Abstract
Genetically distinct hantaviruses have been identified recently in multiple species of shrews (Order Soricomorpha, Family Soricidae) in Eurasia and North America. To corroborate decades-old reports of hantaviral antigens in shrews from Russia, archival liver and lung tissues from 4 Siberian large-toothed shrews (Sorex daphaenodon), 5 Eurasian least shrews (Sorex minutissimus), 12 flat-skulled shrews (Sorex roboratus), and 18 tundra shrews (Sorex tundrensis), captured in the Sakha Republic in northeastern Siberia during July and August 2006, were analyzed for hantavirus RNA by reverse transcription–polymerase chain reaction. A novel hantavirus, named Kenkeme virus, was detected in a flat-skulled shrew. Sequence analysis of the full-length S and partial M and L segments indicated that Kenkeme virus was genetically and phylogenetically distinct from Seewis virus harbored by the Eurasian common shrew (Sorex araneus), as well as all other rodent-, soricid-, and talpid-borne hantaviruses.
Key Words: Hantavirus, PCR, Zoonosis
Hantaviruses (Family Bunyaviridae), the causative agents of hemorrhagic fever with renal syndrome in Eurasia (Yanagihara and Gajdusek 1988) and hantavirus cardiopulmonary syndrome in the Americas (Nichol et al. 1993), have long been known to share long-standing virus–host relationships with specific rodent species (Order Rodentia, Families Muridae and Cricetidae). Previously, the only known exception to the hantavirus-rodent association was Thottapalayam virus (TPMV), isolated from an Asian house shrew (Suncus murinus) captured in India (Carey et al. 1971, Zeller et al. 1989). Recent studies indicate that multiple other species of shrews (Order Soricomorpha, Family Soricidae) from widely separated geographic regions harbor hantaviruses that are far more genetically diverse than hantaviruses carried by rodents, suggesting an ancient coevolutionary and/or adaptive relationship.
Newly identified shrew-borne hantaviruses include Tanganya virus in the Therese's shrew (Crocidura theresae) (Klempa et al. 2007), Camp Ripley virus (RPLV) in the northern short-tailed shrew (Blarina brevicauda) (Arai et al. 2007), Cao Bang virus (CBNV) in the Chinese mole shrew (Anourosorex squamipes) (Song et al. 2007b), Seewis virus (SWSV) in the Eurasian common shrew (Sorex araneus) (Song et al. 2007a), Ash River virus (ARRV) in the masked shrew (Sorex cinereus) (Arai et al. 2008a), Jemez Springs virus (JMSV) in the dusky shrew (Sorex monticolus) (Arai et al. 2008a), and Imjin virus (MJNV) in the Ussuri white-toothed shrew (Crocidura lasiura) (Song et al. 2009). In addition, phylogenetically distinct hantaviruses have been identified in moles (Family Talpidae), including Asama virus in the Japanese shrew mole (Urotrichus talpoides) (Arai et al. 2008b), Oxbow virus in the American shrew mole (Neurotrichus gibbsii) (Kang et al. 2009b), and Nova virus in the European common mole (Talpa europaea) (Kang et al. 2009c).
In an attempt to corroborate decades-old reports of hantaviral antigens in shrews from Russia (Gavrilovskaya et al. 1983, Tkachenko et al. 1983, Miasnikov et al. 1992), archival liver or lung tissues from 4 Siberian large-toothed shrews (Sorex daphaenodon), 5 Eurasian least shrews (Sorex minutissimus), 12 flat-skulled shrews (Sorex roboratus), and 18 tundra shrews (Sorex tundrensis), collected as part of the Beringian Coevolution Project in the Sakha Republic in northeastern Siberia (Fig. 1) during July and August 2006, were analyzed for hantavirus RNA by reverse transcription–polymerase chain reaction (PCR), using primers designed from highly conserved regions of hantavirus genomes (Arai et al. 2008a, Kang et al. 2009b, 2009c). cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen, San Diego, CA) and an oligonucleotide primer (OSM55, 5′-TAGTAGTAGACTCC-3′) designed from the conserved 3′-end of the S, M, and L segments of hantaviruses. First- and second-round PCRs were performed in 20 μL reaction mixtures containing 250 μM dNTP, 2 mM MgCl2, 1 U AmpliTaq polymerase (Roche, Basel, Switzerland), and 0.25 μM of each primer. Initial denaturation at 94°C for 5 min was followed by two cycles each of denaturation at 94°C for 40 s, two-degree step-down annealing from 48°C to 38°C for 40 s, and elongation at 72°C for 1 min, then 32 cycles of denaturation at 94°C for 40 s, annealing at 42°C for 40 s, and elongation at 72°C for 1 min, in a GeneAmp PCR 9700 thermal cycler (Perkin-Elmer, Waltham, MA). Amplicons were separated by electrophoresis on 1.5% agarose gels and purified using the QIAQuick Gel Extraction Kit (Qiagen, Hilden, Germany). DNA was sequenced directly using an ABI Prism 377XL Genetic Analyzer (Applied Biosystems, Foster City, CA). Several amplicons from the same region were sequenced and sequences were cleaned using the Lasergene program version 5 (DNASTAR, Madison, WI).
FIG. 1.
Map of Russia showing the Sakha Republic in Eastern Siberia, where trapping of shrews was conducted as part of the Beringian Coevolution Project during July and August 2006.
All but one sample was negative for hantavirus RNA. The single exception, designated Kenkeme virus (KKMV) strain MSB148794, was a hantavirus detected in a flat-skulled shrew, captured near the Kenkeme River, 40 km west of Yakutsk (62.07003°, 128.93831°). The taxonomic identity of this shrew was confirmed by analysis of the entire 1140-bp cytochrome b mtDNA sequence.
The full-length 1640-nucleotide S-genomic segment of KKMV MSB148794 contained a single open reading frame, encoding a predicted nucleocapsid (N) protein of 429 amino acids (nucleotide positions 48 to 1337), and 47- and 303-nucleotide 3′- and 5′-noncoding regions, respectively. The predicted secondary structure of the KKMV N protein, as determined by various software available in the NPS@ structure server (www.bioinf.manchester.ac.uk/dbbrowser/bioactivity/NPS2.html), most closely resembled that of SWSV strain mp70 and was composed of 44.7% α-helices and 9.1% β-sheets, with the characteristic coiled-coil domain in the amino-terminal region (residues 1–70).
Pairwise alignment and comparison of the full-length KKMV S segment, using ClustalW, exhibited uniformly low nucleotide sequence similarity to representative rodent-associated hantaviruses, ranging from 61.6% for Hantaan virus strain 76–118 and 60.2% for Dobrava virus strain Greece, and 59.3–60.9% for hantaviruses harbored by arvicolid and neotomine/sigmodontine rodents (Table 1). At the amino acid level, the KKMV N protein sequence was most similar to but distinct from that of SWSV, differing by 16.3%. Otherwise, the KKMV N protein differed by 38.1–41.9% and 30.5–55.1%, respectively, from representative rodent- and soricomorph-associated hantaviruses, supporting the notion that it constitutes a distinct hantavirus species, based on criteria set forth by the International Committee for Taxonomy of Viruses (Fauquet et al. 2005) and a more recent exhaustive analysis of hantavirus genomes (Maes et al. 2009).
Table 1.
Sequence Similarities (%) of the Coding Regions of the Full-Length S and Partial M and L Segments of Kenkeme Virus Strain MSB148794 and Representative Rodent- and Soricomorph-Borne Hantaviruses
| |
S segment |
M segment |
L segment |
|||
|---|---|---|---|---|---|---|
| Hantavirus strain | 1290 nt | 429 aa | 1002 nt | 334 aa | 4295 nt | 1431 aa |
| HTNV 76–118 | 61.6 | 61.9 | 67.8 | 67.7 | 69.8 | 75.4 |
| SEOV 80–39 | 61.0 | 59.3 | 66.7 | 65.6 | 69.1 | 74.8 |
| SOOV SOO-1 | 62.2 | 61.4 | 67.4 | 65.9 | 69.5 | 75.5 |
| DOBV Greece | 60.2 | 59.6 | 67.1 | 66.5 | 69.1 | 75.3 |
| PUUV Sotkamo | 60.9 | 59.8 | 60.9 | 54.8 | 65.8 | 69.5 |
| TULV 5302v | 60.9 | 58.2 | 59.3 | 51.5 | 65.5 | 68.8 |
| PHV PH-1 | 59.4 | 58.2 | 58.8 | 49.7 | 65.1 | 68.8 |
| SNV NMH10 | 60.3 | 58.1 | 60.0 | 54.5 | 66.5 | 69.5 |
| ANDV Chile9717869 | 59.3 | 58.8 | 58.4 | 53.6 | 65.9 | 68.6 |
| ARRV MSB73418 | 64.8 | 68.1 | — | — | 73.3 | 83.4 |
| CBNV CBN-3 | 63.4 | 67.5 | 70.8 | 76.3 | 74.9 | 84.8 |
| JMSV MSB144475 | 67.5 | 69.0 | 74.4 | 78.7 | 74.8 | 85.2 |
| SWSV mp70 | 74.9 | 83.7 | — | — | 77.3 | 90.9 |
| RPLV MSB89863 | 58.1 | 45.8 | 66.2 | 68.0 | 71.2 | 78.0 |
| MJNV Cl05-11 | 53.3 | 44.9 | 56.6 | 48.6 | 62.4 | 61.9 |
| TPMV VRC66412 | 52.7 | 45.4 | 54.9 | 47.7 | 61.4 | 61.2 |
| ASAV N10 | 66.4 | 69.5 | 75.0 | 78.4 | 75.1 | 84.3 |
| OXBV Ng1453 | 65.0 | 67.5 | 66.5 | 66.2 | 71.3 | 79.3 |
| NVAV MSB95703 | 54.8 | 48.9 | — | — | 62.4 | 62.3 |
ANDV, Andes virus; ARRV, Ash River virus; ASAV, Asama virus; CBNV, Cao Bang virus; DOBV, Dobrava virus; HTNV, Hantaan virus; JMSV, Jemez Spring virus; KKMV, Kenkeme virus; MJNV, Imjin virus; NVAV, Nova virus; OXBV, Oxbow virus; PHV, Prospect Hill virus; PUUV, Puumala virus; RPLV, Camp Ripley virus; SEOV, Seoul virus; SNV, Sin Nombre virus; SOOV, Soochong virus; SWSV, Seewis virus; TPMV, Thottapalayam virus; TULV, Tula virus; nt, nucleotides; aa, amino acids.
Similarly, low but wider degrees of sequence homology, ranging from 54.9% to 74.4% and 61.4% to 77.3%, respectively, were found in comparing the partial 1002-nucleotide M and 4295-nucleotide L segments of KKMV with other shrew-borne hantaviruses, including ARRV, CBNV, JMSV, SWSV, RPLV, MJNV, and TPMV (Table 1). Differences in the deduced amino acid sequences of the envelope glycoprotein and viral RNA-dependent RNA polymerase also supported KKMV MSB148794 as being a genetically distinct hantavirus.
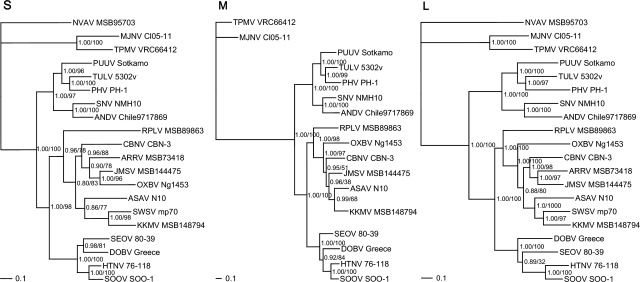
Phylogenetic trees, based on the S-, M-, and L-segment sequences, generated by maximum likelihood and Bayesian methods, implemented in PAUP* (Phylogenetic Analysis Using Parsimony, 4.0b10) (Swofford 2003), RAxML Blackbox webserver (Stamatakis et al. 2008), and MrBayes 3.1 (Ronquist et al. 2003), under the best-fit GTR+I+Γ model of evolution established using jModeltest 0.1.1 (Posada 2008), revealed similar topologies, which were well supported by bootstrap analysis (Fig. 2). KKMV and SWSV occupied separate lineages, in keeping with the phylogenetic relationship between their respective soricid reservoir hosts, S. roboratus and S. araneus. That is, S. roboratus, an oligochaete-eating soricine shrew usually residing in flood-plain habitats, belongs to the Sorex caecutiens group, an evolutionary clade of Palearctic shrews, which is distinct from the S. araneus group.
FIG. 2.
Phylogenetic trees generated by the Bayesian method, under the best-fit GTR+I+Γ model of evolution, based on the full-length S and partial M and L genomic segments of Kenkeme virus (KKMV MSB148794) and other selected hantaviruses. The phylogenetic positions of KKMV are shown in relationship to Cao Bang virus (CBNV CBN-3; EF543524, EF543526, EF543525) from the Chinese mole shrew (Anourosorex squamipes), Camp Ripley virus (RPLV MSB89863; FJ790772, EF540774, EF540771) from the northern short-tailed shrew (Blarina brevicauda), Seewis virus (SWSV mp70; EF636024, EF636026) from the Eurasian common shrew (Sorex araneus), Ash River virus (ARRV MSB73418; EF650086, EF619961) from the masked shrew (Sorex cinereus), Jemez Spring virus (JMSV MSB144475; FJ593499, FJ593500, FJ593501) from the dusky shrew (Sorex monticolus), Imjin virus (MJNV Cl05-11; EF641804, EF641798, EF641806) from the Ussuri white-toothed shrew (Crocidura lasiura), Thottapalayam virus (TPMV VRC66412; AY526097, EU001329, EU001330) from the Asian house shrew (Suncus murinus), Asama virus (ASAV N10; EU929072, EU929075, EU929078) from the Japanese shrew mole (Urotrichus talpoides), Oxbow virus (OXBV Ng1453; FJ539166, FJ539167, FJ593497) from the American shrew mole (Neurotrichus gibbsii), and Nova virus (NVAV MSB95703; FJ539168, FJ593498) from the European common mole (Talpa europaea). Also shown are representative rodent-borne hantaviruses, including Hantaan virus (HTNV 76–118; NC_005218, Y00386, NC_005222), Soochong virus (SOOV SOO-1; AY675349, AY675353, DQ056292), Dobrava virus (DOBV Greece; NC_005233, NC_005234, NC_005235), Seoul virus (SEOV 80-39; NC_005236, NC_005237, NC_005238), Tula virus (TULV 5302v; NC_005227, NC_005228, NC_005226), Puumala virus (PUUV Sotkamo; NC_005224, NC_005223, NC_005225), Prospect Hill virus (PHV PH-1; Z49098, X55129, EF646763), Sin Nombre virus (SNV NMH10; NC_005216, NC_005215, NC_005217), and Andes virus (ANDV Chile9717869; NC_003466, NC_003467, NC_003468). GenBank accession numbers are GQ306148, GQ306149, and GQ306150 for the S, M, and L segments of KKMV MSB148794, respectively. SWSV, ARRV, and NVAV are not shown in the M-segment tree because sequences were unavailable for analysis. The numbers at each node are posterior node probabilities (left of slash) based on 30,000 trees, and maximum-likelihood (ML) bootstrap values (right of slash) based on 1000 bootstrap replicates. Bayesian analysis consisted of two replicate Markov Chain Monte Carlo runs of four chains of 2 million generations, each sampled every 100 generations with a burn-in of 5000 (25%). The scale bar indicates nucleotide substitutions per site.
SWSV, originally identified in the Eurasian common shrew from Switzerland (Song et al. 2007a), has recently been detected in this shrew species in Finland and Hungary (Kang et al. 2009a) and in Germany and Austria (R. Dürrwald and N. Nowotny, personal communication), as well as in Western and Eastern Siberia (Yashina et al. 2009). In addition, SWSV genetic variants have been found in the tundra shrew and Siberian large-toothed shrew in Siberia (Yashina et al. 2009). To our knowledge, however, KKMV represents the first evidence of a genetically distinct hantavirus in a previously unrecognized soricid species from Russia. Nevertheless, in-depth studies are warranted to establish if the flat-skulled shrew is truly the principal reservoir host of KKMV, because S. roboratus is sympatric or parapatric with 12 other Sorex species throughout its geographic range in western and central Russia and Mongolia (Sheftel 2005) and so opportunities exist for cross-species transmission. Investigations to ascertain if genetic variants of KKMV circulate in such sympatric shrew species are now underway.
The discovery of KKMV adds to the rich diversity of recently identified hantaviruses in shrews and lends support to the emerging paradigm-shifting concept that ancestral soricomorphs, rather than rodents, may have served as the early mammalian hosts of primordial hantaviruses. That is, in keeping with all other present-day members of the Bunyaviridae, insects or arthropods may have originally served as vectors of ancient hantaviruses, which became established in soricomorphs. When viewed within this hypothetical context, the evolutionary history of hantaviruses is far more ancient and complex than previously imagined. Although host switching with subsequent host-specific adaptations has occurred during hantavirus evolution (Vapalahti et al. 1999, Nemirov et al. 2002), these events alone do not satisfactorily explain the coexistence and distribution of genetically distinct hantaviruses among species in two taxonomic orders of small mammals spanning four continents (Arai et al. 2008b, Kang et al. 2009b, 2009c).
Many rodent-borne hantaviruses in Eurasia and the Americas appear to be nonpathogenic. Similarly, none of the recently identified shrew-borne hantaviruses has hitherto been shown to cause diseases in humans. However, serological evidence of TPMV infection has recently been reported in a febrile patient (Okumura et al. 2007), suggesting that human infection with other soricid-associated hantaviruses may be possible. Intensive efforts are currently underway in search of shrew-borne hantavirus infection in patients with febrile illnesses and other ill-defined syndromes. Also, as flat-skulled shrews inhabit taiga and meadows in tundra habitats, individuals, particularly mammalogists, forestry workers, and hunters, who venture into such ecotopes, may be at increased risk of exposure to KKMV.
Acknowledgments
This work was supported in part by the U.S. Public Service grants R01AI075057 from the National Institute of Allergy and Infectious Diseases, and P20RR018727 (Centers of Biomedical Research Excellence) and G12RR003061 (Research Centers in Minority Institutions) from the National Center for Research Resources, National Institutes of Health. Shrew samples were collected through grants DEB0196095 and DEB9981915 from the National Science Foundation.
Disclosure Statement
All authors have no competing financial interests. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Arai S. Song J-W. Sumibcay L. Bennett SN, et al. Hantavirus in northern short-tailed shrew, United States. Emerg Infect Dis. 2007;13:1420–1423. doi: 10.3201/eid1309.070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S. Bennett SN. Sumibcay L. Cook JA, et al. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am J Trop Med Hyg. 2008a;78:348–351. [PMC free article] [PubMed] [Google Scholar]
- Arai S. Ohdachi SD. Asakawa M. Kang HJ, et al. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides) Proc Natl Acad Sci USA. 2008b;105:16296–16301. doi: 10.1073/pnas.0808942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DE. Reuben R. Panicker KN. Shope RE, et al. Thottapalayam virus: a presumptive arbovirus isolated from a shrew in India. Indian J Med Res. 1971;59:1758–1760. [PubMed] [Google Scholar]
- Fauquet CM. Mayo MA. Maniloff J. Desselberger U, et al. Virus Taxonomy. Classification and Nomenclature of Viruses. Eighth Report of the International Committee on the Taxonomy of Viruses. London: Elsevier Academic Press; 2005. pp. 704–707. [Google Scholar]
- Gavrilovskaya IN. Apekina NS. Miasnikov YA. Bernshtein AD, et al. Features of circulation of hemorrhagic fever with renal syndrome (HFRS) virus among small mammals in the European U.S.S.R. Arch Virol. 1983;75:313–316. doi: 10.1007/BF01314898. [DOI] [PubMed] [Google Scholar]
- Kang HJ. Arai S. Hope AG. Song J-W, et al. Genetic diversity and phylogeography of Seewis virus in the Eurasian common shrew in Finland and Hungary. Virol J. 2009a;6:208. doi: 10.1186/1743-422X-6-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ. Bennett SN. Dizney L. Sumibcay L, et al. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii) Virology. 2009b;388:8–14. doi: 10.1016/j.virol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ. Bennett SN. Sumibcay L. Arai S, et al. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea) PLoS ONE. 2009c;4:e6149. doi: 10.1371/journal.pone.0006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempa B. Fichet-Calvet E. Lecompte E. Auste B, et al. Novel hantavirus sequences in shrew, Guinea. Emerg Infect Dis. 2007;13:520–552. doi: 10.3201/eid1303.061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes P. Klempa B. Clement J. Matthijnssens J, et al. A proposal for new criteria for the classification of hantaviruses, based on S and M segment protein sequences. Infect Genet Evol. 2009;9:813–820. doi: 10.1016/j.meegid.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Miasnikov YA. Apekina NS. Zuevskii AP. Khitrin AV, et al. The disposition of natural foci of haemorrhagic fever with renal syndrome in different landscape areas of Tiumen Province. Vopr Virusol. 1992;37:161–165. [PubMed] [Google Scholar]
- Nemirov K. Henttonen H. Vaheri A. Plyusnin A. Phylogenetic evidence for host switching in the evolution of hantaviruses carried by Apodemus mice. Virus Res. 2002;90:207–215. doi: 10.1016/s0168-1702(02)00179-x. [DOI] [PubMed] [Google Scholar]
- Nichol ST. Spiropoulou CF. Morzunov S. Rollin PE, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Okumura M. Yoshimatsu K. Kumperasart S. Nakamura I, et al. Development of serological assays for Thottapalayam virus, an insectivore-borne hantavirus. Clin Vaccine Immunol. 2007;14:173–181. doi: 10.1128/CVI.00347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Ronquist F. Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sheftel BL. Advances in the Biology of Shrews II. Vol. 1. Special Publication of the International Society of Shrew Biologists 2005; New York: Distribution of different size groups of red-toothed shrews (Sorex) in the Palearctic Region; pp. 167–178. [Google Scholar]
- Song J-W. Gu SH. Bennett SN. Arai S, et al. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus) Virol J. 2007a;4:114. doi: 10.1186/1743-422X-4-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W. Kang HJ. Song K-J. Truong TT, et al. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerg Infect Dis. 2007b;13:1784–1787. doi: 10.3201/eid1311.070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W. Kang HJ. Gu SH. Moon SS, et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura) J Virol. 2009;83:6184–6191. doi: 10.1128/JVI.00371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. Hoover P. Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, MA: Sinauer Associates; 2003. [Google Scholar]
- Tkachenko EA. Ivanov AP. Donets MA. Miasnikov YA, et al. Potential reservoir and vectors of haemorrhagic fever with renal syndrome (HFRS) in the U.S.S.R. Ann Soc Belg Med Trop. 1983;63:267–269. [PubMed] [Google Scholar]
- Vapalahti O. Lundkvist A. Fedorov V. Conroy CJ, et al. Isolation and characterization of a hantavirus from Lemmus sibiricus: evidence for host switch during hantavirus evolution. J Virol. 1999;73:5586–5592. doi: 10.1128/jvi.73.7.5586-5592.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara R. Gajdusek DC. Hemorrhagic fever with renal syndrome: a historical perspective and review of recent advances. In: Gear JHS, editor. CRC Handbook of Viral and Rickettsial Hemorrhagic Fevers. Boca Raton, FL: CRC Press; 1988. pp. 151–188. [Google Scholar]
- Yashina L. Abramov S. Gutorov V. Dupal T, et al. Seewis virus: phylogeography of a shrew-borne hantavirus in Siberia, Russia. Vector Borne Zoonot Dis. 2010 doi: 10.1089/vbz.2009.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller HG. Karabatsos N. Calisher CH. Digoutte JP, et al. Electron microscopic and antigenic studies of uncharacterized viruses. II. Evidence suggesting the placement of viruses in the family Bunyaviridae. Arch Virol. 1989;108:211–227. doi: 10.1007/BF01310935. [DOI] [PubMed] [Google Scholar]