Abstract
Functionally distinct areas were mapped within the pedunculopontine tegmentum (PPT) of 42 ketamine/xylazine anesthetized rats using local stimulation by glutamate microinjection (10 mM, 5–12 nl). Functional responses were classified as: 1) apnea; 2) tachypnea; 3) hypertension (HTN); 4) sinus tachycardia; 5) genioglossus electromyogram activation or 6) pontine-waves (p-waves) activation.
We found that short latency apneas were predominantly elicited by stimulation in the lateral portion of the PPT, in close proximity to cholinergic neurons. Tachypneic responses were elicited from ventral regions of the PPT and HTN predominated in the ventral portion of the antero-medial PPT. We observed sinus tachycardia after stimulation of the most ventral part of the medial PPT at the boundary with nucleus reticularis pontis oralis, whereas p-waves were registered predominantly following stimulation in the dorso-caudal portion of the PPT. Genioglossus EMG activation was evoked from the medial PPT.
Our results support the existence of the functionally distinct areas within the PPT affecting respiration, cardio-vascular function, EEG and genioglossus EMG.
Authors keywords: Pedunculopontine tegmentum, apnea, functional anatomy, sleep
1. Introduction
The pedunculopontine tegmentum (PPT) is known to participate in a wide range of state-regulating and behavioral functions. PPT plays an important role in rapid eye movement (REM) sleep and REM sleep-related phenomena, including EEG activation, hippocampal theta rhythm and pontine-waves (p-waves) (Datta and Hobson, 1995; Garcia-Rill, 1991; Rye, 1997; Shouse and Siegel, 1992; Steriade and McCarley, 1990; Vertes et al., 1993). Injection of glutamate into the PPT increases REM sleep and wakefulness for hours in unanesthetized rats (Datta et al., 2001a and b) and injection of carbachol increases REM sleep for days in cats (Calvo et al., 1992; Datta et al., 1991;) and rats (Carley and Radulovacki, 1999). Lesions or pharmacological blockade of the PPT diminishes wakefulness and eliminates or alters expression of the tonic and phasic components REM sleep, including p-waves and rapid eye movements (Shouse and Siegel, 1992). The PPT also participates in regulation of motor control (Garcia-Rill, 1991; Winn, 2006), modulation of sensation (Reese et al, 1995), orientation and attention (Rostron et al, 2008), reaction time, and learning and memory (Garcia-Rill, 1991; Datta, 1997; Datta and Hobson, 1995).
The PPT also may play an important role in autonomic regulation. Continuous electrical stimulation within the PPT evoked respiratory depression in anesthetized cats (Lydic and Baghdoyan, 1993), whereas we demonstrated that carbachol injection into the PPT increased respiratory dysrhythmia during sleep in conscious rats (Carley and Radulovacki, 1999; Radulovacki et al., 2004). We further showed that microinjection of glutamate into the PPT of anesthetized rats initiated respiratory disturbances characterized by irregular alternations between tachypnea and bradypnea/apnea (Saponjic et al., 2003; 2005; 2006). Stimulation of the PPT can also evoke cardiovascular reactions, characterized by increased blood pressure (BP) (Padley et al., 2007).
A variety of evidence shows that PPT neurons are, to some extent, anatomically organized into subregions according to their function (Rye, 1997). For example, anatomically a specific antero-ventro-medial region of the PPT reciprocally innervates the basal ganglia (Rye et al., 1987), whereas a dorso-caudal portion of the PPT has specific connectivity to the limbic ventral tegmental area (Grofova and Zhou, 1998; Oakman et al., 1995; Rye 1997; Rye et al., 1987). Lesions of the anterior and posterior PPT produced opposite effects on nicotine-induced locomotion and self-stimulation behavior in rats (Alderson et al., 2008), and electrical stimulation specific to the anterior PPT prior to training improved subsequent learning (Andero et al., 2007).
Our laboratory has demonstrated that local injection of glutamate in anesthetized rats can evoke respiratory dysrhythmia, electroencephalogram (EEG) activation, hippocampal theta-rhythm, or increased phasic events such as p-waves (Saponjic et al., 2003; 2005; 2006). Further, these studies suggested that such phenomena are at least partially differentiable, according to stimulation site, suggesting a functional topography. However, a detailed functional mapping of these activities within the pedunculopontine tegmentum has not been performed. The aim of the present study was to use glutamate microinjections to determine the functional map of respiratory, cardiovascular and electroencephalographic phenomena within the PPT.
2. Methods
Experiments were performed on 42 spontaneously breathing adult male Sprague–Dawley rats, weighing 200–300 g, maintained on a 12-h light–dark cycle, and housed at 25°C with free access to food and water. Principles for the care and use of laboratory animals in research were strictly followed, as outlined by the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences Press, Washington, DC, 1996).
2.1. Surgical Preparation
The rats were anesthetized with a combination of 80 mg/kg ketamine (Abbott Laboratories, North Chicago, IL) and 5 mg/kg xylazine (Phoenix Scientific Inc., St Joseph, MO) by intraperitoneal injection. After a surgical plane of anesthesia was achieved, rats were placed in the stereotaxic apparatus (David Kopf Inst., model 962 A Tujunga, CA). The level of anesthesia was monitored by BP, heart rate and reflexes (corneal and toe-pinch). Supplemental dose of 40 mg/kg ketamine/ 2.5 mg/kg xylazine were used as necessary to maintain a stable plane of anesthesia. No injections were made for at least 15 minutes after any supplemental dose of anesthesia.
Animals were instrumented to record bilateral electroencephalograms (EEGs) using stainless steel screw electrodes located in the frontal (AP, +2.5 mm to bregma, ML, 2 mm to midline; DV, 1 mm ventral to the surface of the brain) and parietal cortex (AP, −2.5 mm to bregma; ML, 2 mm to midline; DV, 1 mm ventral to the surface of the brain), referenced to a screw electrode in the nasion. A bipolar twisted electrode made of teflon-coated stainless steel wires with uninsulated tips of 1 mm and with a 1 mm separation between the uninsulated tips was stereotaxically targeted into the contralateral PPT to record the pontine EEG (AP, −7.8 mm to bregma; ML 1.8 mm to midline; DV, 7 mm ventral to the surface of the brain) (Paxinos and Watson, 2004). The genioglossus electromyogram (EMG) was obtained using teflon-coated wire electrodes with uninsulated tips of 2 mm inserted bilaterally into the base of the tongue 1–2 mm lateral to the frenulum. The electrocardiogram (ECG) was registered by needle electrodes placed in the left axilla and the right flank. The femoral artery was catheterized to monitor blood pressure using Transpac IV transducer (Hospira, Lake Forest, IL).
A unilateral burr-hole osteotomy provided access to the rostral lateral pons, contralaterally to the twisted electrode and the dura was carefully removed. Three-barrel micropipettes were built using standard glass with filament (1 mm×0.25 mm, A-M Systems, Carlsborg, WA) and a vertical puller (Model No 50–239, Harvard Apparatus Ltd., Kent, England) to achieve an overall tip diameter of 10–20 µm. A milli-pulse pressure injector (MPPI-2, ASI, Eugene, OR) was used to inject glutamate (10 mM l-glutamic acid monosodium salt in 0.2 M PBS; ICN Biomedicals, Aurora, OH), 0.2 M PBS, or oil red-O dye (Sigma, St Louis, MO; solution of 7 mg in 1 ml ethanol) to aid histological verification of injection sites.
The MPPI-2 was outfitted with a backpressure unit to prevent injected fluids or extracellular fluid from entering the multibarrel assembly between injections. Typical injection pressure was ~70 psi, while back pressure was ~0.1 psi.
2.2. Recording Procedure
Throughout each experimental protocol, we performed a 10-channel recording comprising: right and left monopolar frontal cortical EEG; right and left monopolar parietal cortical EEG; pontine bipolar EEG; genioglossus EMG; respiration recorded by a thoracic piezoelectric sensor (VelcroR Tab-Infant-Ped; SleepmateR Technologies); ECG; arterial BP and an injection marker (logic level pulse) provided by the pressure injector.
After conventional amplification and filtering (1–20 Hz band-pass for EEG and BP, 1–100 Hz for EMG and ECG, and 1–10 Hz for respiration; CyberAmp 380, Axon instruments/Molecular Devices, Sunnyvale, CA) the analog data were digitized (sampling frequency 200/s) and recorded using SciWorks for Windows software (Datawave Systems, Longmont, CO). Each recording began with a 10 min registration of the baseline activity prior to any injections, which were separated by at least 5 min.
2.3. Experimental Protocol
The pipette was stereotaxically positioned at the expected dorsal margin of the PPT region (Paxinos and Watson, 2004) and was advanced ventrally in 100 µm increments. At each site 5–12 nl of l-glutamate was injected. Injection volumes were directly measured using a dissecting microscope (Wild Heerbrugg, model M5) with a calibrated reticule to observe the movement of the fluid meniscus within the pipette barrel. The dose of the glutamate was chosen according to our previous work (Saponjic et al., 2003; 2005; 2006), which showed the effectiveness of this volume and concentration to evoke a prominent respiratory reaction from the PPT. Further, work by Nicholson (1985) suggests that within the first 35 s the effective diffusion radius is limited to approximately 150 µm.
EEG (cortical and pontine), EMG, BP and respiration were registered prior to and after each glutamate injection. An “effective site” was determined by visually evident perturbations in any of these parameters after the glutamate injection. In this case a minimum 5-min interval was provided prior to advancing the pipette. In all animals at least two repeated glutamate injections were made at any effective site to better document the duration and reproducibility of the response. A sham control also was obtained at each effective site by injection 5–12 nl of PBS. The PBS injection was made either prior to or after the second glutamate injection at the effective site. In every experiment, subsequent glutamate injections reproduced the initial response, whereas all PBS injections failed to do so. Although application of the back pressure to the barrel containing O-red dye raises potential concern of alcohol-leakage to the injection site between injections, the reproducibility of the responses after the repeated glutamate injections argues that this effect was not of significant concern to the interpretation of the present observations.
The effective sites were injected with oil O-red dye for histological verification. In some cases the point of the reaction was marked by biotinylated dextran amine (BDA, Invitrogen, 5% in 0.1 M PBS) at the end of the experiment. BDA was delivered iontophoretically.
The initial target sites within the PPT were varied among experiments in order to provide a balanced sampling of responses across the anatomical extent of the PPT region.
2.4. Data processing
Respiratory pattern analysis was conducted using software developed in our laboratory. An automated adaptive threshold algorithm was used to detect and quantify the inspiratory and expiratory phases of the respiratory cycle, providing the inspiratory, expiratory and total duration of each breath. Apnea was defined as any breath with a duration exceeding the mean of the previous 10 breaths by at least 50%. Tachypnea persisted for at least 3 breaths, was determined by visual scoring. Hypertension (HTN) was assessed visually as a transient BP increase with a return to the baseline. Sinus tachycardia was assessed visually and had a minimum duration of 3 beats. Genioglossus EMG activation was visually assessed with respect to the preceding 10 s baseline. P-waves were visually assessed from the pontine EEG tracings according to the following criteria: 1) a major deflection with the same polarity throughout a recording; 2) a wave duration of 50–150 ms; and 3) a large amplitude that was readily discriminable from the background.
2.5. Histology
At the end of each experiment rats were deeply anesthetized (an additional dose of 40/2.5 mg/kg of ketamine/xylazine was administered if necessary) and perfused intracardially. The perfusion started with a vascular rinse with 0.9% saline until the liver had cleared (at a perfusion speed of 40 ml/min, about 1 min). The perfusion continued with 4% paraformaldehyde solution (300 ml at 40 ml/min, then 30 ml/min), and finally with 10% sucrose solution in phosphate buffer (30 ml/min). The brain was extracted en-bloc, cleared of meninges and blood vessels, and immersed in 4% paraformaldehyde overnight. Brains were immersed in 30% sucrose solution for several days. Then they were cut in a transverse plane in 40 µm thick sections, using a cryostat microtome. To identify the location of microinjection sites in relation to the cholinergic neurons of the PPT, sections were processed for NADPH-diaphorase by incubating for 15 min in a mixture of 2 mg/ml β-NADPH and 1 mg/ml nitroblue tetrazolium (Sigma, St Louis, MO). Air-dried slides were cleared in xylene and coverslipped with Permaslip.
3. Results
Functional responses were observed following 102 glutamate injections in a total of 42 rats. Based upon an initial exploratory and heuristic examination of the data, the responses were classified as: 1) apnea (49 injections in 22 rats); 2) tachypnea (8 injections in 7 rats); 3) hypertension (HTN) (10 injection in 5 rats); 4) ECG sinus tachycardia (5 injections in 2 rats); 5) genioglossus EMG activation (14 injections in 6 rats); 6) p-waves (6 injections in 5 rats ) (Fig. 1A and B). This classification scheme captured over 90% of all responses observed. However, complex responses, including combined apnea and EMG activation (2 cases), apnea and HTN (4 cases), tachypnea and HTN (2 cases), HTN and EMG activation (1 case), tachypnea, HTN and p-waves (1 case) were observed in 10 rats. Advancing the pipette along a dorso-ventral track could elicit responses of different modalities or of different latencies within the same modality. Responses included for analysis as illustrated in Fig. 1B, had variable latencies, but occurred within 5 – 35 s of glutamate injection. To compile a functional map of the “response regions” we employed the pipette location associated with the shortest response latency for that modality within a track of a given experiment. Polymodal reactions to the glutamate tended to occur in the “transitional zones” among the unimodal response areas. Fig. 2 maps the short latency responses of different modalities from all 42 experiments onto a schematic representation of transverse brain sections spaced at 400 µm intervals over the anteroposterior extent of the PPT. The responses are presented in relation to the locations of the NADPH-diaphorase (+) cholinergic cells (filled circles in the background). The responses of different modalities are indicated by a color symbols and mapped to the 400 µm plane nearest to the anatomical 40 µm tissue section corresponding to the tip of the pipette.
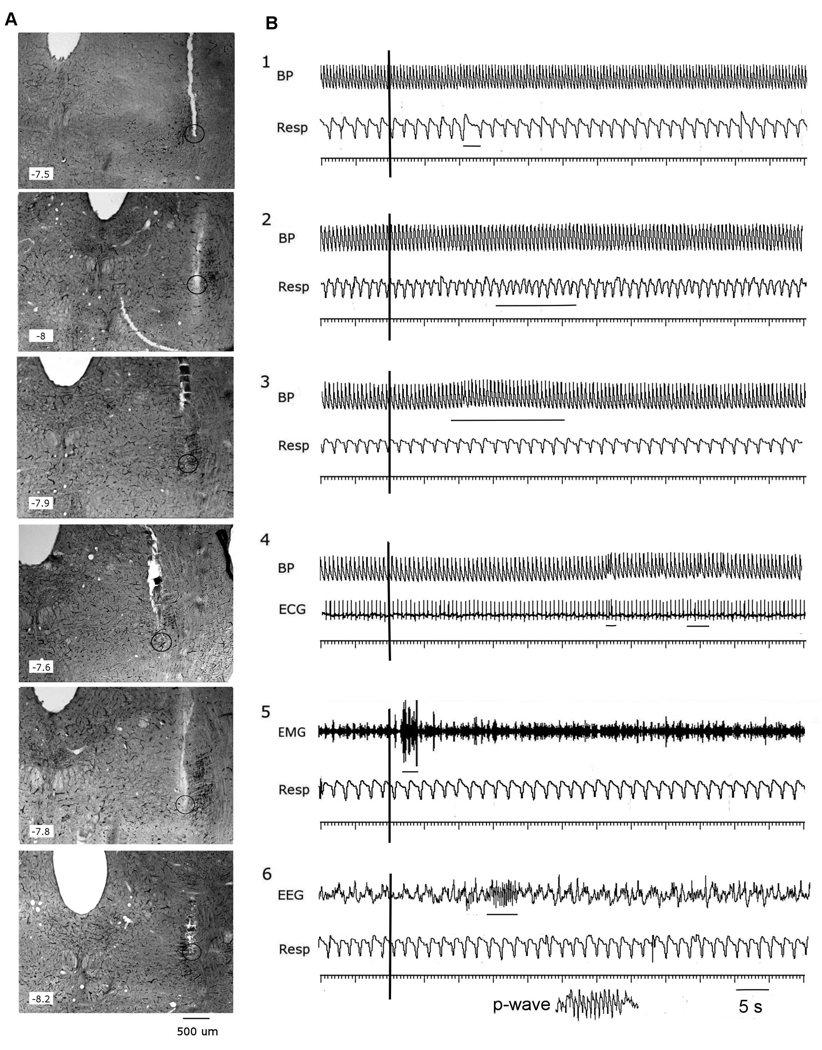
Fig1.
Responses evoked by glutamate microstimulation of the PPT: (A) photomicrographic illustration of various PPT injection sites paired with (B) physiological responses to glutamate microinjection at the depicted site The 40 µm brain sections (A) were stained for NADPH-diaphorase to localize cholinergic neurons with a dark reaction product.
Panel B illustrated six categories of physiologic response: 1. Apnea; 2.Tachypnea; 3.Hypertension (HTN); 4. Sinus tachycardia; 5. Genioglossus EMG activation; 6. P-waves. An injection marker is shown as a vertical rule (at 10 s) on each trace. Responses are marked by thin horizontal lines. On the respiratory traces inspiration is a downward deflection from baseline.
Resp – respiration; BP – blood pressure; ECG – electrocardiogram; EEG – pontine EEG; EMG – genioglossus EMG.
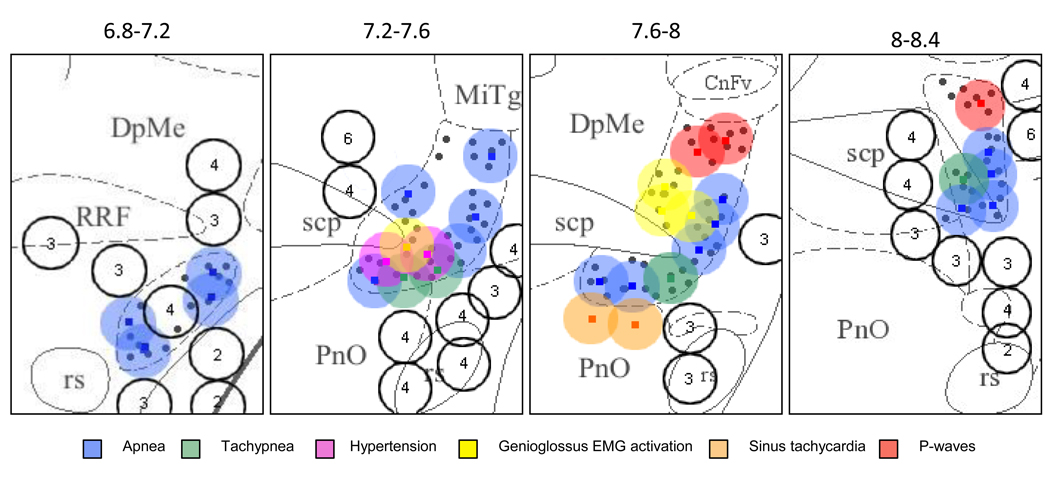
Fig.2.
Schematic map of responses elicited from different sites of the PPT by microinjection of glutamate. Anatomical injection sites were localized with antero-posterior resolution of ±40 µm (the section thickness). For presentation clarity the diagram pools responses collected across multiple experiments by projecting histologically verified pipette locations onto the matching 400 µm coordinate range: 6.8 –7.2; 7.2–7.6; 7.6–8 and 8–8.4 mm from bregma. Injection sites are overlaid on a background demonstrating the location of NADPH-diaphorase positive cholinergic neurons within the PPT. Response types are coded into one of six categories by color. The diameter of each circle represents the estimated short-term glutamate diffusion volume (diameter ~300 µm, see text for details). Unfilled circles represent injections that elicited no response (number within each circle indicate the number of overlapping ineffective injections). Small black filled circles mark the location of cholinergic neurons.
RRF – retrorubral field; PnO – nucleus reticularis pontis oralis; scp – superior cerebellar peduncles; CnFV – cuneiform nucleus; MiTg – microcellular tegmental nucleus; rs – rubrospinal tract.
The semi-transparent circles surrounding the injection sites depict the estimated diffusion distance of the glutamate (about 300 µm in diameter), predicted theoretically by Nicholson (1985) (see Discussion for details).
3.1. Apnea
As depicted in Fig. 2, apneic responses to glutamate microinjection were observed throughout the antero-posterior extent of the PPT region (−6.8 to −8.4 mm from bregma). In the most anterior portion of the PPT (−6.8 to −7.2 mm from bregma) apnea was elicited from dorsolateral areas associated with cholinergic neurons. In the most posterior regions (−8.0 to −8.4 mm from bregma) apneagenic sites were located predominantly in the ventro-lateral part of the PPT. Between these anterior and posterior poles, apneic sites were distributed through the mediolateral and dorsoventral extent of the PPT. However, apnea producing sites were found predominantly in the ventro-lateral area. All apneic sites were closely approximated to areas of cholinergic cells within the PPT.
Sites for delayed apnea (latency >35) surrounded the sites associated with short latency apnea: up to 300 µm dorsally in the anterior PPT; up to 300 µm ventrally in the posterior PPT; and up to 100 µm medially or laterally throughout the PPT. In five experiments, spontaneous apneas were observed during baseline recordings prior to any injections. However, even in these experiments (as shown in Table 1), spontaneous apneas never occurred more than once during a 5-min interval at baseline and had a shorter duration than glutamate-evoked apneas. Thus, spontaneous apnea cannot account for the observed PPT evoked apneas.
Table 1.
Comparison of the apneas at the baseline and post-injection in the group of 8 rats with spontaneous apneas
| Apneas | Post-sigh apneas | |||
|---|---|---|---|---|
| Mean apnea duration (ms) | P value | Mean apnea duration (ms) | P value | |
| Baseline | 1087±590 | 1217±941 | ||
| Post-injection | ||||
| Short latency apneas | 4972±2643 | 8.86 *10−12 | 4458±2537 | 4*10−3 |
| Long latency apneas | 4741±2645 | 9.9 *10−20 | 5198±2711 | 3 *10−3 |
| Total post-injection | 4787±2607 | 2.7 *10−19 | 5056±2676 | 4 *10−3 |
P-values were estimated relative to the baseline values and considered significant if ≤ 0.05.
3.2. Tachypnea
Tachypnea (Fig. 1B) was evoked from several sites at the ventrolateral margin of the PPT, located −7.6 to −8 mm from bregma and in the ventral part of the PPT −8 to −8.4 from bregma (Fig. 2). Tachypneic responses started from 0 to 15 s after the glutamate injection and typically persisted for no more than 35 s. In 2 cases these responses were associated with transient HTN, and either the tachypnea or the HTN could occur first following an individual injection.
3.3. Cardiovascular Disturbance
HTN, lasting approximately 30 s and with a short latency following glutamate injection, was elicited from the ventral half of the PPT in all but the most anterior regions. HTN was observed in 10 cases, and was most often not associated with respiratory pattern changes. Transient sinus tachycardia was elicited from 2 sites residing at the border of the PPT and the nucleus pontis oralis (Fig. 2).
3.4. Genioglossus EMG
Activation of genioglossus EMG was elicited from the medio-posterior part of the PPT (−7.6 to −8 mm from bregma) surrounding the superior cerebellar peduncules (Fig. 2). EMG responses could accompany respiratory reactions (2 cases), but also could be evoked separately from them (4 cases) (Fig. 1B).
3.6. P-waves
Clusters of p-waves were recorded from the contralateral PPT following glutamate injections in the dorso-caudal portion of the PPT (Fig. 2). This response comprised bursts of repetitive monophasic waves of the same polarity, with a typical burst duration of 1–3 s and with an interwave frequency ranging from 5 to 6 Hz (Fig. 1B). In accordance with our previous findings (Saponjic et al, 2003), the sites where glutamate elicited exclusively p-waves were located dorsal to sites from which respiratory responses could be evoked. In 2 cases (in the posterior part of the PPT) both respiratory and p-wave responses were evoked from the same site, but the respiratory response always appeared prior to the p-waves.
4. Discussion
The present study establishes a systematic functional topography for respiratory, cardiovascular, electromyographic and electroencephalographic responses to PPT stimulation by glutamate microinjection. Moreover, our findings demonstrate that, to a reasonable extent, these functional responses to local PPT stimulation are anatomically mapped to partially distinct subregions. Finally, most responses characterized in this study were elicited from the PPT regions associated with numerous cholinergic neurons.
Because the PPT region participates in a host of integrative functions, it is not surprising that local glutamatergic stimulation within this area elicited a variety of autonomic and electrographic responses. However, the various “response regions” revealed a distinct spatial organization.
Across all experiments we consistently observed that advancing the pipette by 100 µm could lead to a change from no response to visually evident response or to a decrease or increase of response latency. Building the functional map of the “response regions” was based on the location of the responses with shortest latency (< 35 s) within a track. These observations also support the likelihood of distinct “response regions”.
Nicholson used a mathematical model to determine that within the first 30 seconds after the injection, the concentration of the drug (glutamate) present 300 µm from the injection site would be no more than 20% of the concentration at the pipette tip (Nicholson, 1985). Using larger infusion volumes (100 nl) in freely moving rats, Datta and coworkers determined that the minimum concentration of glutamate administered to the PPT that produced increased rapid eye movement sleep was ~2 mM, whereas greater than 5 mM glutamate was required to elicit increased wakefulness (Datta et al., 2001b). Here, we employed 10 mM glutamate, suggesting that concentrations would fall below 2 mM at a distance of no more than 300 µm from the pipette tip.
Thus, it is possible that diffusion of glutamate to active sites up to ~300 µm distant from the injection might account for the variable response latencies observed. This consideration limits the practical spatial resolution of our response mapping to ±150 µm. Thus, we expect that injections spaced at 100 µm increments provided a series of partially overlapping areas of glutamate stimulation. From this viewpoint it is not surprising that advancing the pipette 100 µm could sometimes produce complex responses in transition zones.
Thus, we observed a combination of apnea with HTN in 4 cases, apnea with EMG activation in 2 cases and tachypnea with HTN in 2 cases. Based on the discussion above, we speculate that in these cases, the diffusion distance of the glutamate allowed a single injection to affect functionally distinct areas in close proximity to one another.
Additionally, a broad dendritic arborization could influence the location and latency of the responses. It was shown that neurons of the PPT have radiating dendrites with terminals extended up to 500 µm from the soma (Takakusaki et al., 1996). This could cause a broadening or even displacement of apparent response areas based on our injection mapping technique. However, it was shown that PPT dendrites extend primarily into adjacent sensory pathways (Rye et al., 1987; Semba, 1991). Additionally, it is possible, that glutamate receptors of PPT neurons are heavily located on their soma (Honda and Semba, 1995).
In our view, the functional topology of the PPT demonstrated in this study in not surprising. Like other pontine regions, the PPT participates in a wide variety of sensory and autonomic functions, in addition to behavioral state regulation. In terms of cardiorespiratory functions, the PPT receives significant inputs from the medullary NTS (Gilbert and Lydic, 1991); another structure with significant subnuclear functional differentiation (Herbert et al.,1990). Herbert et al. showed that NTS connections to the parabrachial complex of the pons demonstrate a high degree of functional specificity (Herbert et al., 1990). The present findings suggest that such functionally specific connections also may pertain to the PPT, but this determination will require future anatomical studies.
Virtually all glutamate-induced responses were elicited from sites at which cholinergic neurons were located. The distribution of cholinergic neurons within the PPT has been descriptively subdivided into the more dense pars compacta located in the posterior part of the PPT (PPTc) and the less dense pars dissipata (PPTd) in the anterior part of the PPT (Olszewski and Baxter, 1954). It was shown that cholinergic neurons in rat are cytochemically and connectionally distinct from non-cholinergic cells with which they are admixed (Rye et al., 1987). The cellular structure and arborization of the cholinergic neurons within PPT pars compacta (PPTc) differs from that of PPT pars dissipata (PPTd). The cholinergic neurons in the PPTc are smaller than those in the PPTd. According to Lavoie and Parent (1994a and b) “the neuronal processes are shorter and poorly branched in the PPTc, whereas they are longer and more profusely arborized in the PPTd”. Thus, one can expect more local responses within the PPTc. This anatomic difference could also explain the variety of latencies of the PPT responses.
The anatomical pathways by which the PPT activation impacts respiratory, cardiovascular or electroencephalographic processes cannot be directly identified from the present data, although previous neuroanatomical and neurophysiological studies allow us to make some suggestions. The PPT pars compacta is the major cholinergic input to the cardiorespiratory regions of the rostral ventrolateral medulla (RVLM) (Yasui et al., 1990) and individual cholinergic axons arborise extensively within separate nuclei of their termination (Rye et al., 1997). Anatomical tracing studies have confirmed that choline-acetyltransferase positive neurons directly innervate BP regulating areas of the RVLM (Padley et al., 2007). Further, Padley et al. (2007) reported that bilateral injection of bicuculline or excitatory DL-homocysteic acid into the PPT evoked an increase in BP and altered the baroreflex. Therefore, it is possible that the cardiovascular and respiratory responses we observed following PPT glutamate injections resulted from activation of monosynaptic projections from the PPT to the RVLM, although polysynaptic pathways cannot be ruled out. For example, the PPT reciprocally innervates the parabrachial complex (Gilbert and Lydic, 1991; Quattrochi et al. 1998; Semba and Fibiger, 1992) and neurons of the parabrachical complex together with the Kolliker-Fuse nucleus regulate respiratory phase switching. In addition, stimulation of the medial parabrachial or Kölliker-Fuse areas produces expiratory facilitation and apnea, whereas activation of the lateral parabrachial region can produce tachypnea (Chamberlin and Saper, 1994; Dick and Coles, 2000; Takayama and Miura, 1993). Thus, it is reasonable to speculate that activation of medial parabrachial neurons following PPT stimulation may account for apnea, whereas activation of the lateral parabrachial neurons may account for tachypnea. Definitive evaluation of these possibilities will require future investigation, and many other multisynaptic pathways may also account for or contribute to the observed responses.
The present study also showed that neurochemical excitation at certain PPT injection sites evoked an activation of genioglossus EMG. Consistent with this observation, Rukhadze and Kubin (2007) have shown that cholinergic neurons of the PPT, originating in a discrete portion of its pars compacta, send bilateral projections to the motor nucleus of the hypoglossal nerve (Mo12). Thus, it is possible, that these projections contribute to both excitatory and inhibitory modulation of the activity of hypoglossal motoneurons.
The p-waves were registered in the medio-posterior part of the PPT, dorsal to the respiratory “effective” sites. Pharmacological and physiological studies have demonstrated a major role for cholinergic neurons in the generation and transmission of p-waves (Datta et al., 1992; 1997; Henriksen et al., 1972). The influence of glutamate on cholinergic cells of the PPT was shown to evoke either REM sleep and p-waves or wakefulness depending on the dose (Datta and Siwek, 1997; Datta et al., 2001), providing support for the possibility that glutamatergic activation of cholinergic neurons evoked p-waves as we observed. Although we use animals, anesthetized by ketamine, a known NMDA receptor antagonist, we (current work; Saponjic et al., 2003) and others (Datta et al., 2001a and b) demonstrate the possibility to evoke p-waves in ketamine-anesthetized animals by local application of small amounts of glutamate into the PPT. Thus, ketamine is permissive of p-wave expression when administered systemically to achieve surgical anesthesia. However, additional local administration of ketamine directly to the PPT blocks subsequent glutamate-induced p-waves. Thus, NMDA receptors within the PPT may play a role in evoked p-waves.
P-wave generation requires the participation of several different types of neurons (Garcia-Rill, 1991; Datta, 1997; Rye, 1997), but especially relies on bursting neurons. Although NMDA receptors play some role in the generation of bursting patterns, AMPA and metabotropic glutamate receptors as well as GABA receptors also participate in generation of p-waves. As noted above, PPT glutamate injections do not evoke p-waves under nembutal anesthesia.
In summary, the present study establishes a functional heterogenity of responses elicited by glutamate within the PPT, including respiratory, cardiac, vascular, upper airway and EEG effects. This supports a potentially broad role for the PPT in determining state-depending autonomic functions. Moreover, our findings demonstrate that these functional responses to local PPT stimulation are anatomically mapped to partially distinct subregions. Most responses characterized in this study were elicited from the PPT regions associated with numerous cholinergic neurons, but elaboration of the synaptic processes and anatomical pathways by which the effects are mediated will require future investigation.
Acknowledgements
Authors acknowledge Milka Dokic for the technical support of the experiments. The work was supported by NIH grant AG016303.
Abbreviations
- PPT
pedunculopontine tegmentum
- EEG
electroencephalogram
- ECG
electrocardiogram
- EMG
electromyogram
- BP
blood pressure
- HTN
hypertension
- p-waves
pontine waves
- REM
rapid eye movement
- AP
antero-posterior
- ML
medio-lateral
- DV
dorso-ventral
- NTS
nucleus of the solitary tract
- VLM
ventro-lateral medulla
- RVLM
rostro-ventro-lateral medulla
- PB
parabrachial complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors: Irina Topchiy, Jonathan Waxman, Miodrag Radulovacki and David W.Carley do not have any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence this work.
References
- Alderson HL, Latimer MP, Winn P. A functional dissociation of the anterior and posterior pedunculopontine tegmental nucleus: excitotoxic lesions have differential effects on locomotion and the response to nicotine. Brain Struct. Funct. 2008;213:247–253. doi: 10.1007/s00429-008-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Torras-Garcia M, Quiroz-Padilla MF, Costa-Miserachs D, Coll-Andreu M. Electrical stimulation of the pedunculopontine tegmental nucleus in freely moving awake rats: time- and site-specific effects on two-way active avoidance conditioning. Neurobiol. Learn. Mem. 2007;87:510–521. doi: 10.1016/j.nlm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Calvo JM, Datta S, Quattrochi J, Hobson JA. Cholinergic microstimulation of the peribrachial nucleus in the cat. II. Delayed and prolonged increases in REM sleep. Arch Ital Biol. 1992;130:285–301. [PubMed] [Google Scholar]
- Carley DW, Radulovacki M. REM sleep and apnea. In: Mallik BN, Inoue S, editors. Rapid Eye Movement Sleep. New Dehli: Narosa Publishing; 1999. pp. 286–300. [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell. Mol. Neurobiol. 1997;17:341–365. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Calvo JM, Quattrochi JJ, Hobson JA. Long-term enhancement of REM sleep following cholinergic stimulation. Neuroreport. 1991;2:619–622. doi: 10.1097/00001756-199110000-00017. [DOI] [PubMed] [Google Scholar]
- Datta S, Hobson JA. Suppression of ponto-geniculo-occipital waves by neurotoxic lesions of pontine caudo-lateral peribrachial cells. Neuroscience. 1995;67:703–712. doi: 10.1016/0306-4522(95)00081-s. [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J. Neurosci. Res. 2001a;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp. Physiol. 2001b;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir. Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. The pedunculopontine nucleus. Prog. Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- Gilbert KA, Lydic R. Cholinergic reticular mechanisms produce state-dependent decreases in parabrachial respiratory neuron discharge. Neurosci. Abstr. 1991;17:620. [Google Scholar]
- Henriksen SJ, Jacobs BL, Dement WC. Dependence of REM sleep PGO waves on cholinergic mechanisms. Brain Res. 1972;48:412–416. doi: 10.1016/0006-8993(72)90201-6. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Honda T, Semba K. An ultrastructural study of cholinergic and non-cholinergic neurons in the laterodorsal and pedunculopontine tegmental nuclei in the rat. Neuroscience. 1995;68:837–853. doi: 10.1016/0306-4522(95)00177-k. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J. Comp. Neurol. 1994a;344:232–241. doi: 10.1002/cne.903440205. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J. Comp. Neurol. 1994b;344:190–209. doi: 10.1002/cne.903440203. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol. 1993;264:R544–R554. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Nitz DA, Siegel JM. GABA release in the mesopontine central gray as a function of sleep state. Sleep Res. 1993;22:447. [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J. Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. In: Cytoarchitecture of the human brainstem. Karger S, editor. Philadelphia and Montreal: J. B. Lippincott Company; 1954. [Google Scholar]
- Padley JR, Kumar NN, Li Q, Nguyen TB, Pilowsky PM, Goodchild AK. Central command regulation of circulatory function mediated by descending pontine cholinergic inputs to sympathoexcitatory rostral ventrolateral medulla neurons. Circ. Res. 2007;100:284–291. doi: 10.1161/01.RES.0000257370.63694.73. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney, New York: Academic Press; 2004. [Google Scholar]
- Quattrochi J, Datta S, Hobson JA. Cholinergic and non-cholinergic afferents of the caudolateral parabrachial nucleus: a role in the long-term enhancement of rapid eye movement sleep. Neuroscience. 1998;8:1123–1136. doi: 10.1016/s0306-4522(97)00471-5. [DOI] [PubMed] [Google Scholar]
- Radulovacki M, Pavlovic S, Saponjic J, Carley DW. Modulation of reflex and sleep related apnea by pedunculopontine tegmental and intertrigeminal neurons. Respir. Physiol. Neurobiol. 2004;143:293–306. doi: 10.1016/j.resp.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus-auditory input, arousal and pathophysiology. Prog. Neurobiol. 1995;47:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- Rostron CL, Farquhar MJ, Latimer MP, Winn P. The pedunculopontine tegmental nucleus and the nucleus basalis magnocellularis: do both have a role in sustained attention? BMC Neurosci. 2008;9:16. doi: 10.1186/1471-2202-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukhadze I, Kubin L. Mesopontine cholinergic projections to the hypoglossal motor nucleus. Neurosci Lett. 2007;413:121–125. doi: 10.1016/j.neulet.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J. Comp. Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Saponjic J, Cvorovic J, Radulovacki M, Carley DW. Serotonin and noradrenaline modulate respiratory pattern disturbances evoked by glutamate injection into the pedunculopontine tegmentum of anesthetized rats. Sleep. 2005;28:560–570. doi: 10.1093/sleep/28.5.560. [DOI] [PubMed] [Google Scholar]
- Saponjic J, Radulovacki M, Carley DW. Respiratory pattern modulation by the pedunculopontine tegmental nucleus. Respir. Physiol. Neurobiol. 2003;138:223–237. doi: 10.1016/j.resp.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Saponjic J, Radulovacki M, Carley DW. Modulation of respiratory pattern and upper airway muscle activity by the pedunculopontine tegmentum: role of NMDA receptors. Sleep Breath. 2006;10:195–202. doi: 10.1007/s11325-006-0075-9. [DOI] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Shouse MN, Siegel JM. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. Vol. New York: Plenum Press; 1990. [Google Scholar]
- Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J Comp Neurol. 1996;371:345–361. doi: 10.1002/(SICI)1096-9861(19960729)371:3<345::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Takayama K, Miura M. Respiratory responses to microinjection of excitatory amino acid agonists in ventrolateral regions of the lateral parabrachial nucleus in the cat. Brain Res. 1993;604:217–223. doi: 10.1016/0006-8993(93)90372-t. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Expl Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J. Neurol. Sci. 2006;248:234–250. doi: 10.1016/j.jns.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Cechetto DF, Saper CB. Evidence for a cholinergic projection from the pedunculopontine tegmental nucleus to the rostral ventrolateral medulla in the rat. Brain Res. 1990;517:19–24. doi: 10.1016/0006-8993(90)91002-x. [DOI] [PubMed] [Google Scholar]