Abstract
Studies of inhibitory control have focused on inhibition of motor responses. Individuals with ADHD consistently show reductions in inhibitory control and exhibit reduced activity of rLPFC activity compared to controls when performing such tasks. Recently these same brain regions have been implicated in the inhibition of memory retrieval. The degree to which inhibition of motor responses and inhibition of memory retrieval might involve overlapping systems has been relatively unexplored. The current study examined whether inhibitory difficulties in ADHD extend to inhibitory control over memory retrieval. During fMRI 16 individuals with ADHD and 16 controls performed the Think/No-Think (TNT) task. Behaviorally, the Stop Signal Reaction Time task (SSRT) was used to assess inhibitory control over motor responses. To link both of these measures to behavior, the severity of inattentive and hyperactive symptomatology was also assessed. Behaviorally, ADHD individuals had specific difficulty in inhibiting, but not in elaborating/increasing memory retrieval, which was correlated with symptom severity and longer SSRT. Additionally, ADHD individuals showed reduced activity in rLPFC during the TNT, as compared to control individuals. Moreover, unlike controls, in whom the correlation between activity of the rMFG and hippocampus predicts inhibitory success, no such correlation was observed for ADHD individuals. Moreover, decreased activity in rIFG in individuals with ADHD predicted a decrease in the ability to inhibit motor responses. These results suggest that inhibitory functions of rLPFC include control over both memory and motoric processes. They also suggest that inhibitory deficits in individuals with ADHD extend to the memory domain.
Keywords: ADHD, Inhibition, fMRI, Memory, Symptom, Emotion
Introduction
ADHD, a common neuropsychological disorder in both children and adults has detrimental effects on academic, vocational, and social functioning. These individuals often exhibit deficits in inhibitory control, examined mainly in the motor domain, as indexed by tasks such as the Go/No-Go (Gomez, 2003; Borger & Van der Meere, 2000) and Stop-Signal paradigms (Ooosterlaan et al., 1998; Oosterlaan et al., 1996; Nigg, 1999). Increases in Stop-Signal reaction time (SSRT) in individuals with ADHD compared to controls represent one of the largest effect sizes in group comparisons (Ooosterlaan et al., 1998). Hence, examining the nature of inhibitory dysfunction in individuals with ADHD may provide insights into the psychological and neural correlates of inhibitory control in the neurologically normal brain.
Neuroimaging studies demonstrate that regions of the right lateral prefrontal cortex (rLPFC), including superior (SFG), middle (MFG), and most significantly the inferior frontal (IFG) gyri play a critical role in the inhibition of motor responses in neurologically-intact individuals (Aron et al., 2007, Aron & Poldrack, 2006) and become active during performance of both Go/No-Go (Yamaguchi et al., 2008; Garavan et al., 2006; Fassbender et al., 2006; Garavan et al., 1999) and Stop-signal tasks (Yamaguchi et al., 2008; Chevrier et al., 2007; Chamberlain et al., 2009). In contrast, individuals with ADHD show reduced activation, compared to controls, and anatomical correlations in these rLPFC regions when performing such tasks (Booth et al., 2005; Tamm et al., 2004; Casey et al., 1997; Rubia et al., 1999; Rubia et al., 2005; Depue et al., 2010).
While inhibitory control in ADHD individuals has mainly been examined in the motor domain, some studies indicate that these individuals display reduced inhibitory control in interference tasks (Attentional Network Task; Konrad et al., 2006; for a review see; Sergeant et al., 2003). What is less clear is whether inhibitory deficits in ADHD individuals are affected in other psychological/cognitive domains like memory (White & Marks, 2004). One reason for expecting broad inhibitory deficits in ADHD is that rLPFC may support inhibition across different domains (e.g., motor response, emotion, thought, and memory processes; Aron et al., 2004), although research directly examining this issue is scarce. Providing the possibility that an inhibitory brain mechanism which exerts control over multiple domains exists, the ERP N2 component, though to index response inhibition, is correlated with a similar N2 component during NT trials (Mecklinger et al., 2009). Additional evidence suggests rLPFC regions become active when individuals are asked to inhibit or dampen down an emotional response (Phan et al., 2005).
Additional evidence for the role of rLFPC in inhibition in the cognitive domain is provided by neuroimaging studies using the Think/No-Think (TNT) task (Anderson et al., 2004; Depue et al., 2007; for a review see Anderson & Levy, 2009), a task based on the Go/No-Go task, which is often used to measure inhibition in the motoric domain. This task examines the efficacy of inhibiting the retrieval of memories rather than inhibiting a motor response. Examinations of inhibition over emotional memory retrieval have implicated two rLPFC mechanisms (Depue et al., 2007). One localized to rIFG, appears to modulate activity in brain regions that support sensory components of the memory, including the pulvinar (Pul) and fusiform gyrus (FG), while a second localized to rMFG modulates activity in brain regions that support multi-modal components of memory, including the hippocampus (Hip) and amygdala (Amy). Of importance, these rLPFC regions overlap with prefrontal regions typically implicated in response inhibition.
The objective of the current study was to examine inhibitory function outside the motor domain in individuals with ADHD to determine whether they also exhibit dysfunction during inhibition of memory retrieval processes. Furthermore, because these individuals express difficulty in inhibitory control, they are suited well to test our model of inhibition over emotional memory retrieval (Depue et al., 2007), which suggests that regions of rLPFC are important for providing top-down cognitive control over the hippocampus that results in the inhibition of memory retrieval. Accordingly, we examined the neural underpinnings of performance on the TNT task in a group of young adults with ADHD, and compared their brain activation to previously-reported data from a non-ADHD control group (Depue et al., 2007).
In view of the role of rLPFC in inhibition across domains of motor, emotional, and memory processes in non-psychiatric individuals combined with findings of deficient inhibition of motor responses in ADHD individuals, as compared to controls (Casey et al., 1997; Rubia et al., 1999; Rubia et al., 2005), we hypothesized that individuals with ADHD would have difficulty inhibiting memory retrieval and thus, decreased activity in rLPFC. Likewise, based on our prior research (Depue et al., 2007), decreased rLPFC activity would be associated with decreased down-regulation of posterior regions that support memory, during inhibition of memory retrieval (i.e., Pul, FG, Hip, Amy). The latter implies that the negative relationship between activity in rLPFC and posterior sites should be significantly reduced compared to control individuals. In addition, if rLPFC supports inhibitory processes across multiple domains, activity in these regions during the TNT task is expected to correlate with behavioral measures of motor inhibition, which we assessed in our ADHD sample using with the Stop-Signal paradigm. These individual difference measures have previously been used as a powerful tool to understand differences in the inhibition over memory retrieval (Levy & Anderson, 2008; Paz-Alonso et al., 2010; Depue et al., 2006; 2007).
Methods
Participants
Sixteen young adults with ADHD (10 male, 6 female) and 16 healthy controls (10 males, 6 females) all between 18 and 23 years of age, served as participants. A portion of the data from control individuals has previously been presented (Depue et al., 2007). Of note, groups were tested concurrently in the same scanning environment. Groups did not differ in age [t(30)=−.58, p=.57; control mean=19.75, ADHD mean=20.06] nor distribution of gender (10 males and 6 females in each group). A table showing the descriptive characteristics for both groups can be found in S1, as well as full participant selection procedures can be found below.
Initial screening of the unselected sample
An unselected sample of 3,913 undergraduates completed a battery of self-report rating scales that included the Self-Report form of the ADHD Current and Childhood Symptom Scales (Barkley & Murphy, 1998). The initial screening measures were administered to groups of 20–40 individuals as part of the research participation requirement of a large introductory psychology course. Permission was also requested to allow us to send the Other Report version of the Current and Childhood Symptom Scales (Barkley & Murphy, 1998) to the participant’s parent or other primary caregiver during childhood. Approximately 72% of the participants provided consent for the questionnaire to be sent to their parent or caregiver.
Individual assessment of groups with and without DSM-IV ADHD
As part of an ongoing study of neuropsychological functioning in young adults with ADHD, a subset of participants from the initial screening sample were invited to participate in a more extensive individual testing session that included measures of general intelligence, academic achievement, and neuropsychological functioning. Participants who met symptom criteria for any DSM-IV ADHD subtype based on parent or self-report ratings on the Childhood and Current Symptom Scales were invited to complete the individual testing session (N = 207). ADHD ratings by participants and parents were combined based on an algorithm parallel to the procedures used in the DSM-IV field trials for the disruptive behavior disorders (Lahey et al., 1994). In addition, a comparison sample without ADHD (N = 98) was randomly selected from the remainder of the screening sample and invited to participate in the individual assessment.
Identification of groups with and without DSM-IV ADHD combined type/Diagnostic algorithm for the combined type
At the conclusion of the individual assessment session, participants who met criteria for DSM-IV ADHD - combined type and who met all inclusion criteria for the MR protocol were invited to participate in the fMRI study. Individuals who matched our control group in gender and age were then selected. This yielded sixteen individuals with ADHD, who are included in the subsequent analyses. The diagnosis of the combined type in adulthood is complicated by the fact that symptoms of ADHD decline with increasing age, particularly on measures of hyperactivity-impulsivity (e.g., DuPaul et al., 1998; Nolan et al., 1999; Nolan et al., 2001). Therefore, four criteria were used to operationally define participants with the combined type for the fMRI study: (1) Retrospective reports by the participant or the parent indicated that he or she met DSM-IV criteria for the combined type during childhood; (2) the participant currently met criteria for DSM-IV ADHD; (3) the ADHD symptoms led to significant functional impairment, and (4) the onset of the ADHD symptoms was prior to 7 years of age.
Measures of functional impairment
To ensure that participants met DSM-IV criteria C and D specifying that the symptoms of ADHD must lead to significant impairment across settings, all participants completed multiple measures of functional impairment as part of the initial screening. As noted previously, the Current and Childhood Scales and interview include specific questions regarding the impact of ADHD symptoms on the individual’s social, occupational, educational, and overall daily functioning (Barkley & Murphy, 1998). To supplement these items, during the initial screening all participants completed a more detailed impairment questionnaire developed for this study (Willcutt et al., submitted). The impairment scale includes a broader range of questions regarding academic functioning (high school and college grade point average, completion of assignments, retention of academic material), interpersonal relationships (both friendships and romantic relationships), and specific aspects of adaptive functioning such as money management, driving performance, and occupational functioning. Finally, a summary measure of global functioning was obtained during the initial screening by asking the participant and parent to rate the individual’s lowest overall functioning during the past year on a Global Assessment of Functioning Scale that corresponds directly to Axis V in DSM-IV.
The battery of impairment measures was used to derive composite measures of global, academic, social, and occupational functioning, management of daily responsibilities, and driving impairment. Significant impairment in each of these domains was defined by a score at or above the 93rd percentile of the total screening sample on the composite measure.
Intelligence and academic achievement
The Matrix Reasoning subtest from the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, 1997) was administered to assess nonverbal abilities, and verbal abilities were measured by the WAIS-III Vocabulary subtest. A linear transformation was used to rescale the subtest scores to the format typically used to report Verbal and Performance IQ (M = 100, SD = 15), and the mean of these scores was used as an estimate of Full Scale IQ. The Woodcock-Johnson Tests of Achievement, Third Edition (WJ-III; Woodcock, et al., 2001) was used to assess academic achievement in mathematics (Calculations and Math Fluency) and reading-related domains (Letter-Word Identification, Word Attack, and Spelling). Reading disability was defined by a standard score below 85 on the Letter-Word Identification subtest, and math disability was defined by a score below 85 on the Calculations subtest.
Criteria for the comparison group
The comparison group for the fMRI study included 16 individuals who did not meet current or lifetime criteria for any DSM-IV ADHD subtype based on the rating scales. The control and ADHD samples were matched as a group on age, sex, and academic year.
Exclusion Criteria
Potential participants were excluded from both groups if they reported a previous diagnosis of a Learning Disability (LD) or met our study criteria for an LD on the measures of reading or math achievement (Woodcock-Johnson III) described in the subsequent section. Individuals with any psychiatric disorder (Depressive/Anxiety disorders, bipolar, Obsessive-Compulsive Disorder, Post-Traumatic Stress Disorder and Tourettes) were also excluded, as assessed by a self report questionnaire identifying past or present conditions, as were potential participants who had an estimated Full Scale IQ < 80, were pregnant, were left handed, had metal in their body that could not be removed (e.g., cardiac pacemaker), had a previous history of seizures or a head injury with loss of consciousness, or any other contraindication for the MR environment. All participants of the ADHD group (N=16) were currently taking stimulant medication and asked to abstain from taking their medication 24 hours prior to scanning. At the time of scanning abstinence was assessed, if the participant had recently taken (<24hr) their medication they were excluded from the study.
Procedure
Think/No-Think
Anderson and Green’s (2001), Think/No-Think paradigm was utilized using face-picture pairs (Depue et al., 2006; 2007). Forty faces (female) previously normalized as having a neutral expression were used. Forty images were selected from the International Affective Picture Series (IAPS), negative in emotional content (Lang et al., 1995). Pictures were selected at a median level of negative affect on a scale of 1–9 (mean = 4.1, SD = .55). Due to the IAPS having no relatedness scores, two independent raters selected pictures to have as minimal relatedness in content as possible, in order to eliminate possible grouping effects. The experiment was designed with E-Prime software from Psychology Software Testing, which was used to display the stimuli and record performance on a Dell computer.
The experimental procedure was divided into three phases: training, experimental, and testing. In the training phase, participants learned 40 face picture pairs, which were displayed for 4 s. Participants first viewed each pair and, after 20 pairs, were shown a face and asked to select which of two pictures was originally paired with the face. Both of the two pictures came from the training phase so that novelty of one choice could not be used as a potential alternative cue for recognition. This procedure continued in sets of 20 until the participant could recognize the correct picture previously paired with a face with 97.5% accuracy (39 items) over all 40 pairs. In the experimental phase, participants saw the face for only 32 of the 40 pairs, half of these being relegated to the Think Condition, and half to the No-Think condition. In both conditions, a trial consisted of a face for 3.5 s, and then a 500 ms inter-trial interval. The color of a border around the faces indicated the condition: green for Think trials and red for No-Think trials.
As in Anderson and Green (2001), in the Think condition, participants were told “Think of the picture previously associated with the face”, whereas in the No-Think condition they were told “Do not to let the previously associated picture come into consciousness”. Within each condition (Think/No-Think), participants viewed faces 12 times, randomly distributed across all 512 trails. The 8 faces not shown in the experimental phase served as a 0-repetition baseline.
During the test phase, participants were shown each of the faces and told to write down a brief description of the picture originally associated with it. These data provided the accuracy measures.
Stop-signal Task
Only ADHD participants completed the Stop-signal task (Logan & Cowan, 1984) and thus, it was used for correlation purposes for these individuals only. On primary task trials, the letters X or O are presented in the center of the monitor for 500 ms, and the participant responds by pressing the corresponding key on the keyboard. On stop-signal trials the same visual stimulus appears, but an auditory tone is also presented shortly after the X or the O appears on the screen. The participant is instructed to press the X or O key as rapidly as possible for each trial, but to inhibit the key press on each of the trials on which the tone is presented. The primary dependent measure is stop-signal reaction time, a measure of the duration of the inhibitory process (Logan & Cowan, 1984; Nichols & Waschbusch, 2004). Longer SSRTs indicate greater difficulty inhibiting/cancelling an ongoing response.
Image Acquisition/Analysis
Image Analysis
Standard image Acquisition and Analysis procedures for FSL can be found in full detail in S2.
Percent signal change (ΔS) analyses were performed using FSL’s (Analysis group, FMRIB, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl/) Featquery signal change processing tool. Featquery was used to interrogate ΔS of regions of interest (ROIs) defined for the control individuals by our prior results (Depue et al., 2007) and defined for the ADHD individuals based on results of the current whole brain analysis (NT>T). ROIs included: right middle frontal gyrus (rMFG, controls only), right inferior frontal gyrus (rIFG), right BA10 (fronto-polar cortex) bilateral amygdala (Amy), bilateral hippocampus (Hip), bilateral pulvinar (Pul) and bilateral fusiform gyrus (FG). Next, associated ΔS was calculated using a 5mm3 sphere around the peak of activation within the ROIs based on our results of our whole brain NT>T SPMs, for each individual. This ΔS is derived from lower level analyses for each individual’s contrast, so as to not contaminate the results from the mixed effects model used at the higher level group comparison that includes between subjects variance. Regions included right-sided rMFG, rIFG, rBA10 and bilateral posterior regions: Amy, Hip, Pul, and FG. These peak-based spheres were then interrogated within our modeled experimental paradigm to examine differences between NT and T conditions versus a fixation baseline to establish the ΔS that was related to each condition (NT or T). Peaks within ROIs were selected individually for each group based on the NT>baseline contrast. The only exception to this approach was the peak used for rMFG, which did not show a significant increase in activation in ADHD individuals. To provide as unbiased a peak as possible, data for both ADHD and control individuals were combined and the maximal site of activation for the NT>baseline contrast was selected (MNI= x=36, y=31, z=30). Parameter estimates were then converted to ΔS values before reporting. To assess paired correlations across the raw time series, we extracted ΔS from the NT>baseline contrast for each brain region within an individual (intra-individual). This ΔS from individual brain regions was correlated for all pairs of brain regions (i.e., rMFG and Hip) on an individual basis. These resulting r-values were transformed to Zvalues which were then correlated with the variable of interest (i.e., symptomatology, SSRT, inhibition index). This approach is similar to the approach outlined by Koshino and colleagues (2005).
Results
Behavioral Results
Behavioral accuracy was determined from the final recall test. A two-way ANOVA with the factors of Group (ADHD, Control) and Condition (Think, No-Think) revealed no main effect of Group (p=.64), a significant main effect of Condition [F(1,31)=17.24, p=.0001], such that NT items were recalled significantly less than T items, and a trend towards significance for the interaction of Group x Condition [F(1,31)=2.81, p=.07]. Because the groups differed in baseline levels of recall (57.5% vs. 62.5%; ADHD, controls respectively), t-tests were used to compare conditions within group (T vs. NT, T vs. baseline, NT vs. baseline), as well as across groups for each condition (ADHD vs. Control). ADHD individuals showed a significant trend for greater recall during T than NT items [t(15)=1.48, p=.08] and T than baseline items t(15)=1.46, p=.10] but no difference between NT items and baseline items [t(15)=.14, p=.89]. Control individuals exhibited greater recall for T than NT items [t(15)= −4.29, p=.0006], a trend for greater recall of T than baseline items t(15)=1.49, p=.07], and a significant reduction of NT relative to baseline items [t(15)= −2.28, p=.02]. Significant group differences emerged both for lower recall in the NT condition for control individuals than ADHD individuals [t(30)=2.12, p<.05], as well as, an increased reduction for NT trials relative to baseline (NT-Base) for control individuals than ADHD individuals [t(30)=2.06, p<.05]. Stop-signal results for the ADHD individuals can be found in Supplementary Materials (S3).
Group Comparisons of Memory Retrieval Inhibition: Whole Brain Analysis
Our recently outlined model (Depue et al., 2007), suggested that two sets of regions are involved in inhibiting emotional memory retrieval: 1) increased activity in rIFG correlates with decreased activity in sensory-related regions, including the pulvinar (Pul) and the fusiform gyrus (FG), and 2) increased activity in rMFG correlates with decreased activity of multi-modal memory regions, including the hippocampus (Hip) and amygdala (Amy). Furthermore, the two prefrontal regions (rIFG, rMFG) show correlations with rBA10. Of note, we refer to rIFG and rMFG as prefrontal regions and Pul, FG, Hip and Amy as posterior regions for the remainder of the paper. Whole brain analyses for the contrast of NT>T trials in ADHD individuals showed the same regions of activity previously observed in controls (Depue et al., 2007), with the exception of rMFG (see S4).
To establish whether these differences were robust, we directly compared brain activation for the two groups for the contrast of NT>T trials. Group differences in prefrontal brain regions revealed an area of rMFG that control individuals activate to a significantly greater degree than ADHD individuals. Conversely, posterior brain regions yielded greater activation in the ADHD as compared to the control group within regions of Pul, FG, the parahippocampal gyrus (PHG), the Hip, and Amy. Importantly, the area of rMFG is thought to be important for communication with the Hip and Amy (Depue et al., 2007). Similarly, the finding of increased activity in ADHD individuals within Pul, FG, PHG, Hip, and Amy include all posterior regions decreased during inhibition over memory retrieval in the control group.
Because the contrast of NT>T includes signal from both NT and T trails we wished to determine the activity in each condition (T, NT) independently compared to the fixation baseline in a whole brain group comparison analysis (Z=2.81, p<.005). The group difference in rMFG occurred because of greater activation for the control than the ADHD group for NT trials (p<.005), while no significant difference was apparent for T trials. Decreased activation in rLPFC in the ADHD group appears to be specific to inhibitory processing, and does not extend to cognitive control required to maintain or elaborate emotional memories. Group differences in posterior brain regions occurred because the ADHD group as compared to controls, showed increased activation on NT trials for all posterior brain regions (p<.005), whereas no differences were observed for T trials. Hence, these analyses reveal that group differences in brain activation are driven by NT trials.
Comparison of ROI Signal Change
Our prior study (Depue et al., 2007) suggested that the time course of activation in both the prefrontal and posterior regions varied systematically across the multiple attempts (i.e, 12 trials) at cognitive control. To investigate this issue, percent signal change (ΔS) for each of the ROIs: two prefrontal (rIFG, rMFG) and four posterior regions (Pul, FG, Hip, Amy) was extracted for the NT>baseline condition on a quartile-by-quartile basis to investigate how the ΔS evolves over the time course. Because we previously established that group differences were apparent in NT trials as compared to T trials vs. baseline, we conducted these analyses on the NT>baseline contrast, in order to examine the signal specifically related to NT trials.
ADHD individuals show significantly less activation of rIFG compared to baseline, compared to control individuals, during the second quartile (Fig. 3A). More strikingly, ADHD individuals did not significantly activate rMFG above baseline for any quartile and showed significantly less activation than control individuals, who activate rMFG above baseline during the latter three quartiles (Fig. 3B). Furthermore, within posterior regions, ADHD individuals’ exhibit increased activation compared to baseline, whereas control individuals show reduced activity below baseline, most significant in the third and fourth quartile (Fig. 3C–F). These results indicate that, as compared to controls, ADHD individuals’ exhibit reduced activation in prefrontal regions, predominantly rMFG, which is accompanied by greater activity in posterior regions that support memory representation.
Fig. 3.
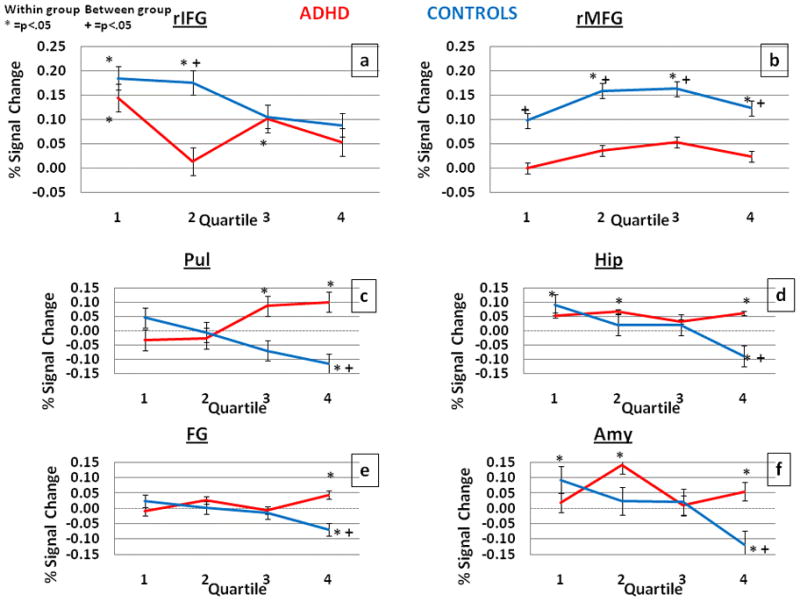
Comparison of percent signal change for the contrast of NT>baseline in ADHD (red) and controls (blue) for the critical ROIs involved in inhibition of emotional memory retrieval. * indicates statistically significant within group comparisons, while + indicates between group (p<.05). Error bars represent standard error.
Although as a group, the ADHD individuals showed no behavioral inhibition over memory retrieval below baseline recall, some individuals (N=5) did show behavioral inhibition during the recall test. Using the same methodology as above, the patterns of ΔS for this subset of ADHD individuals was examined to see if they differed from the other ADHD individuals (N=11) and were more similar to controls. The results (see S5) indicate that these individuals exhibit significant increased signal (p<.05) in rMFG in the second and third quartile and reduced signal below baseline (p<.05) in the Hip and Amy in the fourth quartile.
Interestingly, no group differences in ΔS emerged for rBA10 and while ADHD individuals showed little ΔS in rMFG, they still exhibited correlations of rBA10 and rMFG, as well as rIFG (see S6). Importantly, this finding replicates our previous model in which BA10 showed correlations with prefrontal regions in control individuals.
Correlations across Brain Regions, Behavior and Symptoms: Individual Differences
If prefrontal regions exert control by communicating with posterior regions to inhibit emotional memory retrieval, one would predict significant negative correlations between activity in prefrontal and posterior regions in controls that would be absent or diminished in the ADHD group. The correlation of activity in these brain regions with behavioral performance likewise would also be reduced or absent in ADHD as compared to control individuals. All group differences between magnitudes of correlations were assessed by Fisher’s Z.
These predictions were tested in three different analyses. First, we correlated the mean ΔS (NT>baseline) between pairs of ROIs across individuals (inter-individual) (correlation values positioned next to the orange and green arrows in Figure 4). Across control individuals, there was a significant negative correlation between activity in prefrontal and posterior regions (rIFG and Pul/FG, rMFG and Hip/Amy), whereas none of these correlations were significant across the ADHD individuals. Moreover, these correlations significantly differed or indicated a trend between the groups for all regions [rIFG-Pul (p<.05), rIFG-FG (p<.06), rMFG-Hip (p<.001), rMFG-Amy (p<.00001).
Fig. 4.
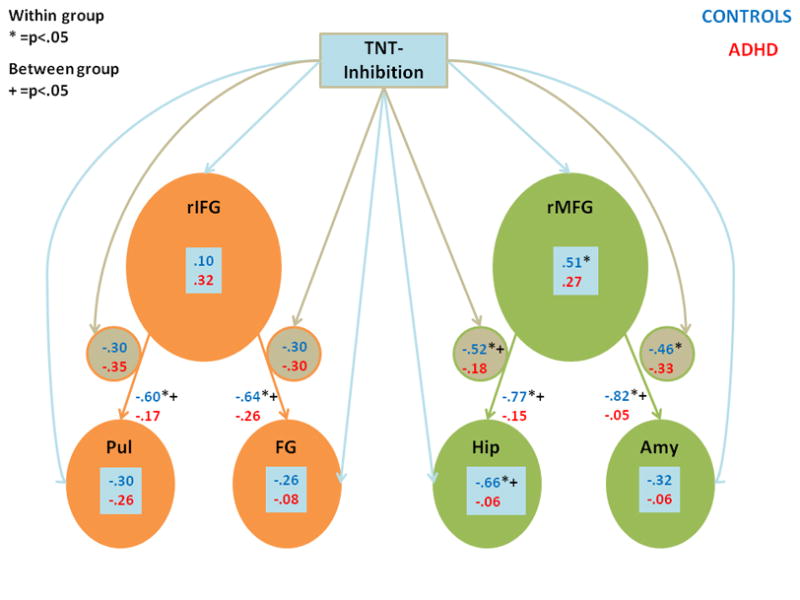
Correlations between activity in brain regions (ROIs) and inhibition of memory. Bonferroni corrected correlations between brain regions’ mean percent signal change across individuals (inter-individual) is shown next to arrows connecting those brain region (i.e., next to orange and green arrows). Bonferroni corrected correlations between mean percentage signal change for a given brain region (inter-individual) and retrieval inhibition (measured as a percentage of NT trials correctly suppressed) are shown in blue boxes (connected to the retrieval inhibition rectangle by blue arrows). Correlations between the time series of activity across brain regions (intra-individual) within an individual and retrieval inhibition (correlations in small circles, connected to the retrieval inhibition rectangle by brown arrows). Results for the control group are shown in blue, while those for the ADHD group are shown in red. * indicates statistically significant within group comparisons, while + indicates between group determined by Fisher’s Z (p<.05).
Second, to examine how behavioral performance correlates with the activity of these brain regions as assessed by mean ΔS (NT>baseline) within that region, we calculated an inhibition index, which was the percentage of successfully inhibited NT items compared to the percentage of recalled baseline items. As shown in Figure 4 (correlation values shown within blue text boxes), across control individuals (inter-individual), increases in inhibition of memory retrieval correlated with great activity in rMFG (r=.51), and less activity in the Hip (r=−0.66). In contrast, for ADHD individuals, there was no significant correlation between activity in these regions and inhibition of memory retrieval, leading to a significant difference in the size of the correlations between the groups for the correlation of the Hip and inhibition index (p<.005).
Finally, we calculated paired correlations across the raw time series ΔS (NT>baseline) for pairs of brain regions within an individual (intra-individual) and converted these to Z-values, this approach is similar to the approach outlined by Koshino and colleagues (2005). These Z-values were then correlated with an individual’s inhibition index (correlations within small circles in Figure 4). Within control individuals, the greater the negative association between the time series of activation between rMFG - Hip and between rMFG - Amy, the greater the inhibition of memory retrieval. These correlations were not significant for ADHD individuals. As a result, a significant group difference emerged in the size of the correlations between rMFG - Hip and inhibition index (p<.05).
In sum, these three correlational analyses suggest that the negative relation of activity between prefrontal and posterior brain regions, especially between rMFG and the Hip, in control individuals is related to the ability to inhibit emotional memory retrieval. Although the lack of any significant correlations in the ADHD group might be indicative of a lack of power of our measures, this is not likely to be the case because, as discussed next, these measures of brain functioning did correlate with other behavioral measures.
If, as predicted, activity in these brain regions is related to the ability to exert inhibitory control, then within the ADHD group one should observe a significant relationship with the behavioral severity of ADHD symptomatology, as well as other behavioral measures of inhibitory function. To investigate this issue, correlations were computed between brain activity with lifetime Likert scores for both inattentive and hyperactive symptoms of ADHD, as well as with inhibition over motor responses on a standard Stop-Signal reaction time task (SSRT) (S2), all of which are shown in Table 1. Measures of brain activity were the same measures reported above; mean ΔS (NT>baseline) across individuals for a given ROI, as well as correlation of the raw time series ΔS (NT>baseline) for pairs of brain regions within an individual. For comparison, associations of brain activity with inhibition of emotional memory retrieval are also shown in Table 1. To assure that these correlation were not caused by skewed distribution of ΔS or outliers we present scatter plots in the supplemental material for all significant correlations found in Table 1 (see S7).
Table 1.
Bonferroni corrected correlations between brain activity and symptomatology. Red indicates a correlation that differs significantly from 0 (df = 15, p<.05). INHIB=percentage NT trials successfully inhibited; INATT=lifetime number of inattentive symptoms on a Likert scale; HYP=lifetime number of hyperactive symptoms on a Likert scale.
| ADHD | MFG | HIP | AMY | IFG | PUL | FG | MFG- HIP | MFG- AMY | IFG- PUL | IFG- FG | INHIB | INATT | HYP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INATT | −.15 | .52 | .37 | −.31 | .39 | .18 | .42 | .36 | .21 | .08 | −.56 | 1 | |
| HYP | −.15 | .34 | .31 | −.35 | .34 | .13 | .26 | .18 | .16 | .42 | −.50 | .48 | 1 |
| SSRT | −.40 | .26 | .26 | −.48 | .30 | .28 | .10 | .33 | .55 | .15 | −.58 | .11 | .25 |
Correlations in Table 1 indicate that, in general, ADHD symptomatology is related to the paired correlation between activity in prefrontal and posterior brain regions: the higher the symptomatology, the less the negative correlated activity between prefrontal and posterior regions. Inattentive symptomatology is related to the Hip and the MFG-Hip correlation, whereas hyperactive symptomatology, which correlated with inattentive symptomatology, related more strongly to the IFG-FG correlation. Moreover, increasing levels of both inattentive and hyperactive symptomatology are significantly related to poorer inhibition over emotional memory retrieval. Finally, poorer inhibition over motor responses, as measured by SSRT, was related to a decreased ability to inhibit emotional memory retrieval. Yet performance on SSRT was related mainly to the rIFG and rIFG-Pul pathway as opposed to the rMFG-Hip pathway, suggesting possible distinctions within rLPFC supporting inhibition.
Discussion
The results of our study provide evidence that lateral regions of the right prefrontal cortex are involved in inhibitory control over both memory and motor processes. This conclusion was supported by numerous aspects of the pattern of behavior and brain activation in ADHD individuals as well as the differences observed from control individuals. First, the groups differed specifically in brain activation associated with prefrontal inhibition of posterior brain regions. Controls showed significantly greater activation in rLPFC areas, most notably in rMFG, and significantly reduced activation in posterior regions, compared to ADHD individuals during trials requiring inhibition of emotional memory retrieval (NT trials). These prefrontal group differences, characterized by reduced activity in ADHD individuals, were most apparent across quartiles in rMFG and in the second quartile for rIFG. We interpret these findings as indicating an inability of ADHD individuals to consistently maintain cognitive/inhibitory control. Group differences in activation in posterior regions were most apparent during the fourth quartile where ADHD individuals showed increased activity compared to control individuals, who exhibit brain activation significantly below baseline. Our prior behavioral work, as well as others (Depue et al., 2006, Hanslmayr, 2009) has suggested that successful inhibition over memory retrieval only occurs with a high number of repeated attempts (10) while a moderate number (5) does not. Thus, by the fourth quartile (between 10–12 attempts) control individuals show reduced activity below baseline in posterior regions, whereas ADHD individuals never show such reduction, perhaps as a result of poor cognitive/inhibitory control exerted by rLPFC. There were no differences between groups in prefrontal or posterior brain activation during trials not requiring inhibition of posterior brain regions (T trials). Therefore, reductions in rLPFC activity in ADHD individuals were specific to memory retrieval processes requiring inhibition.
Second, significant group differences in correlations occurred because controls showed significant negative correlations of activation between prefrontal (increased activation) and posterior (decreased activation) brain regions (i.e., rIFG Pul/FG, rMFG Hip/Amy) during NT trials, whereas none of these correlations were significant across ADHD individuals. Moreover, correlated activity in rMFG Hip significantly predicted success in inhibiting memory retrieval in control but not ADHD individuals. These group differences held not only for across-individual (inter-individual) analyses between brain regions, but also for within-individual (intra-individual) analyses of the relation across the time series between pairs of regions and successful inhibition of memory retrieval.
Third, within the ADHD group, greater symptomatology predicted a reduced correlation in activity between prefrontal and posterior regions. Greater inattentive symptomatology was related to a reduced correlation in activity between the rMFG and hippocampus, whereas greater hyperactive symptomatology predicted a reduction in the correlation in activity between rIFG and FG. The latter finding is not surprising in view of the relation of rIFG activation and motor activity generally. Importantly, increasing levels of both types of symptomatology related significantly to poorer inhibition over emotional memory retrieval. The importance of these interrelations lies in the fact that both inattentive and hyperactive symptomatology in ADHD have also been interpreted as due to reduced inhibition (Barkley, 1994), and so the intercorrelation of symptom severity, reduced inhibition over emotional memory retrieval, and motor disinhibition, suggest a generalized inhibitory deficit across several domains in individuals with ADHD.
Fourth, inhibitory influence over motor responses, as measured by the SSRT task, was significantly related in ADHD individuals to both (i) the inability to inhibit memory retrieval, and (ii) reduced activation of rLPFC regions and their correlated activity with posterior brain regions, which is consistent with previous research (Rubia et al., 1999; Rubia et al., 2005). However, longer SSRT was related mainly to less correlated activity between the rIFG and Pul rather than correlated activity between the rMFG and Hip/Amy, which appears to be more important for inhibiting emotional memory retrieval. This pattern is consistent with other studies that have found rIFG activation to play a critical role in motor response inhibition (Aron et al., 2007; Aron & Poldrack, 2006). Thus, activity in rIFG and its correlates show a relation with inhibition in the stimulus-response domain, whereas, activity in rMFG and its correlates show a relation with inhibition in cognitive domains (e.g., memory retrieval).
These findings suggest that a strong relation between the two domains of inhibition (motor and cognitive) exists, and that the possibility that each of these domains may rely on somewhat separable prefrontal regions (rIFG vs. rMFG, respectively). A significant amount of research supports the possibility of such a dorsal/ventral division within the LPFC (Sakagami & Wantanabe, 2007; Badre & D’esposito, 2007; Morris et al., 1999). Dorsal areas of LPFC, particularly areas 9/46 (including MFG) have been shown to modulate cognitive functions that support top-down cognitive control (Sakagami & Wantanabe, 2007). Anatomically, areas 9/46 connect to hippocampal and parahippocampal regions through the fornix and retrosplenial cortex (Morris et al., 1999; Petrides et al., 2007) and are notable in doing so, as such connections do not exist for more ventral prefrontal regions that modulate motor processes. In contrast, ventral areas of LPFC, particularly BA areas 44, 45, 47 (which incorporate IFG), have been shown to modulate stimulus representation, including the selection, judgment, and categorization of such stimuli (Sakagami & Wantanabe, 2007; Morris et al., 1999), through anatomical connections to the inferior temporal lobe; and as discussed above, motor response modulation. This dorsal/ventral division of the LPFC also appears to be reflected in our data.
The current data also demonstrate how a translational approach in which a model derived from neurologically-intact individuals can be applied to a relevant clinical population to inform both our understanding of the organization of the neurologically-normal brain as well as that of the clinical population. The selective disruption of activity in rMFG in ADHD individuals, as well as the lack of correlation in this area with activity in the hippocampus, supports our previous suggestion that these regions represent critical circuitry underlying the inhibition of emotional memory retrieval. Furthermore, our results suggest that inhibitory deficits in individuals with ADHD are not limited to the domain of motor control, but may also apply to internal representations of information (e.g., memories), which may have implications regarding the control of attention/memory and academic achievement.
A major issue that is not clear from the current data is the specificity of dysfunction of rLPFC, and especially area 9/46, in individuals with ADHD, provided we looked at inhibitory tasks only. One possibility is that dysfunction of rLPFC affects inhibitory processes, per se, across multiple domains. Alternatively, rLPFC dysfunction might extend more generally to neural mechanisms involved in cognition that do not only necessarily involve inhibitory modulation. Evidence that rLPFC is dysfunctional in ADHD individuals is found in the literature in specific tasks requiring inhibitory control (Boothe et al., 2005; Rubia et al., 1999), as well as, cognitive control more generally (Valera et al., 2005; Epstein et al., 2007; Smith et al., 2008; Durston et al., 2007; Stevens et al., 2006; Schneider et al., 2006). Further research will need to compare performance and brain activity (especially of rLFPC) for tasks carefully matched in demand, but which vary in the requirement of inhibition (see Hampshire et al., 2010 regarding rIFG).
Although the current study has provided important insights on inhibitory control mechanisms in the brain, the limitations of the data should be noted. It is important to note that some of our analyses examining the effects of NT and T conditions compared to a fixation baseline need to be interpreted with a word of caution. Any contrast comparing a task condition (NT) to an unconstrained baseline may contain an element of uncertainty because of the lack of information about the cognitive processes a participant is engaged in at the time of the fixation baseline. Therefore, our analyses of NT and T conditions versus a fixation baseline which are imperative to understanding the relative activation/deactivation of certain brain regions (e.g., hippocampus), need to be cautiously interpreted until future studies include a more constrained baseline, in which causes of relative activation/deactivation of these brain regions can be better ascribed. Similarly, one of the most important group differences we report is the reduction of activity in the lateral prefrontal cortex for ADHD compared to control individuals for the contrast of NT-baseline activity. Because of the limitations of the fMRI technique, we must acknowledge the fact that baseline differences between the two groups may exist. However, other aspects of our data suggest that these group differences are indeed driven by group differences in NT activity and not baseline activity because no group differences were observed for activation of T trials. If the baseline differed between groups, we would have been likely to show group differences on T trials as well. Third, the ROI for rMFG was established from the overall combined group maximal peak activation. Because the ADHD individuals did not significantly activate this region, it must be noted that correlations within the ADHD individuals between this region and others are likely to produce null results. However, the ADHD individuals still exhibited enough variance within rMFG to produce significant paired correlations with the hippocampus and symptomatology, as well as a trend with SSRT, indicating these correlations are still important for establishing and testing the previous model we have outlined. Fourth, all participants were currently prescribed medication. While they withdrew from stimulant medication prior to scanning, the long-term effects of stimulants on brain chemistry/anatomy, as well as withdrawal effect are not well known. Fifth, our sample consisted of mixed gender individuals, while gender did not correlated with any functional activity, this null result could be due to low power to detect such correlations. Lastly, because our control population was collected under a different protocol, we did not collect SSRT data from them. Thus, the results of the SSRT task can only be associated to ADHD individuals and not the control sample.
In sum, ADHD individuals’ exhibit reduced activity in the rLPFC when inhibiting emotional memory retrieval, which was also reflected behaviorally. Furthermore, the negative correlation of activity of this prefrontal with posterior regions involved in memory processing observed in control individuals was reduced in the ADHD individuals. Significant correlations between ADHD symptom severity and behavioral measures of inhibition in both the memory and motor domain with decreased activity of areas of rLPFC, suggests that this region may support inhibitory control across multiple domains. Furthermore, this inhibitory control may possibly involve a dorsal/ventral division within the rLPFC, with more dorsal regions supporting inhibitory control over cognitive processes, while more ventral regions support inhibitory control over stimulus-response processing. Whether the reduced activity of rLPFC in individuals with ADHD affects only inhibitory processes or also influences a wider range of functions involved in cognitive control requires further research.
Supplementary Material
Fig. 1.
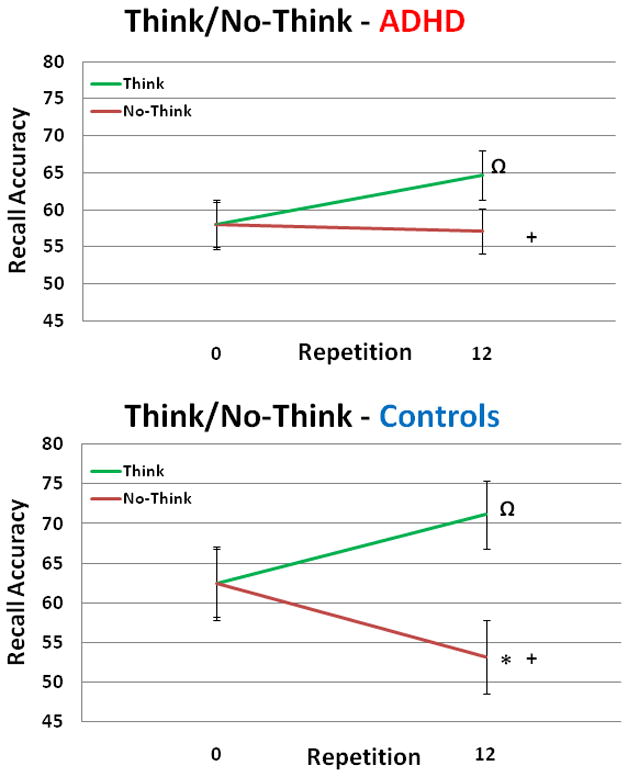
Behavioral results from both ADHD and control individuals. Ω,* indicates statistically significant within group comparisons (p<.10, p<.05), while + indicates statistically significant between group comparisons (p<.05). ADHD individuals’ baseline recall = 58%, Think trials = 64.7% and No-Think trials = 57.1%. For Control individuals, baseline recall = 62.5%, Think trials = 71.1% and No-Think trials = 53.2%. Errors bars represent standard error.
Fig. 2.
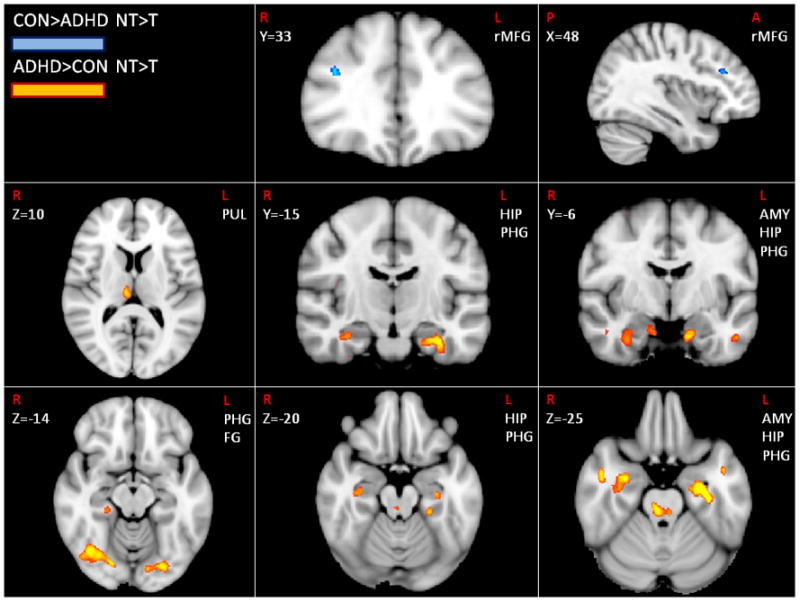
Brain regions showing group differences during NT>T trials. Orange indicates greater activity in ADHD individuals, while blue identifies greater activity in control individuals (Z>2.81, p<.005, cluster threshold of p<.05 except for hippocampus, amygdala and thalamus).
Acknowledgments
NIMH grant R01 MH 070037 (Banich, P.I.) provided support for data collection and analysis as well as salary support for all but the fourth authors. Appreciation is given to Bruce Pennington for insights into the disorder and also given to Deb Singel and Yiping Du for MR technical issues. We would also like to thank the anonymous reviewers for their careful attention and comments regarding the manuscript.
Footnotes
Financial Disclosures
All authors acknowledge that there are no competing conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brendan E. Depue, Email: depue@colorado.edu.
Gregory C. Burgess, Email: greg.burgess@colorado.edu.
Erik G. Willcutt, Email: erik.willcutt@colorado.edu.
Luka Ruzic, Email: anotherluka@gmail.com.
Marie T. Banich, Email: marie.banich@colorado.edu.
References
- 1.Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MC, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MC, Levy BJ. Suppressing unwanted memories. Current Directions in Psychological Science. 2009;18:189–194. doi: 10.1177/0963721417689881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aron AR, Poldrack RA. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Badre D, D’esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 8.Banich MT, Burgess GC, Depue BE, Ruzic L, Bidwell LC, Hitt-Laustsen S, Willcutt EG. The Neural Basis of Sustained and Transient Attentional Control in YoungAdults with ADHD. Neuropsychologia. 2009;47:3095–3104. doi: 10.1016/j.neuropsychologia.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkley RA. Impaired delayed responding: A unified theory of attention-deficit hyperactivity disorder. In: Routh Donald K., editor. Disruptive behavior disorders in childhood. New York, NY, US: Plenum Press; 1994. [Google Scholar]
- 10.Barkley RA, Murphy K. Attention-deficit hyperactivity disorder: A clinical workbook. 2. New York: Guilford Press; 1998. [Google Scholar]
- 11.Booth JR, et al. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 12.Borger N, Van der Meere J. Motor control and state regulation in children with ADHD: A cardiac response study. Biological Psychology. Special Issue: Error processing and adaptive responding. 2000;51:247–267. doi: 10.1016/s0301-0511(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 13.Casey BJ, Castellanos FX, Giedd JN, Marsh WL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain SR, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biological Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Depue BA, Banich MT, Burgess GC, Bidwell LC, Willcutt EG. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Research: Neuroimaging. 2010;182:231–237. doi: 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Depue BE, Banich MT, Curran T. Suppression of emotional and nonemotional content in memory. Psychological Science. 2006;17:441–447. doi: 10.1111/j.1467-9280.2006.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- 19.DuPaul JG, Anastopoulos AD, Power TJ, Reid R, Ikeda MJ, McGoey KE. Parent Ratings of Attention-Deficit/Hyperactivity Disorder Symptoms: Factor Structure and Normative Data. Journal of Psychopathology and Behavioral Assessment. 1998;20:83–102. [Google Scholar]
- 20.Durston S, et al. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 21.Epstein JN, et al. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. Journal of Child Psychology and Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 22.Fassbender C, Simones-Franklin C, Murphy K, Hester R, Meaney J, Robertson IH, Gravan H. The role of a right fronto-parietal network in cognitive control: Common activations for “cues-to-attend” and response inhibition. Journal of Psychophysiology. 2006;20:286–296. [Google Scholar]
- 23.Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Research. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Garavan H, Ross TJ, Stein EA. Right hemisphere dominance for inhibitory control: An Event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez R. Underlying processes in the poor response inhibition of children with attention-deficit/hyperactivity disorder. Journal of Attention Disorders. 2003;6:111–122. doi: 10.1177/108705470300600303. [DOI] [PubMed] [Google Scholar]
- 26.Hanslmayr S, Leipold P, Pastotter B, Bauml KH. Anticipatory signatures of voluntary memory suppression. Journal of Neuroscience. 2009;29:2742–2747. doi: 10.1523/JNEUROSCI.4703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: Evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Koshino H, Carpenter PA, Minshew NJ, Cherkasky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-funcitoning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Lang PJ, Bradley MM, Cuthbert BN. The International Affective Picture System (IAPS) Gainesville: University of Florida, Center for Research in Psychophysiology; 1995. [Google Scholar]
- 30.Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd G, et al. DSM IV field trials for attention deficit/hyperactivity disorder in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- 31.Levy BJ, Anderson MC. Individual differences in the suppression of unwanted memories: the executive deficit hypothesis. Acta Psychologia. 2008;127:623–635. doi: 10.1016/j.actpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Logan GD, Cowan WB. On the ability to inhibit thought and action a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 33.Mecklinger A, Parra M, Waldhauser GT. ERP correlates of intentional forgetting. Brain Research. 2009;1255:132–147. doi: 10.1016/j.brainres.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 34.Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol. 1999;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Nichols SL, Waschbusch DA. A review of the validity of laboratory tasks used to assess symptoms of ADHD. Child Psychiatry Hum Dev. 2004;34:297–315. doi: 10.1023/B:CHUD.0000020681.06865.97. [DOI] [PubMed] [Google Scholar]
- 36.Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: Replication with DSM-IV combined type, extension, and qualification. Journal of Abnormal Child Psychology. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- 37.Nolan EE, Volpe RJ, Gadow KD, Sprafkin J. Developmental, gender, and comorbidity differences in clinically referred children with ADHD. Journal of Emotional and Behavioral Disorders. 1999;7:11–20. [Google Scholar]
- 38.Nolan EE, Gadow KD, Sprafkin J. Teacher reports of DSM-IV ADHD, ODD, and CD symptoms in school children. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:241–249. doi: 10.1097/00004583-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: A meta-analysis of studies with the stop task. Journal of Child Psychology and Psychiatry. 1998;39:411–425. [PubMed] [Google Scholar]
- 40.Oosterlaan J, Sergeant JA. Inhibition in ADHD, aggressive, and anxious children: A biologically based model of child psychopathology. Journal of Abnormal Child Psychology. 1996;24:19–36. doi: 10.1007/BF01448371. [DOI] [PubMed] [Google Scholar]
- 41.Paz-Alonso PM, Ghetti S, Matlen BJ, Anderson MC, Bunge SA. Memory suppression is an active process that improves over childhood. Frontiers in Human Neuroscience. 2009;3:24. doi: 10.3389/neuro.09.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural Substrates for Voluntary Suppression of Negative Affect: A Functional Magnetic Resonance Imaging Study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 44.Rubia K, Overmeyer S, Taylor E, Brammer MJ, Williams SCR, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 45.Rubia K, Smith AB, Brammer MJ, Tonne B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 46.Sakagami M, Wantanabe M. Annals of the New York Academy of Sciences. Malden, MA, US: Blackwell Publishing; 2007. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Reward and decision making in corticobasal ganglia networks. [DOI] [PubMed] [Google Scholar]
- 47.Schneider M, Retz W, Coogan A, Thome J, Rosler M. European Archives of Psychiatry and Clinical Neuroscience. Supplement 1. Vol. 256. 2006. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD) A neurological view; pp. I/32–I/4I. [DOI] [PubMed] [Google Scholar]
- 48.Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neurosci Biobehav Rev. 2003:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Smith AB, Taylor E, Brammer M, Halari R, Rubia K. Reduced activation in right lateral prefrontal cortex and anterior cingulate gyrus in medication-naive adolescents with attention deficit hyperactivity disorder during time discrimination. Journal of Child Psychology and Psychiatry. 2008;49:977–985. doi: 10.1111/j.1469-7610.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 50.Stevens MC, Pearlson GD, Kiehl KA. An fMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. American Journal of Psychiatry. 2008;164:1737–1749. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- 51.Tamm L, Menon V, Ringel J, Reiss AL. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 52.Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional Neuroanatomy of Working Memory in Adults with Attention- Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 53.Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 54.White HA, Marks W. Updating memory in list-method directed forgetting: Individual differences related to Adult Attention-Deficit/Hyperactivity Disorder. Personality and Individual Differences. 2004;37:1453–1462. [Google Scholar]
- 55.Woodcock R, McGrew K, Mather N. Woodcock-Johnson III. Itasca, IL: Riverside Publishing Company; 2001. [Google Scholar]
- 56.Yamaguchi S, Zheng D, Oka T, Bokura H. The key locus of common response inhibition network for no-go and stop signals. Journal of Cognitive Neuroscience. 2008;20:1434–1442. doi: 10.1162/jocn.2008.20100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


