abel MF, Damiano DL, Pannunzio M, Bush J. Muscle-tendon surgery in diplegic cerebral palsy: functional and mechanical changes. J Pediatr Orthop. 1999;19:366. [PubMed] [Google Scholar]
- 93.Boyd R, Graham HK. Botulinum Toxin A in the management of children with cerebral palsy: indications and outcome. Eur J Neurol. 1997;4(supp 2):S15. [Google Scholar]
- 94.Arnold AS, Anderson FC, Pandy MG, et al. Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. J Biomech. 2005;38:2181. doi: 10.1016/j.jbiomech.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 95.Delp SL, Hess WE, Hungerford DS, et al. Variation of rotation moment arms with hip flexion. J Biomech. 1999;32:493. doi: 10.1016/s0021-9290(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 96.Hicks JL, Schwartz MH, Arnold AS, et al. Crouched postures reduce the capacity of muscles to extend the hip and knee during the single-limb stance phase of gait. J Biomech. 2008;41:960. doi: 10.1016/j.jbiomech.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canale ST, Beaty JH. Operative Pediatric Orthopedics. Mosby-Year Book; 1995. [Google Scholar]
- 98.Chang WN, Tsirikos AI, Miller F, et al. Distal hamstring lengthening in ambulatory children with cerebral palsy: primary versus revision procedures. Gait Posture. 2004;19:298. doi: 10.1016/S0966-6362(03)00070-5. [DOI] [PubMed] [Google Scholar]
- 99.Seniorou M, Thompson N, Harrington M, et al. Recovery of muscle strength following multi-level orthopaedic surgery in diplegic cerebral palsy. Gait Posture. 2007;26:475. doi: 10.1016/j.gaitpost.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 100.Wolf TM, Clinkscales CM, Hamlin C. Flexor carpi ulnaris tendon transfers in cerebral palsy. J Hand Surg (Br) 1998;23:340. doi: 10.1016/s0266-7681(98)80054-5. [DOI] [PubMed] [Google Scholar]
- 101.Carlson MG, Athwal GS, Bueno RA. Treatment of the wrist and hand in cerebral palsy. J Hand Surg (Am) 2006;31:483. doi: 10.1016/j.jhsa.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 102.Rayan GM, Young BT. Arthrodesis of the spastic wrist. J Hand Surg (Am) 1999;24:944. doi: 10.1053/jhsu.1999.0944. [DOI] [PubMed] [Google Scholar]
- 103.Sutherland DH, Santi M, Abel MF. Treatment of stiff-knee gait in cerebral palsy: a comparison by gait analysis of distal rectus femoris transfer versus proximal rectus release. J Pediatr Orthop. 1990;10:433. [PubMed] [Google Scholar]
- 104.Vogt JC. Split anterior tibial transfer for spastic equinovarus foot deformity: retrospective study of 73 operated feet. J Foot Ankle Surg. 1998;37:2. doi: 10.1016/s1067-2516(98)80003-3. [DOI] [PubMed] [Google Scholar]
- 105.Dodgin DA, De Swart RJ, Stefko RM, et al. Distal tibial/fibular derotation osteotomy for correction of tibial torsion: review of technique and results in 63 cases. J Pediatr Orthop. 1998;18:95. [PubMed] [Google Scholar]
- 106.Gage J, Novacheck T. An update on the treatment of gait problems in cerebral palsy. J Pediatr Orthop B. 2001;10:265. [PubMed] [Google Scholar]
- 107.Kay RM, Rethlefsen SA, Hale JM, et al. Comparison of proximal and distal rotational femoral osteotomy in children with cerebral palsy. J Pediatr Orthop. 2003;23:150. [PubMed] [Google Scholar]
- 108.Song HR, Carroll NC. Femoral varus derotation osteotomy with or without acetabuloplasty for unstable hips in cerebral palsy. J Pediatr Orthop. 1998;18:62. [PubMed] [Google Scholar]
- 109.Mubarak SJ, Valencia FG, Wenger DR. One-stage correction of the spastic dislocated hip. Use of pericapsular acetabuloplasty to improve coverage. J Bone Joint Surg Am. 1992;74:1347. [PubMed] [Google Scholar]
- 110.McNerney NP, Mubarak SJ, Wenger DR. One-stage correction of the dysplastic hip in cerebral palsy with the San Diego acetabuloplasty: results and complications in 104 hips. J Pediatr Orthop. 2000;20:93. [PubMed] [Google Scholar]
- 111.Graham HK. Painful hip dislocation in cerebral palsy. Lancet. 2002;359:907. doi: 10.1016/s0140-6736(02)08015-7. [DOI] [PubMed] [Google Scholar]
- 112.Saraph V, Zwick EB, Zwick G, et al. Effect of derotation osteotomy of the femur on hip and pelvis rotations in hemiplegic and diplegic children. J Pediatr Orthop B. 2002;11:159. doi: 10.1097/00009957-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 113.Davids JR, Mason TA, Danko A, et al. Surgical management of hallux valgus deformity in children with cerebral palsy. J Pediatr Orthop. 2001;21:89. doi: 10.1097/00004694-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 114.Andreacchio A, Orellana CA, Miller F, Bowen TR. Lateral column lengthening for planovalgus foot deformity in ambulatory children with cerebral palsy. J Pediatr Orthop. 2000;20:501–5. [PubMed] [Google Scholar]
- 115.Noritake K, Yoshihashi Y, Miyata T. Calcaneal lengthening for planovalgus foot deformity in children with spastic cerebral palsy. J Pediatr Orthop B. 2005;14:274. doi: 10.1097/01202412-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Thomson JD, Banta JV. Scoliosis in cerebral palsy: an overview and recent results. J Pediatr Orthop B. 2001;10:6. [PubMed] [Google Scholar]