Abstract
Intestinal transplantation is often the only alternative form of treatment for patients dependent on total parenteral nutrition for survival. Although a limited number of intestinal transplantations have been performed, results with FK 506 immunosuppression are comparable to those for other organ transplants. The impact of successful intestinal transplantation on gastroenterology will likely be similar to the impact of kidney and liver transplantation on nephrology and hepatology.
Keywords: intestine, FK 506, allograft
THE DEVELOPMENTAL PHASE
The First Attempts
Lillehei first reported an experimental model for isolated intestinal transplantation in dogs in 1959 (1). The first description of multivisceral canine transplantation (liver, stomach, pancreaticoduodenal complex, small and large intestine) (2) followed a year later.
Despite advances in immunosuppression that permitted other organ transplantations, transplantation of the intestine and related organs was not clinically successful. Results of clinical trials using azathioprine and cyclosporin (CyA), for example, proved dismal. Of all isolated intestinal transplantation attempts, only two clinical cases (Kiel, Paris) (3, 4) enjoyed a long-term survival under CyA immunosuppression, although multiple rejections and assorted complications kept the patients hospitalized for prolonged periods of time.
Following the multivisceral transplantations, one patient (Pittsburgh) (5) survived for more than six months before dying of lymphoproliferative disease, and a patient in Innsbruck (6) lived for almost a year before succumbing to metastases from an underlying disease. However, in each of these cases, the intestinal component of the grafts provided life-supporting function and allowed independence from total parenteral nutrition (TPN), thereby proving the feasibility, but not the practicality, of intestinal transplantation in humans.
The Concept of Transplantation of Organ Clusters
The intraabdominal organs resemble the residual grapes of a grape cluster (7, 8). The central stem of the vine consists of the celiac axis, the superior mesenteric artery, the superior mesenteric vein, and the portal vein. The organs, like grapes, can be removed without disturbing the integrity of the vine. Thus, when various organs are removed from the multivisceral graft, the remaining organs constitute a custom complex that satisfies the needs of the patient. In addition, including the liver with the graft protects other organs transplanted from the same donor against rejection (2, 9).
The cluster concept is of practical value. Because the intraabdominal organs have an intimate anatomical and physiological relationship, they must often be replaced in combinations that do not include bowel. Such procedures have been used to treat massive upper abdominal malignancies that necessitated upper abdominal exenterations (resection of the liver, stomach, spleen, pancreaticoduodenal complex, and frequently, parts of the ascending and transverse colon). Previously, graft replacement was usually accomplished with a cluster containing the liver and pancreaticoduodenal complex. The duodenum and at times part of the jejunum served as conduits. These intestinal pieces proved durable, subject to rejection, and capable of regeneration even after severe rejection.
A PIVOTAL CASE
At the University of Western Ontario, Grant et al reported the unequivocal success of a liver–small intestinal cluster transplant that enabled the recipient to resume normal activity without parenteral nutrition (10). These results were attributed to the protective effect of the contemporaneously transplanted liver. Consequently, the intestinal transplant was initially considered successful only in combination with a liver transplant.
INTESTINAL TRANSPLANTATION UNDER FK 506 IMMUNOSUPPRESSION
The current era of intestinal transplantation began with clinical trials under FK 506 immunosuppression (11). FK 506 is a macrolide antibiotic derivative from the fungus Streptomyces tsukubaensis. Its immunosuppressive action is similar in nature (although much more potent) to that of CyA, but the chemical structures of the two differ, and FK 506 binds to a different immunophilin (12). Although FK 506 and CyA share some side effects (nephrotoxicity, neurotoxicity, diabetogenicity), FK 506 does not cause gingival hyperplasia or hirsuitism and has absorption characteristics superior to those of CyA (13). Clinical trials of intestinal transplantation under FK 506 began after the successful introduction of FK 506 as a rescue agent in patients for whom cyclosporin therapy was ineffective (14). Transplantation under FK 506 was then adopted as a primary form of treatment for liver, kidney, heart, and lung recipients (15).
The experimental results of intestinal transplantation were encouraging (16). A clinical trial demonstrated the feasibility and consistent success of intestinal transplantation, whether isolated or in combination with other organs. In fact, isolated intestinal transplantation proved as efficacious as combination transplants. Furthermore, the procedure was easier to perform and followed a less complicated postoperative course (17). This report is based on the information gathered from the intestinal transplantation trial under FK 506 at the University of Pittsburgh.
INDICATIONS
The generic indication for intestinal transplantation is intestinal failure, which is defined as the inability to maintain nutrition through the enteral route due to loss of native intestine absorptive surface or function. Loss of absorptive surface (short gut syndrome) is by far the more common of the two main causes of intestinal failure. In children, this was most frequently due to intestinal resection following necrotizing enterocolitis, gastroschisis, volvulus, and intestinal atresia (18). Pseudoobstruction and generalized Hirschprung’s disease were other indications, even though the intestine is anatomically intact.
In adults referred to us for transplantation, the leading cause of Short gut syndrome was Crohn’s disease, followed by thrombotic disorders and trauma (12). The most common cause of loss of function in adults when intestine was present was pseudoobstruction, followed by radiation enteritis.
The liver needs to be replaced only if associated severe liver disease or an inborn error correctable with liver transplantation is present.
PREOPERATIVE EVALUATION AND SELECTION OF THE APPROPRIATE OPERATION
In addition to precise knowledge of the underlying disease, the entire gastrointestinal (GI) tract must be mapped in detail to decide which organs need to be replaced. Endoscopy, GI contrast studies, motility studies, and angiography are frequently used for this purpose.
The condition of the native liver must be assessed to determine whether transplantation is necessary. When in doubt, a liver biopsy should be performed. The presence of deep jaundice, severe hepatomegaly, and/or portal hypertension indicates that the liver must be replaced. Extensive fibrosis and cholestatic injury also point to irreversible damage and therefore require transplantation. In both cases, the liver and other organs needed for transplant should be obtained from the same donor.
In borderline cases, lack of data makes judgement more difficult. Mild jaundice may be reversible with successful intestinal transplantation, as we have seen once in our series. In the case of thrombotic disorders, natural anticoagulant deficiencies—in particular protein C, protein S, and anti-thrombin III deficiency—should be systemically evaluated. These proteins are largely produced in the liver. Therefore, replacing the liver with a phenotypically normal liver will correct these problems because they are of hepatic origin.
Often one can determine which organ cluster needs to be transplanted, or whether the intestine alone will suffice, only after a thorough exploration of the organs during the operation. The donor surgeons are asked to bring back the entire multivisceral graft whenever possible, thereby exploiting the flexible cluster concept of removing extraneous organs when the abdominal exploration is completed.
CONTRAINDICATIONS
Unless they are totally resectable with the transplant, infections and malignancies are absolute contraindications. The investigative nature of these transplants makes patients in extremis poor candidates, although humanitarian instincts have sometimes prevented their exclusion. A combination of inferior vena cava and portal vein thrombosis, particularly in the context of previous surgery, has proven extremely difficult to handle technically and thus represents a high surgical risk. Multiple previous laparotomies complicate the procedure but do not constitute a contraindication to transplantation. Narcotic dependence is common among these patients, and rehabilitation before transplantation is strongly recommended.
DONOR SELECTION
Heart-beating cadavers with normal intestines are used as donors. They must have the same ABO blood type [to avoid humoral graft versus host disease (GVHD) complications] and be approximately the same size as the recipient. Histocompatibility locus antigen (HLA) matching is random. If the recipient is seronegative for cytomegalovirus (CMV) and the donor is CMV-positive, he or she could contract severe CMV -graft enteritis, despite the use of acyclovir and Ganciclovir (DHPG) prophylaxis. However, these donors cannot be exluded in the case of children, given the scarcity of available grafts. Fortunately, most pediatric donors are CMV seronegative.
In multiple organ recovery procedure, the heart, lungs, and kidneys are usually removed for transplantation in other patients. University of Wisconsin (Belzer) safely preserves the graft for at least 10–12 h. The graft is not subjected to immunoalteration with OKT3 pretreatment or by any other means. The organ supply for isolated intestinal grafts from cadaveric donors is currently plentiful. In the future, however, transplantation of intestinal segments from living related donors may be an attractive option because the segments may be immunologically more compatible.
RECIPIENT PROCEDURES
Solitary Intestine
When performing abdominal exploration and resection of the specimen, surgeons almost always must contend with adhesions and scars from multiple previous laparotomies. Careful dissection and hemostasis is therefore important to ensure successful transplantation. The infrarenal abdominal aorta is usually the source of arterialization of solitary intestinal grafts or of intestine combined with other viscera. The venous outflow of the isolated grafts is directed into the native portal system whenever possible, although some investigators have proposed drainage into the systemic venous circulation as an alternative and technically more expedient approach. However, drainage into the portal system is feasible almost without exception (19) (Figure 1). This method is preferable to systemic drainage because it does not produce an Eck fistula (portaprival) effect on the liver (20).
Figure 1.
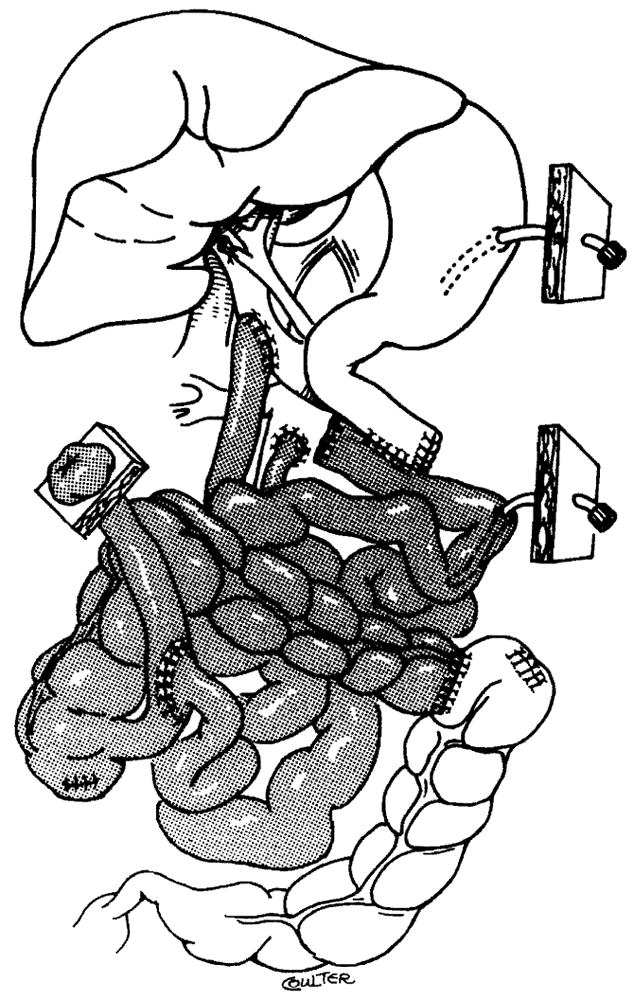
Isolated intestinal transplant.
Multiple Organ Grafts
In combined liver-intestinal and multivisceral grafts, the native liver is stripped from the native vena cava, which remains intact (Figures 2 and 3). In combined liver-intestinal transplantation, an end-to-side portocaval shunt is performed to drain the retained host splanchnic organs via the native portal vein during the intraoperative time when the venous outflow is blocked. Outflow of the graft is accomplished by anastomosing the host and graft inferior vena cava with the piggyback technique (21) (Figure 4). If the anatomical situation is favorable, the portocaval shunt performed at the beginning of the operation to prevent venous stasis in the native organs is taken down after the graft is revascularized. The native portal vein is then anastomosed to the side of the donor portal vein to maintain complete hepatopetal flow of the portal blood from both the graft and the retained host viscera. In some cases, a technically perfect portoportal anastomosis of this type cannot be performed (usually as a result of size discrepancy), and the previously constructed portocaval shunt that drains the native organs is left in place.
Figure 2.
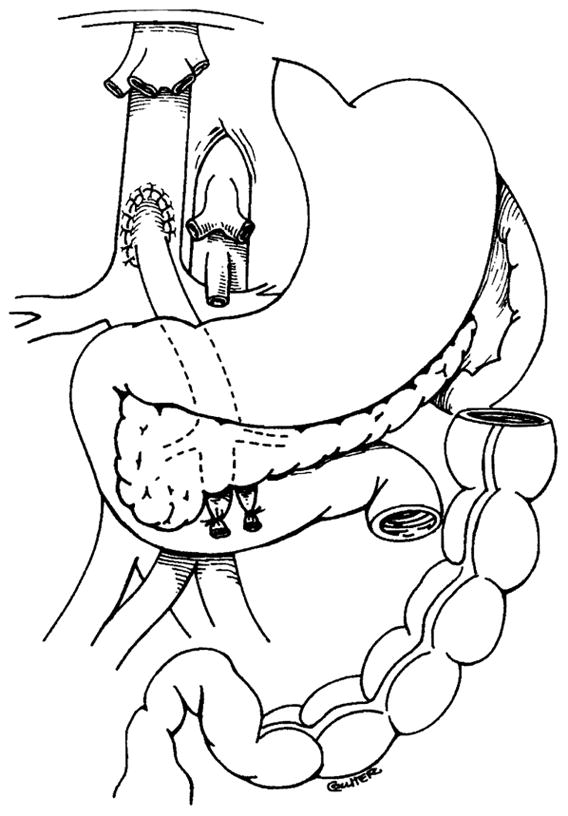
Resection of liver with preservation of the native inferior vena cava (a portocaval shunt has been constructed).
Figure 3.

Multivisceral transplant.
Figure 4.
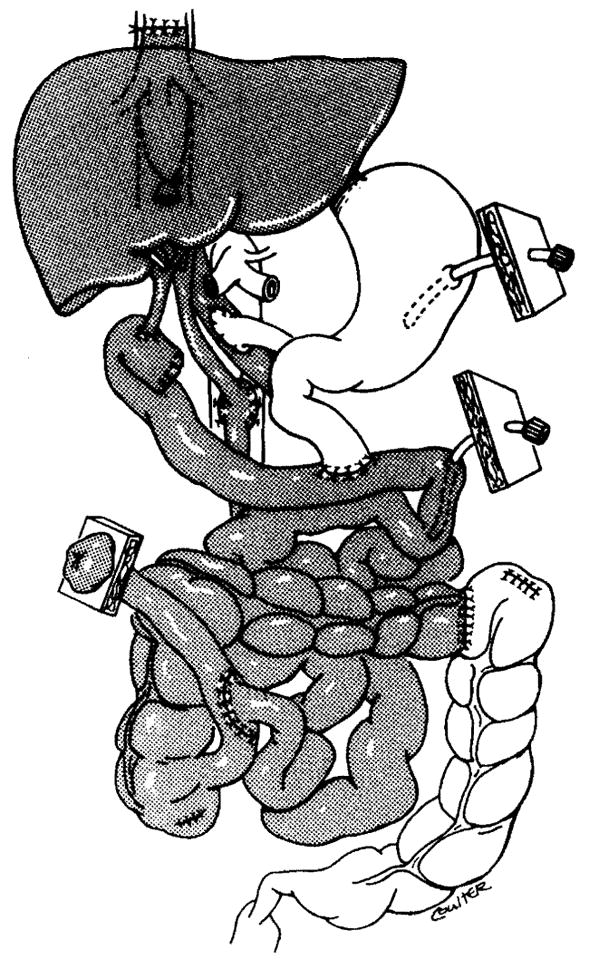
Combined liver-intestinal transplant.
Gastrointestinal continuity is established with conventional techniques. A stoma is constructed using the graft that provides venting and allows physicians easy access when perfoming endoscopies and biopsies. The stoma is closed when the graft stablizes and frequent endoscopies are no longer necessary, usually several months after transplantation. In liver-intestinal clusters, the donor bile duct is drained into a Roux en Y loop, which is constructed using donor jejunum (Figure 4).
PREVENTION, MONITORING, AND TREATMENT OF REJECTION
Rejection is the most common complication of intestinal transplantation, occurring in most clinical cases. Immunosuppression is based on FK 506, which is administered intravenously (0.1 mg/kg per day) immediately after the operation and then orally (0.3 mg/kg per day) when tolerated. The dose is decreased if nephrotoxicity occurs or if FK plasma levels are greater than 3 ng/ml and is increased if the recipient rejects the transplant. An intravenous infusion of PGE1 given for five days after transplantation is considered weakly immunosuppressive and thought to reduce nephrotoxicity. Administration of a bolus of prednisolone at the time of transplantation is followed by a high dose (200 mg) that is tapered to a maintenance dose of 20 mg over 5 days. Tapering of the steroid dosages may continue until they can be stopped. Steroid discontinuation is particularly important for children because these hormones adversely affect growth. However, weaning children off steroids must be done with great caution.
Clinical signs of rejection include fever, graft dysmotility [low or (more often) high stomal output], and changes in stoma color. Other common complications such as infection and ischemic injury have the same clinical presentation. Endoscopy with multiple mucosal biopsies under vision is the best means by which to confirm or refute a diagnosis of rejection. Surveillance endoscopies and biopsies through the stoma are usually performed twice a week for one month after transplantation and once a week for 1–2 months thereafter. Endoscopic signs of rejection include erythema and induration of the graft, which is covered with exudate or pseudomembranes if rejection is severe.
Histologically, acute rejection presents as a widening of the lamina propria with inflammatory cells, primarily blastic lymphocytes accompanied by smaller lymphocytes, eosinophils, and macrophages with or without neutrophils. Epithelial cell necrosis, goblet and Paneth cell depletion, and epithelial regenerative changes also occur. More severe rejection presents with focal ulceration, which can evolve into mucosal sloughing. Treatment of rejection varies according to its severity. Augmentation of the baseline FK 506 and steroid dose is sufficient to treat mild rejection, whereas steroid bolus and/or a temporary period of high doses of prednisolone are required for moderate or severe rejection. If this medication proves ineffective, antilymphocyte globulin (ALG) may be adminstered as a last resort. Repeated endoscopies and biopsies are used to monitor treatment effectiveness.
Repeated severe acute rejections can lead to chronic rejection characterized by arterial occlusive disease. A full thickness biopsy or arteriography must be performed to confirm this diagnosis. Mucosal biopsy shows distortion of the mucosal architecture with villous blunting, widening, and fibrosis of the lamina propria; loss of goblet and Paneth cells; focal ulceration; and metaplasia. Although hyperacute or humoral rejection may occur in experimental animals, neither has been detected in clinical cases. Five transplants of isolated (n = 1) and combined (n = 4) intestinal grafts have been performed in the presence of a positive cytotoxic cross match with no adverse consequences.
In our series, definite GVHD occurred only once, in a patient whose immunosuppressive therapy was greatly reduced in the setting of surgical infection. Subclinical GVHD, manifested as transient skin rash with fever of unknown etiology, has been suspected in intestinal transplantation patients but has not been well documented. Rejection of the liver is diagnosed and treated as in liver transplantation.
PREVENTION, MONITORING, AND TREATMENT OF INFECTION
Infection is the second most common complication of intestinal transplantation. Careful attention must be paid to the details of surgical technique because major contamination during surgery in the presence of immunosuppression will most likely result in a surgical infection. Failure of a single anastomosis will also lead to infection, which is usually the first of several complications leading to death.
Both donor and recipient undergo enteral decontamination and receive intravenous antibiotic prophylaxis. The recipient receives enteral treatment for 4–6 weeks postoperatively, but intravenous antibiotics are administered for only 5–7 days unless a specific reason warrants continued dosages. Even if surgical technique is perfect, the recipient runs a high risk of bacterial or fungal infection from the intestinal graft because rejection, ischemic injury, or a viral infection can open the mucosal barrier to enteral microorganisms. The resulting septicemia (translocation) can cause contamination of indwelling venous and arterial lines, which need to be cultured and replaced frequently. These lines must be used for long periods during postoperative convalescence and can consequently act as the primary source of infection.
All body fluids are frequently cultured. In particular, stool cultures are performed twice a week for 6–8 weeks posttransplant, and if bacterial growth is significant (> 106 colonies/ml), the enteral decontamination is modified accordingly. Low-dose trimethoprim-sulfamethoxazole is used to prevent pneumocystis pneumonia. However, since no prophylaxis effectively combats viruses, the best strategy is to avoid excessive immunosuppression. Intravenous DHPG and oral or intravenous acyclovir are administered liberally for cytomegalovirus and Epstein-Barr virus prophylaxis, but they seem only to postpone and perhaps lessen the severity of these infections, rather than prevent them.
Infection must be recognized early and treated promptly. All histological specimens obtained in an infection workup are sent for viral cultures, stains, and polymerase chain reaction (PCR) to search for viruses. If a virus is identified, treatment begins immediately. Although specific treatment for some viruses such as respiratory syncytial virus and adenovirus is not available, drastic reduction of immunosuppression can ameliorate viral infections.
NUTRITION
A feeding jejunostomy or gastrostomy is routinely placed at the time of transplantation. Enteral nutrition begins as soon as intestinal motility is established and increased as tolerated. Formulas used include Vivonex TEN®, Peptamen®, and Modified Compleat®. The physician may treat the presence of glucose in the stool and/or high stomal output in the absence of rejection or infection by reducing the rate of enteral feedings and using antidiarrheal drugs. Intravenous nutrition is administered liberally, gradually decreased, and stopped only when the enteral feedings satisfy the nutritional needs of the patient.
Adult patients usually resume their normal eating habits quickly. Children, on the other hand, may refuse to eat, either because they never learned how or because they are afraid to do so. Teaching them how to eat is a difficult task, although it is easier if they were already ingesting food prior to transplantation.
ASSESSMENT OF GRAFT FUNCTION
Simple clinical observations such as maintenance of body weight during the course of enteral feedings, serum albumin, and enteral output play a valuable role in the assessment of graft function. FK 506 plasma levels are a poor man’s test of its absorptive capacity, and the absence of sugar or presence of trace quantities in the enteral output indicates good carbohydrate absorption. The presence of greater amounts of sugar indicates an inability to tolerate the feedings.
The D-xylose absorption test provides a better assessment of carbohydrate absorption, which is depressed for four weeks after transplantation and normalizes over several months. Improvement of D-xylose absorption over time is a positive sign. Gradual deterioration, on the other hand, has been associated with chronic rejection. Moderate or severe graft rejection and/or cytomegalo-virus infection depress the D-xylose absorption curve. However, the results must be interpreted cautiously in cases of delayed gastric emptying, intestinal dysmotility, and impaired renal function because these also have multifactorial etiologies.
The level of fat absorption usually falls below normal but rarely reaches levels at which steatorrhea is produced. Serum level measurements of vitamins A, D, and E and 72-h stool fat excretion measure fat absorbtion. Indirect methods such as serum albumin and prealbumin as well as anthropometric measurements are usually used to calculate protein absorption levels.
Motility can be grossly assessed either by the enteral output or with radiological studies. Motility studies are performed periodically to evaluate the motility patterns of the graft components. Assessment of the liver function is as in liver transplantation.
RESULTS
Forty-five intestinal transplants were performed at the University of Pittsburgh under FK 506 immunosuppression between May 1990 (when the program started) and April 1993. Follow up is provided through June 1, 1993. Twenty-three adults (mean age 33.2 years, range 19–58 years) and 22 children (mean age 3.5 years, range 0.5–15.5 years) participated in the program. Two patients died on the operating table during attempted multivisceral transplantation. Fifteen patients underwent isolated intestinal transplantation, 21 underwent combined liver-intestinal transplantation, and 7 underwent multivisceral transplantation. The large bowel was included in the graft of the last 13 patients in this series.
Two retransplantations were performed: one solitary intestinal and one combined liver-intestinal. Cumulative one-year patient survival was 82% (adults: 89.4%; children: 75.6%) and graft survival was 73% (adults: 79.3%; children: 68%). One-year patient survival for isolated intestinal transplantation and combined transplantation was 90.9 and 76.2%, respectively. One-year graft survival was 70.0 and 72.7%, respectively. Of the seven recipients of the multivisceral transplant procedure, six are alive. No retransplantations were performed in this group.
Eleven patients died posttransplantation: five of acute rejection and sepsis before (n = 3) or after (n = 2) removal of their grafts, three of sepsis due to technical failure, and the other three of lymphoproliferative disease (n = 1), pneumonia (n = 1), and TPN catheter-related pulmonary embolus (n = 1).
The frequency and severity of acute rejection of the intestine did not vary between the recipients of solitary vs combination grafts. In combined liver-intestinal grafts, the frequency and severity of acute liver rejection was approximately the same as that reported by us and others in recipients of isolated liver grafts. Patients who received an isolated graft tolerated enteral feedings and became TPN-independent sooner than did combined transplant recipients. Mean intensive care unit and hospital stay were also shorter for isolated intestinal graft recipients.
Three recipients of a combined liver-intestinal graft developed lymphoproliferative disease, resulting in one fatality. Thirty-two patients are still alive, of which 25, including the longest-surviving patient (3 years), are totally free of TPN and follow an unrestricted, regular diet. Six patients still require partial TPN support, but only one remains fully dependent on TPN.
CONCLUSION
Transplantation of the intestine, whether alone or in combination with other intraabdominal organs under FK 506 immunosuppression, yields results comparable to those obtained from more commonly performed transplantations. This procedure represents a reasonable alternative for the treatment of intestinal failure, provided that the complexity of patient care and the commitment required to provide this care are taken into account.
Acknowledgments
Our work was supported by research grants from the Veterans Administration and by Project Grant No. DK 29961 from the National Institutes of Health in Bethesda, Maryland.
Literature Cited
- 1.Lillehei RC, Goott B, Miller FA. The physiological response of the small bowel of the dog to ischemia including prolonged in-vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543–60. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA., Jr Mass homo-transplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: report of a case. Clin Transplant. 1989;3:89–91. [Google Scholar]
- 4.Goulet O, Revillon Y, Brousse N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940–43. doi: 10.1097/00007890-199204000-00046. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. J Am Med Assoc. 1989;261:1449–57. [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder P, Goulet O, Lear PA. Small-bowel transplantation: European experience. Lancet. 1991;336:110–11. doi: 10.1016/0140-6736(90)91621-g. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374–86. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slarzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–44. [PMC free article] [PubMed] [Google Scholar]
- 9.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–74. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 10.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–84. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 11.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–34. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–87. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE. Debate: FK 506 vs cyclosporine. Transplant Proc. 1993;25(1):511–12. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Todo S, Fung J, et al. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–4. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann, Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multi-visceral versus isolated small bowel allo-transplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 17.Todo S, Tzakis A, Reyes J, et al. Small intestinal transplantation in humans with or without the colon. Transplantation. 1993 doi: 10.1097/00007890-199403270-00012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzakis AG, Todo S, Reyes J, et al. Intestinal transplantation in children under FK 506 immunosuppression. J Pediatr Surg. 1993;28:1040–43. doi: 10.1016/0022-3468(93)90514-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzakis AG, Todo S, Reyes J, et al. Piggyback orthotopic intestinal transplantation. Surg Gynecol Obstet. 1993;176:297–98. [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr Probl Surg. 1983;20:692–745. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649–52. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]


