Abstract
Tumor necrosis factor α (TNFα) is a key pathogenic factor in Crohn’s disease and rheumatoid arthritis. TNFΔARE mice express high levels of TNFα and present Crohn’s-like ileitis and arthritis. Alterations in the chemokine network could underline the TNF-driven ileitis. The aim of this study was to evaluate the role of TNF and chemokines in ileitis using ectromelia virus CrmD, a protein that binds TNFα and a limited number of chemokines. We generated transgenic mice expressing CrmD in intestinal epithelial cells (vCrmD mice) and crossed them with the TNFΔARE mice to test whether CrmD could affect TNF-driven inflammatory processes. During homeostasis, only the number of B cells in the lamina propria was reduced by CrmD expression. Interestingly, CrmD expression in the intestine markedly attenuated the inflammatory infiltrates in the ileum of TNFΔARE mice, but did not affect development of arthritis. Our results suggest that CrmD affects development of ileitis by locally affecting both TNF and chemokine function in the ileum.
Keywords: Intestine, TNF, leukocyte, CrmD, poxvirus, Crohn’s disease
Introduction
The immune system within the mucosa is one of the first barriers to recognize and respond to pathogens, and relies upon a controlled interplay between gut epithelial and immune cells1. Cytokines are essential mediators of the interactions between activated cells and non-immune cells, including epithelial, endothelial, and mesenchymal cells. TNFα has pleiotropic effects affecting both innate and adaptive responses through the induction of cytokines and chemokines, which recruit inflammatory leukocytes, and the activation of the mucosal endothelium2,3. Alterations in the expression levels of TNFα are associated with several immune-related disorders including inflammatory bowel disease (IBD) and rheumatoid arthritis. IBD is characterized by continuous inflammatory reactions leading to the disruption of the intestinal epithelial barrier. IBD includes ulcerative colitis (UC) and Crohn’s disease (CD). TNFα overexpression is observed in patients suffering from arthritis, CD, and UC, many of whom have been successfully treated with anti-TNF drugs4–7. However, better immunomodulators are required to improve the outcome of the treatment.
Several animal models have been developed to study IBD8 including the TNFΔARE mouse model. TNFΔARE mice lack the AU-rich element that destabilizes the TNF mRNA in the 3′-untranslated region of the TNF gene9, and therefore, stromal and bone marrow-derived cells from the TNFΔARE mice express high levels of TNFα mRNA and protein under basal conditions and upon stimulation with LPS9. TNFΔARE mice develop Crohn’s-like IBD and arthritis and express cytokines that are also found in human CD patients9,10. The Crohn’s-like IBD observed in the TNFΔARE mice is highly penetrant and restricted to the terminal ileum. The first signs of ileitis are observed at 6–8 weeks of age in both sexes9,10.
The effector mechanisms associated with TNF function that account for the marked inflammatory infiltrates observed in the TNFΔARE mice are still not defined. They could include generalized activation of endothelium and the increased expression of factors involved in cell recruitment such as chemokines, small, basic, chemo-attractant cytokines that play a pivotal role in the recruitment of leukocytes during development, homeostasis, and inflammation. Chemokines are classified into C, CC, CXC, and CX3C subfamilies according to the relative positioning of the N-terminal cysteine residues11. Most chemokines are secreted, with the exception of CXCL16 and CX3CL1, which contain a transmembrane domain11. To date, however, there is no direct evidence that chemokines are effectors in TNF-driven diseases.
In this study we probe into the role of TNF and chemokines in ileitis using a virus-derived immunomodulatory protein: the cytokine response modifier D (CrmD). CrmD is encoded by ectromelia virus (ECTV), an orthopoxvirus that causes mousepox and is closely related to the human pathogens variola virus (VARV) and monkeypox virus (MPXV). VARV and MPXV encode a related protein CrmB. CrmD and CrmB bind with high affinity to human, mouse, and rat TNFα, mouse LTα, and a small group of CC and CXC chemokines12. CrmD and CrmB inhibit both TNFα cytotoxic effect and chemotaxis in vitro 12. Several studies with poxvirus models of infection have shown that CrmB and other related poxvirus TNF receptors (CrmC, CrmE and T2) contribute to virus virulence and it has been suggested that these viruses may use the secreted TNFα receptors to reduce inflammatory responses during infection13. Studies done with recombinant VARV or MPXV CrmB indicate a protective effect in lipopolysaccharide (LPS)-induced shock14 showing a role for CrmB in the inhibition of the innate immune response. To date there is no information regarding the potential use of CrmD/B as an immunomodulator during homeostasis or chronic inflammation.
We have investigated the role of CrmD in vivo by expressing CrmD in mouse intestinal epithelial cells (IEC). Expression of CrmD only affected the B cell population within the intestinal LP during homeostasis. However, CrmD expression markedly reduced all leukocyte populations observed in the ileum of the TNFΔARE mouse model9. These changes were associated with reduced expression of many chemokines shown to be upregulated in the ileum of TNFΔARE mouse model. Together these results demonstrate that CrmD blocks development of TNFα-driven inflammation and that TNFα causes intestinal inflammation via upregulation of chemokines.
Results
Chemokines responsible for leukocyte recruitment are upregulated in the ileum of the TNFΔARE mice
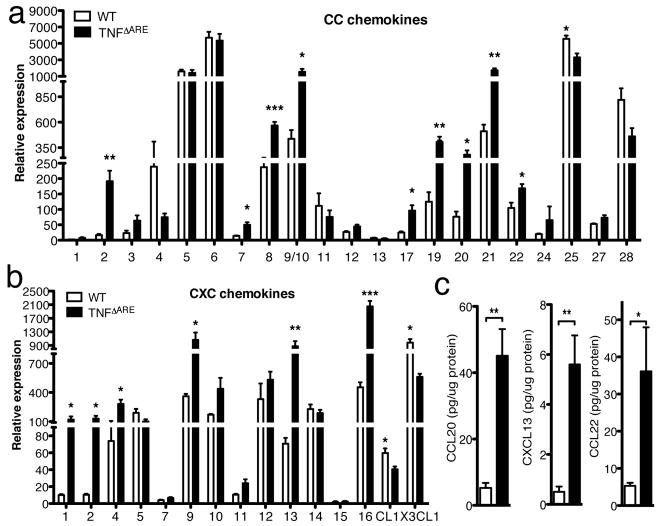
TNFΔARE mice have rich inflammatory cell infiltrates in the ileum which are absent in wild-type (WT) mice. To date, there is no information regarding the pattern of chemokine expression and its potential implication in the recruitment of the inflammatory infiltrates in this model of TNF-driven disease. To examine if the previously observed cellular changes were associated with modifications in the expression pattern of chemokines, we extracted total RNA from the distal 5 cm of the small bowel of 11 and 13 weeks old age-matched WT and TNFΔARE mice and examined the relative mRNA levels of all murine chemokines by Q-PCR. TNFΔARE mice expressed significantly higher levels of CC chemokines (CCL2, CCL7, CCL8, CCL9/10, CCL17, CCL19, CCL20, CCL21 and CCL22) than WT mice (Figure 1a). CXC chemokines CXCL1, CXCL2, CXCL4, CXCL9, CXCL13, and CXCL16 were also significantly upregulated in the TNFΔARE ileum (Figure 1b). However, CL1 and CX3CL1 expression was downregulated (P<0.05, Figure 1b). The fold increase in chemokine expression observed is shown in Table 1. To assess whether some of the chemokines were indeed significantly augmented we analyzed CCL20, CCL22 and CXCL13 protein levels in ileum extracts from WT and TNFΔARE mice by ELISA. As shown in Figure 1c, all three chemokines were found significantly increased in the terminal ileum of TNFΔARE mice (P<0.005). These results indicate that several chemokines are markedly upregulated in the ileum of TNFΔARE mice.
Figure 1. Analysis of chemokine expression patterns in the ileum of TNFΔARE mice.
a & b) Differential expression of CC (a) and CXC (b) chemokines in the ileum of TNFΔARE and WT mice (n=3, normalized to ubiquitin). The expression of several chemokines is increased in the ileum of TNFΔARE mice. (* P<0.05, ** P<0.01, *** P<0.001) c) CCL20, CXCL13 and CCL22 protein levels in extracts from the terminal ileum of TNFΔARE (n=5) and WT mice (n=3) (** P<0.01).
Table 1.
Average fold increase in chemokine expression in the ileum of TNFΔARE mice compared to WT mice.
| CCL | Fold increase | CXCL | Fold increase |
|---|---|---|---|
| 1 | 2.8 | 1 | 12.1 |
| 2 | 11.7 | 2 | 12.6 |
| 3 | 2.8 | 4 | 3.8 |
| 4 | 0.3 | 5 | 0.5 |
| 5 | 0.9 | 7 | 1.6 |
| 6 | 0.9 | 9 | 2.9 |
| 7 | 3.6 | 10 | 2.6 |
| 8 | 2.4 | 11 | 2.3 |
| 9/10 | 3.5 | 12 | 1.6 |
| 11 | 0.7 | 13 | 12.4 |
| 12 | 1.7 | 14 | 0.8 |
| 13 | 0.7 | 15 | 1.3 |
| 17 | 3.9 | 16 | 4.5 |
| 19 | 3.2 | ||
| 20 | 3.7 | CL | Fold increase |
| 21 | 3.4 | 1 | 0.7 |
| 22 | 1.6 | ||
| 24 | 3.3 | CX3CL | Fold increase |
| 25 | 0.6 | 1 | 0.6 |
| 27 | 1.4 | ||
| 28 | 0.6 |
Generation of transgenic mice expressing CrmD in the IEC
TNFα plays a pivotal role in the inflammatory process observed in TNFΔARE mice. CrmD interacts with high affinity with TNFα, LTα and a subset of chemokines, including mCCL20, mCCL25, mCCL27, mCCL28, mCXCL11, mCXCL12β, mCXCL13 and CXCL1412 (A.A., unpublished observation). CrmD inhibits both the cytotoxic effect of TNFα and hCCL25-mediated chemotaxis in vitro12.
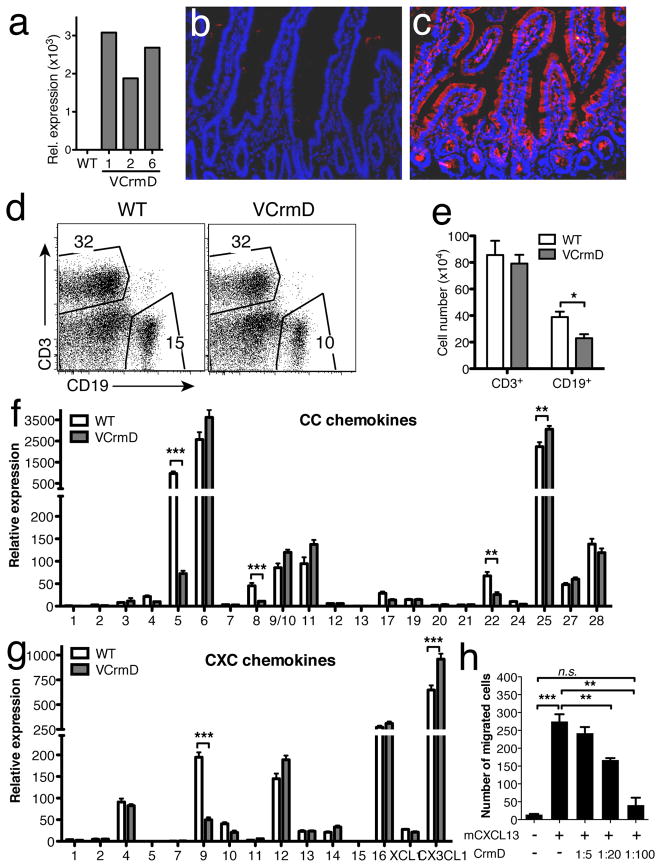
To analyze the potential immunomodulatory role of CrmD in vivo during homeostasis and inflammation we expressed it under the control of the villin promoter (vCrmD), targeting its expression to the IEC of both the small and large bowel15. We obtained four vCrmD founders from which three lines (1, 2, and 6) were derived. Expression of CrmD mRNA was assessed by Q-PCR. Line 1 expressed the highest level of mRNA followed by line 6 and 2 (Figure 2a). CrmD expression was detected by immunofluorescence in the crypts and epithelial cells in the jejunum of lines 1 and 6 (Figure 2c and not shown) but not in the intestine of WT mice (Figure 2b). Since CrmD is secreted, positive staining was also observed in the intestinal LP (Figure 2c). vCrmD mice were healthy, reproduced well, and did not display any gross abnormalities.
Figure 2. The effect of transgenic expression of CrmD on leukocyte subsets in the LP.
a) CrmD mRNA expression in different lines of vCrmD mice. The values were normalized to ubiquitin in each sample. b & c) Immunostaining of CrmD in the small intestine of WT (b) and vCrmD (c) mice. d) Representative FACS analysis of T and B cells within the LP of vCrmD and WT mice. e) Absolute cell number of T and B cells in vCrmD mice within the CD45+ cell subset (n=5 for each group; * P<0.05). f & g) Differential expression of CC (f) and CXC (g) chemokines in the ileum of vCrmD and WT mice (n=3, normalized to ubiquitin; ** P<0.01, *** P<0.001). h) CrmD inhibition of mCXCL13-mediated chemotaxis. Isolated splenocytes were incubated with 100nM mCXCL13 in transwell plates in the absence or presence of CrmD. The molar ratio mCXCL13:CrmD is indicated. The number of migrated cells is represented. CrmD is able to significantly (P<0.01) inhibit mCXCL13-mediated chemotaxis in a dose dependent manner. One representative experiment of three, performed in triplicate, is shown.
Expression of CrmD in the intestinal epithelium decreases number of B cells within the LP
Then we analyzed the effect of CrmD expression on the leukocyte populations of the intestine LP during homeostasis. Age-matched WT and vCrmD mice from line 1 were utilized. Leukocytes were isolated from the LP of the small and large bowel, including the cecum, as previously described15 and the different leukocyte populations were analyzed by flow cytometry. LP from WT mice harbors B cells, T cells, and myeloid cells16. Myeloid cells can be subdivided by analyzing the cell surface markers CD11b and CD11c. One population is characterized by expression of high levels of CD11c and MHC class II (MHCII) (Supplemental Figure 1c, CD11b+/CD11chigh) whereas the other has low levels of CD11c (CD11b+/CD11clow). The CD11b+/CD11clow population can be further analyzed and subdivided into three subsets depending on the expression of MHCII and Gr-1. In WT mice, the majority (>70%) of the CD11b+/CD11clow cells are eosinophils (Eo, MHCIIlow or null/Gr-1low). The remaining cells are neutrophils (No, MHCIIlow/Gr-1high) and macrophages (Mac, MHCIIhigh/Gr-1low)17.
Expression of CrmD did not affect the T cell or B cell relative numbers, whereas it resulted in a significant (P<0.05) reduction in total B cell numbers in the LP of vCrmD mice compared to controls (Figure 2e). In order to investigate which B cell population was decreased, we looked for IgM- and IgA-producing cells and we found that relative and absolute numbers of IgM+ B cells were decreased in vCrmD transgenic mice (Supplementary Figure 1a&b). Further analysis did not reveal any significant differences in the relative (Supplementary Figure 1c) or absolute number (Supplementary Figure 1d) of the other leukocyte populations within the LP of the small bowel of vCrmD compared to WT mice. We did not observe differences in relative or absolute number of leukocytes in the LP of the large bowel (not shown). The IEL compartment of both small and large bowel and the PP did not present significant changes either (not shown). Conventional histological analysis of the different segments of the intestine of vCrmD mice did not reveal any gross morphological abnormality (not shown). To investigate the mechanisms by which CrmD affected selectively B cell recruitment we analyzed the effect of CrmD on chemokine expression (Figure 2f&g) and chemotaxis (Figure 2h). CrmD expression did not affect the expression of most chemokines. However, significant changes were observed in the expression of a few chemokines (CCL5, CCL8, CCL22, CXCL9, CCL25 and CX3CL1), most notably (over 3 fold changes) CCL5 and CXCL9. These changes did not result in reduction of cell recruitment probably due to the redundancy of the chemokine network and the higher expression level of other chemokines involved in T cell recruitment such as CCL25. The expression of the main chemokines involved in B cell recruitment (CCL20, CCL21, CXCL12 and CXCL13) was not affected by CrmD. To test if the changes in the number of B cells observed in the vCrmD mice could be due to direct inibition of B-cell chemoattractants we performed chemotaxis experiments with splenic B cells and CXCL13. As shown in Figure 2h, CrmD inhibited chemotaxis mediated by mCXCL13 in a dose dependent manner, suggesting that the reduced levels of B cells in the intestine of vCrmD mice could result from direct inhibition of chemokine function by CrmD.
Together these results indicate that CrmD expression by IEC induced a decrease in B cell population probably by direct inhibition of CXCL13 function but did not significantly alter most leukocyte populations in the gut.
Expression of CrmD by IEC reduces the inflammatory infiltrates present in the ileum of the TNFΔARE mice
To study the effect of CrmD on a TNFα-driven chronic inflammatory process we crossed vCrmD mice from line 1 with TNFΔARE mice9, to obtain mice CrmD transgenic and heterozygous for TNFΔARE (CrmD/TNFΔARE mice). Heterozygous TNFΔARE mice develop Crohn’s like disease in the terminal ileum and inflammatory arthritis, due to an intrinsic defect in the posttranscriptional regulation of TNFα mRNA9.
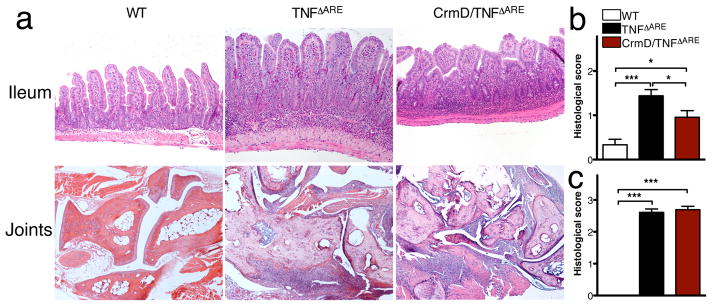
Expression of CrmD during homeostasis (vCrmD mouse) did not result in histological changes with respect to control littermates (not shown). To evaluate if epithelial expression of CrmD would affect the pathology observed in the intestine of TNFΔARE mice, we analyzed the terminal ileum of age-matched WT (n=3), TNFΔARE (n=15) and CrmD/TNFΔARE (n=9) (10–14 weeks old). As expected, the ileum of TNFΔARE mice displayed histopathological changes characteristic of inflammatory bowel disease such as villus blunting, mucosal and submucosal inflammation with the presence of neutrophils and lymphoplasmacytic infiltrates (Figure 3a). Patchy transmural inflammation and the appearance of lymphoid aggregates and granulomata were also observed (not shown). These criteria were individually analyzed and scored semiquantitavely (see methods). Interestingly, histological analysis of the terminal ileum from CrmD/TNFΔARE mice revealed significant changes in the inflammatory pathology. Although mucosal abnormalities were still observed, the intestinal inflammation was significantly decreased by CrmD expression (Figure 3a&b). There was improvement of the villus blunting, decrease in the density of mucosal and submucosal inflammatory infiltrates and in the number of lymphoid aggregates. This reduction in inflammation occurred despite the continuous and constitutive expression of TNFα by stromal and hematopoietic cells in the TNFΔARE mice9.
Figure 3. CrmD expression reduces the inflammation observed in the ileum but not in the joints of TNFΔARE mice.
a) Representative pictures of the terminal ileum (upper panel) and joints (lower panel) of WT (left), TNFΔARE (middle) and CrmD/TNFΔARE (right) mice. Notice the reduction of inflammation observed in the ileum of CrmD/TNFΔARE (right) mice compared to that of TNFΔARE (middle) and the formation of the inflammatory pannus and areas of cartilage and bone erosion in the joints from both TNFΔARE and CrmD/TNFΔARE mice. b) Histological score indicating the development of intestinal inflammation based on the analysis of WT (n=3), TNFΔARE (n=15) and CrmD/TNFΔARE (n=9) mice. c) Histological score indicating the development of joint inflammation based on the analysis of WT (n=5), TNFΔARE (n=9) and CrmD/TNFΔARE (n=9) mice (* P<0.05, *** P<0.001).
To test whether CrmD expression in the intestine could affect disease development in the periphery, we analyzed joints from WT (n=5), TNFΔARE (n=9) and CrmD/TNFΔARE (n=9) mice at 10–14 weeks of age. In agreement with previous reports9, the joints of TNFΔARE mice displayed severe pathological features of chronic inflammatory arthritis, including hyperplasia of the synovial membrane and presence of polymorphonuclear infiltrates. We also observed pannus and fibrous tissue formation, subchondral bone erosion, and articular cartilage destruction (Figure 3a&c). At the histological level, CrmD/TNFΔARE mice exhibited the same features observed in TNFΔARE littermate controls (Figure 3a&c), suggesting that the CrmD inhibitory properties were restricted to the intestine.
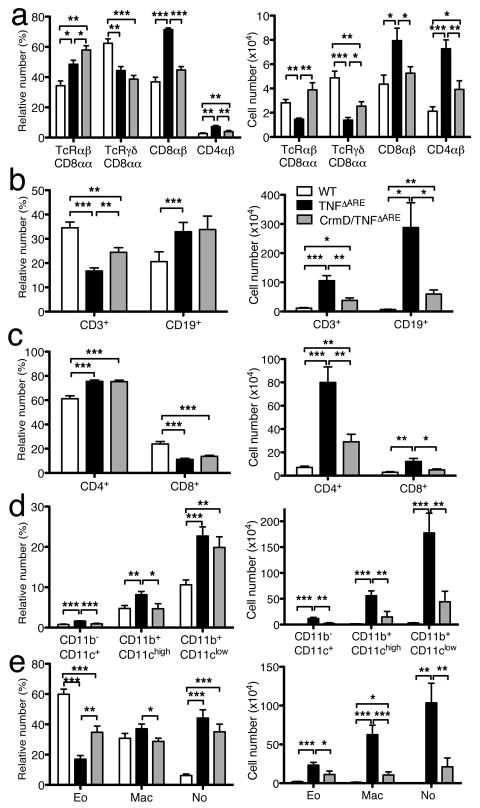
TNF-driven ileitis is associated with the marked reduction of CD8αα-expressing IELs and the increased representation of conventional CD8αβ IELs18. In order to evaluate if CrmD expression in IEC alters the phenotype observed in the IEL of TNFΔARE mice, we performed flow cytometry. IEL from the ileum of control mice consist primarily of CD8+ T cells with a low percentage of CD4+ T cells (Figure 4a left panel). CD8+ T cells can be divided in CD8αβ T cells and CD8αα T cells, which include both TCRαβ and TCRγδ T cells. As expected, TNFΔARE mice showed a decrease in the relative number (Figure 4a left) as well as in the absolute number of CD8αα T cells and an increase in the CD8αβ and CD4 populations (Figure 4a right). Expression of CrmD corrected most of the changes induced by TNF expression, with the exception of those associated with CD4+ T cells and CD8αα+ TCRγδ+ T cells whose values were significantly different from those found in controls (Figure 4a).
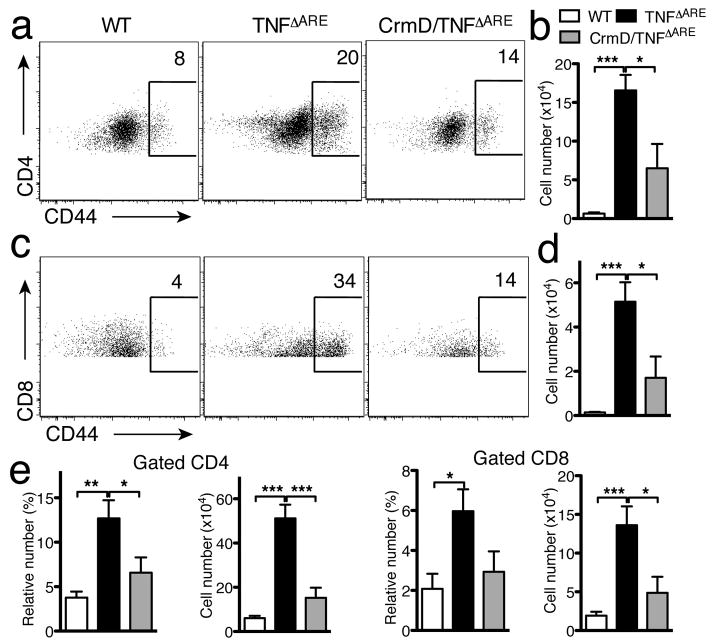
Figure 4. CrmD expression reduces the inflammatory infiltrate observed in the ileum of TNFΔARE mice.
a) Relative (left) and absolute (right) numbers of intraepithelial T-cell subsets in WT (n=4), TNFΔARE (n=5) and CrmD/TNFΔARE (n=6) mice (* P<0.05, ** P<0.01, *** P<0.001). b) Relative (left) and absolute (right) numbers of T and B cells in ileum of TNFΔARE and CrmD/TNFΔARE mice. c) Relative (left) and absolute (right) numbers of CD4 and CD8 T cells in ileum of TNFΔARE and CrmD/TNFΔARE mice. d & e) Relative (left) and absolute (right) numbers of DC and myeloid cell (d), Eo, Mac and No (e) in the ileum of CrmD/TNFΔARE mice. (WT, n=9; TNFΔARE, n=12, CrmD/TNFΔARE n=11; * P<0.05, ** P<0.005, *** P<0.0005). No, neutrophils; Eo, eosinophils; Mac, macrophages.
We then purified the LP leukocyte populations from the ileum of WT, TNFΔARE, and CrmD/TNFΔARE mice (8–11 weeks old) and analyzed the different populations by flow cytometry. In normal LP, the relative number of CD3+T cells is higher than the number of CD19+B cells (Figure 4b left). This relation is altered in the TNFΔARE mice, which have proportionally more B than T cells. The total numbers of B and T cells in TNFΔARE mice were significantly higher than those found in WT mice (P<0.05 and P<0.005 respectively) (Figure 4b right). TNFΔARE mice showed a higher percentage of CD4+ cells and a lower percentage of CD8+ cells compared to WT mice (P<0.005, Figure 4c left), consistent with previous studies18. The absolute cell numbers of these cell populations were also increased (CD4+ P<0.0005, and CD8+ P<0.005, Figure 4c right). Expression of CrmD markedly attenuated the changes associated with TNFα expression. There was no difference between WT and CrmD/TNFΔARE mice in the total cell number of CD8+ cells. However, the absolute number of CD3+, CD19+ and CD4+ T cells was higher in CrmD/TNFΔARE mice than in WT mice (P<0.05 and P<0.005, respectively). CrmD/TNFΔARE mice presented fewer T and B cells than TNFΔARE mice (Figure 4b). CrmD/TNFΔARE mice had a reduction in the total cell number of CD3+ (P<0.005), CD19+ (P<0.05), CD4+ (P<0.005), and CD8+ (P<0.05) cells in the ileum compared to the TNFΔARE mice (Figure 4b&c). The ratio of CD4+/CD8+ cells in the CrmD/TNFΔARE mice was similar to that observed in the TNFΔARE mice.
Analysis of the myeloid cell populations in the distal ileum showed that the relative and absolute number of CD11b+/CD11clow, CD11b+/CD11chigh and CD11b−/CD11c+ cells was increased in the TNFΔARE compared to WT mice (P<0.0005, Figure 4d). Interestingly, the absolute number of all these subsets was markedly reduced in CrmD/TNFΔARE mice compared to TNFΔARE mice (P<0.005, Figure 4d right). Analysis of the CD11b+/CD11clow population showed that TNFΔARE mice had more neutrophils, eosinophils, and macrophages than WT mice (P<0.005, Figure 4e). The absolute numbers of these cells were markedly decreased in CrmD/TNFΔARE mice compared to TNFΔARE mice (Figure 4e right). These results indicate that expression of CrmD in the intestine significantly reduced the inflammatory infiltrates promoted by TNFα.
The effect of CrmD expression is associated with a reduction of CD44+ T cells in LPL and MLN
It was recently reported that dysregulated production of TNF results in the expansion of both CD8+ and CD4+ T cell subsets expressing higher levels of CD44, which have a critical role in the development of ileitis in the TNFΔARE mice18,19. To evaluate if the reduction in ileitis observed in CrmD/TNFΔARE mice correlated with a decrease of CD44+ T cells, we performed flow cytometric analysis in the LPL and in the draining MLN. As expected, the relative and absolute numbers of CD4+CD44+ T cells (Figure 5a&b) as well as CD8+CD44+ T cells (Figure 5c&d) were increased in LPL from TNFΔARE mice. However, both populations were decreased in the ileum of CrmD/TNFΔARE mice. Similarly, the relative and absolute numbers of CD4+CD44+ T cells as well as CD8+CD44+ T cells in the MLN, which were increased in TNFΔARE mice, were significantly decreased in CrmD/TNFΔARE mice to levels compared to WT mice (Figure 5e). Together the results suggest that CrmD expression ameliorates TNF-induced disease by reducing the accumulation of cells critical for development of disease.
Figure 5. CrmD expression induces a reduction of CD44+ T cells.
a–d) Representative plot (a&c) and absolute number (b&d) of CD4+CD44high (a&b) and CD8+CD44high (c&d) cells in the ileum of WT (n=9), TNFΔARE (n=12) and CrmD/TNFΔARE (n=11) mice (* P<0.05, ** P<0.01, *** P<0.001). e) Relative and absolute number of CD4+CD44high and CD8+CD44high cells in the MLN of WT (n=7), TNFΔARE (n=9) and CrmD/TNFΔARE (n=8) mice (* P<0.05, ** P<0.01, *** P<0.001).
CrmD impairs TNFα-mediated changes in chemokine expression
The reduction in the total cell number of leukocytes by CrmD could be due to the direct interaction of CrmD with chemokines. CrmD binds to CCL20, CCL22, CCL25, CCL27, CCL28, CXCL12, CXCL13 and CXCL14, but of these, only CCL20, CCL22 and CXCL13 are increased in TNFΔARE mice (Figure 1). CrmD has been shown to inhibit chemotaxis induced by CCL2512, CCL27 (S.M.P. and A.A., unpublished observation), and CXCL13 (this study, Figure 2h), but its ability to block the remaining chemokines has not been demonstrated to date. However, even if CrmD could inhibit chemotaxis induced CCL20 and CCL22, it would be difficult to explain the drastic changes in some leukocyte populations (i.e, neutrophils) observed in the CrmD/TNFΔARE mice, given that these ligands do not affect these cells. An alternative hypothesis would be that the changes were caused by inhibition of TNFα-mediated induction of cytokine and chemokine expression.
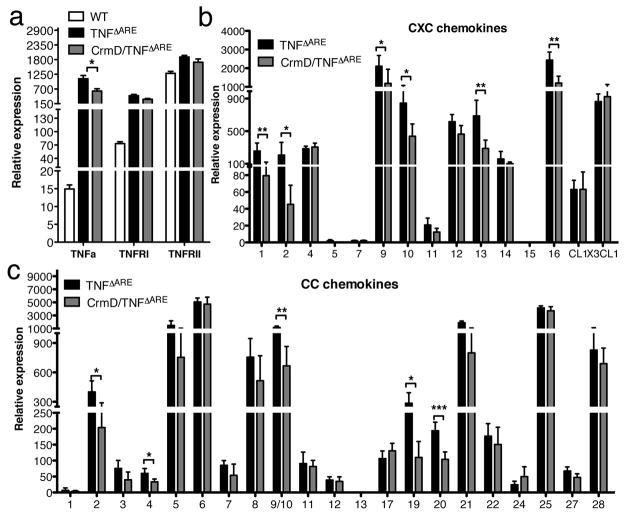
To test this hypothesis, we compared the levels of TNFα, TNFRI, TNFRII, and chemokine mRNA in the ileum of WT, TNFΔARE, and CrmD/TNFΔARE mice. As expected, we detected significantly higher levels of TNFα in the ileum of the TNFΔARE than in WT mice (P<0.001, Figure 6a). TNFα receptor I (TNFRI) and TNFRII mRNA levels were also upregulated in the TNFΔARE mice (P<0.001, Figure 6a). CrmD slightly reduced the expression of TNFα mRNA in the ileum of CrmD/TNFΔARE mice(P<0.05, Figure 6a). However, the serum levels of TNFα were not significantly different between TNFΔARE mice (56 ± 38 pg/ml n=35) and CrmD/TNFΔARE mice (43 ± 42 pg/ml n=10) indicating again a localized effect of CrmD in the gut. The enhancement in TNFα expression was accompanied by an increase in the expression of certain chemokines. Interestingly, CrmD impaired the induction of CXCL1, CXCL2, CXCL9, CXCL10, CXCL13, CXCL16, CCL2, CCL4, CCL9/10, CCL19, and CCL20 expression (Figure 6b&c, see P values). The fold increase in chemokine expression observed is shown in Table 2. These results suggest that CrmD inhibits TNF-induced chemokine expression. The fact that TNFα levels are very low during homeostasis would explain why expression of CrmD did not affect the general recruitment of leukocyte populations during homeostasis in vCrmD mice, with the exception of B cells, which was probably due to direct chemokine inhibition. This second mechanism may also contribute to the reduction in B cells observed in the CrmD/TNFΔARE mice.
Figure 6. Effect of CrmD on TNFα-mediated induction of TNFα and chemokine expression in the ileum of TNFΔARE mice.
a) TNFα, TNFRI and TNFRII mRNA levels in the ileum of WT, TNFΔARE and CrmD/TNFΔARE mice. TNFα, tumor necrosis factor α; TNFR, tumor necrosis factor receptor. b & c) Differential expression of CXC (b) and CC (c) chemokines in the ileum of TNFΔARE and CrmD/TNFΔARE mice. The expression of several chemokines is reduced in the ileum of CrmD/TNFΔARE versus TNFΔARE mice. (n=5, normalized to ubiquitin, * P<0.05, ** P<0.01, *** P<0.001).
Table 2.
Average fold increase in chemokine expression in the ileum of CrmD/TNFΔARE mice compared to TNFΔARE mice.
| CCL | Fold increase | CXCL | Fold increase |
|---|---|---|---|
| 1 | 0.6 | 1 | 0.3 |
| 2 | 0.5 | 2 | 0.2 |
| 3 | 0.5 | 4 | 1.1 |
| 4 | 0.6 | 5 | 0.2 |
| 5 | 0.5 | 7 | 1.0 |
| 6 | 0.9 | 9 | 0.6 |
| 7 | 0.6 | 10 | 0.5 |
| 8 | 0.7 | 11 | 0.6 |
| 9/10 | 0.6 | 12 | 0.8 |
| 11 | 0.9 | 13 | 0.4 |
| 12 | 0.9 | 14 | 0.6 |
| 13 | 1.3 | 15 | 0.7 |
| 17 | 1.2 | 16 | 0.5 |
| 19 | 0.4 | ||
| 20 | 0.5 | CL | Fold increase |
| 21 | 0.4 | 1 | 1.0 |
| 22 | 0.9 | ||
| 24 | 2.0 | CX3CL | Fold increase |
| 25 | 0.9 | 1 | 1.1 |
| 27 | 0.7 | ||
| 28 | 0.8 |
Discussion
In this study we have used transgenic mice expressing a virus-encoded protein to probe the mechanisms underlying TNF-driven inflammatory disease development in the intestine. We show that TNFα has a profound impact on chemokine expression and that expression of CrmD in the TNF-driven disease background is associated with a marked reduction in the induction of chemokine expression mediated by TNFα, indicating inhibition of TNFα function. The reduction in the accumulation of the inflammatory infiltrate could also be due to direct and selective inhibition of chemokine function by CrmD as observed for B cells. Our results indicate that CrmD expression affects the course of a TNF-driven disease by blocking TNF and thereby reducing chemokine expression and function. They also point to a direct role of chemokines in TNF-driven ileitis, suggesting the possible use of chemokines as new targets for therapy.
The levels of TNFα in the mouse intestine during homeostasis are low (Figure 6a). TNFα is increased during inflammatory conditions, playing a pivotal role in the initiation and maintenance of inflammation in the intestine6,20. TNFΔARE mice, which express elevated levels of TNFα, develop a Crohn’s-like disease in the ileum, characterized by massive cellular infiltrates composed of mononuclear cells and polymorphonuclear cells. We hypothesized that these cells were recruited into the ileum due to the local stimulation by TNF of chemokine synthesis. We found that several chemokines were indeed upregulated, including CCL2, CCL7, CCL8, CCL9/10, CCL17, CCL19, CCL20, CCL21, CCL22 CXCL1, CXCL2, CXCL4, CXCL9, CXCL13, and CXCL16. Many of these chemokines are directly induced by TNFα21–23.
To analyze the relevance of TNFα and chemokines in this model of inflammation we used the virus-encoded molecule CrmD, which exhibit a unique ability to interact simultaneously with both TNFα and some chemokines12. The expression of CrmD under the control of the villin promoter resulted in minor changes in chemokine expression (Figure 2f&g) and did not affect the major leukocyte populations present within the LP during homeostasis, with the exception of B cells (Figure 2e), suggesting that inhibition of TNFα by CrmD during homeostasis does not have a significant effect on the recruitment of the different leukocyte populations. Some of these changes, in particular that of CXCL9 may contribute to the reduction in T cell numbers observed in the CrmD/TNFΔARE mice and to the overall phenotype observed. These results also indicate that direct inhibition of chemokine function by CrmD does not affect the recruitment and retention in the intestine of most leukocyte populations. The only exception is binding and inhibition of chemokines responsible for B cell migration, such as CXCL13, a chemokine bound and inhibited by CrmD (12 and Figure 2h).
The expression of CrmD in the intestine of TNFΔARE mice dramatically altered the course of disease in the ileum, but not in the joints. The CrmD/TNFΔARE mice had less ileitis than their TNFΔARE littermates, but developed arthritis as the TNFΔARE mice (Figure 3). Strikingly, the absolute number of inflammatory cells present in the ileum was drastically reduced (Figure 4&5). Among the cell populations affected by CrmD are cells critical for ileitis development (Figure 5). These changes were associated with lower levels of TNFα mRNA, and a significant reduction of several chemokines shown to be upregulated in the TNFΔARE mice (Figure 6).
CrmD binds simultaneously to TNFα through the N-terminal cysteine rich domain of CrmD whereas binding to mouse chemokines CCL20, CCL22, CCL27, CCL28, CXCL11, CXCL12β, CXCL13 and CXCL14 occurs through the C-terminal domain of CrmD12 (S.M.P and A.A. unpublished observations). The C-terminal domain has been also described in other EV proteins and has been termed SECRET (for smallpox virus-encoded chemokine receptor)12. Among the chemokines bound by CrmD only CXCL13, CCL20 and CCL22 were upregulated in the ileum of TNFΔARE mice, suggesting that binding and inactivation of CXCL13 and possibly CCL20 by CrmD could account for some of the reduction observed in B cells in vCrmD mice and in the CrmD/TNFΔARE compared to the TNFΔARE mice. In fact, direct inhibition of CXCL13 by CrmD was observed (Figure 2h). However, direct binding of CrmD to chemokines could not account for the significant reduction observed in absolute numbers of neutrophils and macrophages, because the chemokines involved in the recruitment of such cells (CXCL1 and CXCL2 for neutrophils and CCL1–CCL9 for monocyte/macrophages24) are not bound by CrmD. It is also unlikely that the decrease in T cell numbers was due to the ability of CrmD to bind CCL25, because recent experiments carried out using mice deficient in CCL25 or its receptor CCR9, do not support a significant role for these molecules in the TNFΔARE model18. Moreover, CCL25 expression is not increased in the TNFΔARE compared to wt mice, and the T cell numbers are not affected in the vCrmD intestine.
Our results suggest that the main mechanism accounting for disease reduction in the CrmD/TNFΔARE mice is the local inactivation of TNFα by CrmD, since TNFα is the driving cytokine in the disease process and deletion of TNFα receptors abolishes disease in the TNFΔARE mice9. We attribute this decrease to reduced amounts of bioactive TNFα (which can regulate its own expression) and to reduced numbers of TNFα-producing leukocytes. The inactivation of TNFα is local, as disease is not reduced in the joints and the systemic levels of TNFα are not decreased in the CrmD/TNFΔARE mice.
Results shown here indicate that the reduction in ileitis is associated with changes in the number of lymphocytes in the intraepithelial and lamina propria compartments. These findings are important because such cells play important effector roles in the pathogenesis observed in TNFΔARE mice. CD8αβ IEL produce TNFα and IFNγ which induce the epithelial barrier dysfunction in TNFΔARE mice18, whereas CD8αα IEL appear to have regulatory functions25,26. Accumulation of both CD4+CD44+ and CD8+CD44+ effector cells in the LPL leads to maintenance and perpetuation of the ileitis18,19. CrmD expression in IEC not only decreased the number of effector CD8αβ cells in IEL, but also inhibited the accumulation of effector cells in the LPL leading to the generalized reduction in the ileitis observed in the CrmD/TNFΔARE mice.
What could account for these changes? Our results indicate that CrmD expression inhibits the expression of TNFα and several chemokines induced by TNF and that this is associated with pronounced and generalized decrease in leukocyte infiltration in the ileum. Since CrmD is able to bind to and inhibit TNFα, we believe that direct inhibition of TNFα function is key to the reduction in the expression of both TNFα and chemokines that are upregulated in the TNFΔARE mice. The reduction in TNFα mRNA levels in CrmD/TNFΔARE mice (compared to those found in TNFΔARE mice) is modest but significant and could be due to reduced recruitment of TNF-secreting leukocytes. The reduced expression and reduced functional activity of TNFα could thus explain the reduced expression of chemokines critical for recruiment of leukocytes to the intestine of CrmD/TNFΔARE mice. The identification of the chemokine subsets implicated in these responses is subject of ongoing investigation in our laboratory. Other possible mechanisms could include direct inhibition by CrmD of chemokine function as discussed above; reduced vascular activation and reduced expression of non-chemokine chemoattractants.
In summary, we show in this study that gut-specific expression of the ectromelia virus-encoded molecule CrmD significantly attenuates the TNF-induced ileitis observed in TNFΔARE mice. These results establish that the intestinal pathology observed in the TNFΔARE mice is largely caused by local, rather than systemic activity of TNF and suggest that chemokines are important factors controlling disease in this model.
Methods
Mice
CrmD was amplified by PCR from Ectromelia virus DNA using primers CRMD1 (5′-TAACGTACGGCCGCCACCATGATGAAGATGACACCATCATACATCTTGTTGG-3′) and CRMD2 (5′-TAAACGCGTTCAATCTCTTTCACAATCATTTGGTGG-3′) containing BsiWI and MluI sites respectively. The amplified product was cloned downstream of the villin promoter27 and sequenced to ensure that no mutations were present. The transgene was released with SalI, microinjected into C57BL/6J (The Jackson Laboratory, Bar Harbor, Maine) eggs, which were transferred into oviducts of ICR foster mothers (Charles River Laboratories, Wilmington, Mass). Identification of the transgenic mice was accomplished by PCR amplification of mouse tail DNA using the following specific primers vCrmD-F (5′-TTCTTCCTCTGGGCCTCAAGCCTGGC-3′) and vCrmD-R (5′-TTACATTTCCCATTAATGGGTGTATACGG-3′). TNFΔARE mice have been described previously9. CrmD/TNFΔARE mice were obtained by crossing TNFΔAREmice and vCrmD mice from line 1. The CrmD/TNFΔARE mice were genotyped with primers vCrmD-F, vCrmD-R, TNFΔARE-F (5′-TGAGGTGCAATGCACAGCCTTCCTC-3′) and TNFΔARE-R (5′-AAAGGTAATTAGGGTTAGGCTCC-3′).
Immunostaining
To detect CrmD expression in the jejunum of vCrmD mice, the tissue was fixed in 2% paraformaldehyde containing 20% sucrose overnight and frozen in OCT. Immunostaining was performed as previously described28. Briefly, rabbit polyclonal primary antibodies targeted to CrmD were incubated for 1 h at room temperature, followed by incubation with the appropriate fluorescent-labeled secondary antibodies for 30 min.
Histological scoring
The last 5 cm of the terminal ileum and joints were fixed by immersion in 10% phosphate-buffered formalin and then processed for paraffin sections. Routinely, 5 μm sections were cut and stained with H&E and two 1.5–2 cm sections of ileum were histologically evaluated in a blinded fashion. A semiquantitative scoring system, described previously29 with some modifications, was used to obtain the Histological score. Scores were given for 5 histological changes in the ileum: (1) inflammatory infiltrates (neutrophils, lymphocytes, plasma cells, and macrophages in the mucosa and submucosa); (2) lymphoid aggregates; (3) villus distortion (flattening and/or widening of normal mucosal villous architecture); (4) transmural infiltration and; (5) granulomata. Scores were given for 4 histological changes in the joints: (1) Synovial proliferation; (2) presence of neutrophils; (3) articular cartilage destruction and; (4) subchondral bone resorption. In addition, the presence or absence the pannus was noted. Scores ranged from 0 to 3 (0, absent; 1, mild; 2, moderate and 3, severe) and the total histological score per mouse was calculated by the summation of each parameter score for each mouse (data represented as mean ± SEM).
Isolation of lamina propria leukocytes (LPL) and intraepithelial lymphocytes (IEL)
IEL and LPL were isolated as previously described with some modifications17. Briefly, the section of the intestine to be analyzed was removed, flushed with ice-cold calcium- and magnesium-free Hank’s balanced salt solution (HBSS), and freed of fat, mesentery, and PP. The tissue was then cut into small pieces about 2 cm in length and washed 2 times with HBSS and then, incubated at 37°C with shaking for 20 minutes in HBSS containing 1 mmol/L dithiothreitol for IEL extraction. Then, the tissue was incubated with PBS with 1.3 mM EDTA for 1 h at 37°C. Fragments of intestine were further incubated in 7 ml of 2 mg/ml collagenase D (Roche) in RPMI for 1 h at 37°C and lamina propria leukocytes were isolated after 38% Percoll density gradient. For the vCrmD mice, the analysis was performed using the whole small intestine and the large bowel in separate preparations. In the case of the TNFΔARE and CrmD/TNFΔARE mice, only the ileum (distal 10 cm of the small bowel) was analyzed. Cells from the MLN and PP were obtained by homogenizing the tissue using a 70 μm cell strainer.
FACS analysis
Cells from LP, IEL, PP or MLN were incubated with Fc block (eBiosciences, San Diego, CA) for 15 min at room temperature, and were then stained with different antibodies to detect different subsets of leukocytes. The antibodies used were from BD Biosciences (San Jose, CA) and eBiosciences (San Diego, CA). Events were acquired on a Becton Dickinson FACScan (Becton Dickson, Franklin Lake, NJ) and analyzed using FloJo software (Tree Star, Ashland, OR).
RNA extraction and quantitative PCR (qPCR)
We extracted total RNA from the distal ileum (distal 5 cm of the small bowel) using the RNeasy Midi Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The cDNA obtained from these samples was analyzed by qPCR as previously described 17.
Chemotaxis assay
Splenocytes were obtained from wt C57BL/6J following standard procedures. 100nM mCXCL13 (R&D Systems, Minneapolis, USA) was placed in the lower compartment of 96-well ChemoTx System plates (Neuro Probe Inc., MD, USA) with or without affinity purified recombinant CrmD in RPMI 1640 containing 1% FBS. CrmD was purified as previously12. Splenocytes were placed on the upper compartment of the 96-well ChemoTx System plate. Both chambers were separated by a 3μm pore size filter. The cells were incubated at 37oC during 3 hours. Following the incubation period, the presence of cells in the lower chamber was determined by light microscopy.
Protein Quantitation
TNFα immunoreactivity was measured in the serum and CXCL13, CCL20 and CCL22 levels were measured in intestinal tissue extracts by ELISA (R&D Systems, Inc. Minneapolis, MN). ELISA assay was done according to the manufacturer’s instructions. The amount of chemokine is expressed as pg/μg tissue protein.
Statistical analysis
Statistical analysis of the data was performed using Prism 4.0c (GraphPad Software, San Diego, CA). Data were given as means ± SME unless otherwise stated. Unpaired Student’s t test was used to determine statistical significance. Differences were considered significant when P<0.05.
Supplementary Material
a) Representative FACS analysis of IgA+ and IgM+ leukocyte subsets within the LP of vCrmD and WT mice. b) Absolute cell number of IgA- (upper panel) or IgM-producing cells (lower panel) in WT and vCrmD mice within the CD45+ cell subset (n=5 for each group; * P<0.05). c) Representative FACS analysis of leukocyte subsets within the LP of vCrmD and WT mice. d) Absolute cell number of leukocyte subsets in vCrmD mice within the CD45+ cell subset (second panels), CD3+ subset (first panel) and CD11b+ subset (lower panel) (n=5 for each group; * P<0.05). No, neutrophils; Eo, eosinophils; Mac, macrophages.
Acknowledgments
We thank Yasmin G. Hernandez for technical help and Jay Unkeless and Ali Alejo for discussions and critical reading of the manuscript. We thank Sergio M. Pontejo for providing the anti-CrmD antibody. Supported by NIH grants P01 DK072201 (SAL and LM), New York Crohn’s Foundation and CCFA (LM), and from the Instituto de Salud Carlos III, Spanish Ministry of Health (AV-B).
Nonstandard abbreviations
- CrmD
cytokine response modifier D
- DC
dendritic cell
- DTT
dithiothreitol
- Eo
eosinophils
- ECTV
Ectromelia virus
- HBSS
Hank’s balanced salt solution
- IEL
Intraepithelial lymphocytes
- IEC
Intestinal epithelial cells
- LPL
Lamina propria leukocytes
- LPS
lipopolysaccharide
- Mac
macrophages
- MPXV
monkeypox virus
- No
neutrophils
- NK
natural killer
- TNFα
Tumor necrosis factor α
- vCKBP
viral chemokine binding protein
- VARV
variola virus
- WT
Wild-type
Footnotes
Disclosure: A. Alcami is an inventor of a patent application on the potential therapeutic use of the anti-chemokine activity of CrmD.
Authorship
AVB, SL: conception and design of the study;
AVB, AM, LS, FM, RAG, NT, GCF, SL: generation, collection, assembly, analysis and/or interpretation of data;
AA, GK: material support;
AVB, AM, SL: drafting of the manuscript;
NH, RAG, LM, GK, AA: revision of the manuscript;
AVB, AM, SL: approval of the final version of the manuscript.
References
- 1.Dahan S, Roth-Walter F, Arnaboldi P, Agarwal S, Mayer L. Epithelia: lymphocyte interactions in the gut. Immunol Rev. 2007;215:243–253. doi: 10.1111/j.1600-065X.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Deventer SJ. Tumour necrosis factor and Crohn’s disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piguet PF, Vesin C, Guo J, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499–3505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008:CD006893. doi: 10.1002/14651858.CD006893. [DOI] [PubMed] [Google Scholar]
- 6.Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol. 2007;102:2058–2069. doi: 10.1111/j.1572-0241.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 7.Yadav PK, Liu Z. Current strategies for the treatment of ulcerative colitis. Recent Pat Inflamm Allergy Drug Discov. 2009;3:65–72. doi: 10.2174/187221309787158407. [DOI] [PubMed] [Google Scholar]
- 8.Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn’s disease. Trends Mol Med. 2003;9:218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 9.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 10.Kontoyiannis D, Boulougouris G, Manoloukos M, Armaka M, Apostolaki M, Pizarro T, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J Exp Med. 2002;196:1563–1574. doi: 10.1084/jem.20020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 12.Alejo A, Ruiz-Arguello MB, Ho Y, Smith VP, Saraiva M, Alcami A. A chemokine-binding domain in the tumor necrosis factor receptor from variola (smallpox) virus. Proc Natl Acad Sci U S A. 2006;103:5995–6000. doi: 10.1073/pnas.0510462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Argüello MB., AAaAA . Secreted tumour necrosis factor inhibiors encoded by poxviruses. In: Digard PE AANaRERe, editor. SGM symposium. Vol. 64. Cambridge University Press; 2005. [Google Scholar]
- 14.Gileva IP, Nepomnyashchikh TS, Antonets DV, Lebedev LR, Kochneva GV, Grazhdantseva AV, et al. Properties of the recombinant TNF-binding proteins from variola, monkeypox, and cowpox viruses are different. Biochim Biophys Acta. 2006;1764:1710–1718. doi: 10.1016/j.bbapap.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 17.Shang L, Thirunarayanan N, Viejo-Borbolla A, Martin AP, Bogunovic M, Marchesi F, et al. Expression of the chemokine binding protein M3 promotes marked changes in the accumulation of specific leukocytes subsets within the intestine. Gastroenterology. 2009;137:1006–1018. 1018, e1001–1003. doi: 10.1053/j.gastro.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostolaki M, Manoloukos M, Roulis M, Wurbel MA, Muller W, Papadakis KA, et al. Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn’s disease. Gastroenterology. 2008;134:2025–2035. doi: 10.1053/j.gastro.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 19.Collins CB, Ho J, Wilson TE, Wermers JD, Tlaxca JL, Lawrence MB, et al. CD44 deficiency attenuates chronic murine ileitis. Gastroenterology. 2008;135:1993–2002. doi: 10.1053/j.gastro.2008.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andoh A, Yagi Y, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008;14:5154–5161. doi: 10.3748/wjg.14.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright TW, Pryhuber GS, Chess PR, Wang Z, Notter RH, Gigliotti F. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J Immunol. 2004;172:2511–2521. doi: 10.4049/jimmunol.172.4.2511. [DOI] [PubMed] [Google Scholar]
- 22.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 23.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 24.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 25.Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A. 2003;100:5324–5329. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–1497. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 28.Martin AP, Canasto-Chibuque C, Shang L, Rollins BJ, Lira SA. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol. 2006;177:7296–7302. doi: 10.4049/jimmunol.177.10.7296. [DOI] [PubMed] [Google Scholar]
- 29.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121:1428–1436. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a) Representative FACS analysis of IgA+ and IgM+ leukocyte subsets within the LP of vCrmD and WT mice. b) Absolute cell number of IgA- (upper panel) or IgM-producing cells (lower panel) in WT and vCrmD mice within the CD45+ cell subset (n=5 for each group; * P<0.05). c) Representative FACS analysis of leukocyte subsets within the LP of vCrmD and WT mice. d) Absolute cell number of leukocyte subsets in vCrmD mice within the CD45+ cell subset (second panels), CD3+ subset (first panel) and CD11b+ subset (lower panel) (n=5 for each group; * P<0.05). No, neutrophils; Eo, eosinophils; Mac, macrophages.