Abstract
Background
Management of type 1 diabetes could be significantly improved with the availability of computerized insulin algorithms for home use.
Methods
This was a 1-year open label randomized control trial involving 123 adult subjects with type 1 diabetes (hemoglobin A1c values 7.5–11%) assigned to either the insulin guidance software (ACCU-CHEK® [Roche, Indianapolis, IN] Advisor) for personal data assistant (experimental group) or the control group. The primary aim of the study was to see if subjects using insulin dosing advisor software will improve glucose control over 1 year. The principal end point was an improvement in A1c at 6 and 12 months by ≥0.4%.
Results
Baseline demographics were similar in the two groups. Mean A1c was 8.54 ± 0.11% in the control group and 8.42 ± 0.11% (P = 0.4265) in the experimental group. The mean A1c was significantly lower from 3 to 12 months in the experimental group (P < 0.02). A1c reduction of ≥0.6% was maintained at 12 months in the experimental group. Also, a significantly higher number of subjects achieved A1c <7.5% in the experimental group from 3 to 9 months. Within target range glycemia (70–150 mg/dL) was higher in the experimental group at 3–9 months without any change in insulin dose or weight. Above target range glycemia was lower in the experimental group throughout the study. Frequency of testing per day was higher in the experimental group. Nocturnal hypoglycemia was not different between groups; however, the experimental group experienced more severe hypoglycemic events.
Conclusions
This is the first report that shows improved glycemic control can be maintained over 12 months in patients with type 1 diabetes by using Advisor with no change in insulin dose and weight.
Introduction
Studies have shown that reducing hemoglobin A1c levels can delay and/or reduce the overall risk of micro- and macrovascular complications associated with both type 1 and type 2 diabetes.1–5 Implementation of intensive diabetes management is expensive, though there is a significant reduction in risk of long-term complications and cost.6,7 Although the benefits of optimal glucose control seem clear, the risk of severe hypoglycemia can be a barrier to achieving this goal.1,4,5 In fact, there is nearly a threefold increase in hypoglycemia with intensification of treatment in type 1 diabetes.1 This paradox has created a need for new technology that will facilitate optimal glucose control by recommending appropriate insulin doses while decreasing the risk of hypoglycemia.
Diabetes prevalence is rising, and there are more than 21 million people, with both type 1 and 2 diabetes (diagnosed and undiagnosed), in the United States.8–12 With a limited number of endocrinologists or diabetes specialists available in the United States, most clinical diabetes care is provided by primary care physicians.13 Tools to help patients adjust their insulin dose at home should help in improving their glucose control. Several technologies such as continuous glucose monitors (sensors) and glucometers (self-monitoring of blood glucose [SMBG]) are on the market and have been shown to help patients improve glucose excursions, reduce glucose variability, decrease time spent in hypoglycemia and hyperglycemia, and improve A1c levels.14–18 Other software available on insulin pumps can also guide patients with adjustment of insulin dose, especially meal-time boluses.19 To the best of our knowledge, there is no Food and Drug Administration-approved tool for patients with type 1 diabetes on multiple daily injections that can give insulin dosing suggestions.
This is the first long-term (1-year) randomized clinical trial that assesses the impact of the ACCU-CHEK® (Roche, Indianapolis, IN) Advisor insulin dosing guidance software (Advisor) on glucose control and hypoglycemia in patients with type 1 diabetes.
Research Design and Methods
A total of 123 adult subjects with type 1 diabetes with a baseline A1c of 7.5–11% were enrolled in the study at the Barbara Davis Center for Childhood Diabetes at the University of Colorado at Denver Health Sciences Center (Aurora, CO). The primary aim of the study was to see if subjects using insulin dosing Advisor software will improve glucose control over 1 year. Two patients screen-failed (entry criteria not met) before randomization, leaving 60 subjects randomized to the control group and 61 subjects randomized to the experimental group (Advisor). Subjects were randomized based on lottery system using sealed envelopes. All patients had baseline blood work that included a complete blood count, complete metabolic panel, creatinine kinase, and an A1c. The mean A1c at baseline was 8.54 ± 0.11% in the control group and 8.42 ± 0.11% in the experimental group (P = 0.4265).
Subjects were randomized on a 1:1 basis to either the experimental or control group. All subjects in the experimental group received the same training for the insulin guidance software program for personal data assistant (PDA). All subjects were given a glucose meter and an unlimited supply of test strips for SMBG. Women who were pregnant or planning to become pregnant were excluded from participation in the study, as were patients on insulin pumps, those taking glucocorticoid therapy, and those diagnosed with cancer, liver disease, anemia, or hepatitis. Patients who exercised more than 5 days a week or often traveled internationally were also excluded.
The protocol was approved by the Colorado Multiple Institutional Review Board. All subjects signed an informed consent form before being enrolled in the study.
Visits
All subjects were asked to attend seven in-clinic visits (baseline, 2 weeks, 6 weeks, 3 months, 6 months, 9 months, and 12 months) and participate in three telephone visits (4.5 months, 7.5 months, and 9.5 months) throughout the course of the study. Data for blood glucose values, testing frequency, hyperglycemic excursions, hypoglycemic events (all, nocturnal, and severe), insulin dose, weight and body mass index, hospitalizations, emergency room visits, and illnesses were recorded at each in-clinic visit. All subjects completed a patient satisfaction questionnaire, and the experimental group also completed an Advisor questionnaire.
As part of their routine clinical care, any additional phone visits were equally encouraged in both groups.
Advisor insulin guidance software
Subjects randomized to the experimental group received a PDA loaded with the insulin guidance software (Fig. 1). The concept behind the Advisor software is similar to bolus calculators currently used on many insulin pumps. At baseline (visit 1), a health care provider and/or certified diabetes educator reviewed the features of the software on the PDA and loaded a subject-specific insulin dosing algorithm into the software based on the physician's recommendations. The software program allowed the health care provider to enter demographic data such as age, height, and weight that could potentially affect the insulin sensitivity factor already programmed into the device. The program advised basal, bolus, and correction insulin dosages based on individual patients' prescriptions in addition to being alerted for SMBG testing. Subjects in the experimental group were also asked to input their blood glucose values into the PDA via the touch screen. Subjects then received a recommended insulin dose based on their prescription, which was programmed by the health care provider. The patients were asked to either agree with the recommended insulin dose or disagree, and manually enter the insulin dose they took for a given event. All the data from the glucose meters and the PDAs were downloaded at every visit.
FIG. 1.

Advisor for PDA.
Glucose target ranges
Glucose values were captured in one of the following categories to assess target glycemia and pie charts were created. Within target range (WTR) glucose values were those between 70 and 150 mg/dL (3.89–8.33 mmol/L).21 Below target range (BTR) glucose values were defined as ≤69 mg/dL (3.83 mmol/L), and above target range (ATR) glucose values were those values above 150 mg/dL (8.33 mmol/L). These glucose levels were chosen based on our previous research on SMBG downloads.21
Hypoglycemia
Hypoglycemia was defined as glucose values ≤59 mg/dL (3.27 mmol/L). Severe hypoglycemia was defined as subjects needing assistance as previously described by the Diabetes Control and Complications Trial Research Group.1
A1c and other laboratory measurements
The A1c values were measured by the DCA 2000® Analyzer, distributed by Bayer Corp. (Elkhart, IN). The DCA 2000 A1c assay gives accurate and precise results over a range of total hemoglobin from 7 to 24 g/dL. All subjects had hemoglobin concentrations well within these values. Normal A1c values are 3.4–6.2%.18 The complete blood count (LH750, Beckman Coulter, Hialeah, FL), complete metabolic panel (AU-5200, Olympus Japan Co. Ltd., Tokyo, Japan), and creatinine kinase (AU-800, Olympus) were performed by Quest Diagnostics (Denver, CO).
Statistical analysis
Wilcoxon rank sum and χ2 tests of independence were used to compare continuous and categorical variables, respectively, at baseline. The χ2 test of independence was used to compare the number of discontinued subjects between the two groups. A general linear mixed model approach suggested by Cnaan et al.20 was used to model the A1c curves over time for the two groups. A general linear mixed model approach with an unstructured covariance structure and preplanned contrasts was used to compare the experimental and control groups on percentage of glucose readings within, above, and below target, percentage of total hypoglycemic events, basal insulin dose, weight, and body mass index. Paired t tests were used to test the within-group change in weight from baseline to 12 months among those who completed all 12 months. Fisher's Exact test was used to compare the number of patients achieving target values between groups at each time point. Poisson regression was used to compare the frequency of severe hypoglycemic events between the two groups. Data are presented as least squares mean ± SE unless otherwise noted. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Results were considered significant at P < 0.05 using a two-sided alpha.
Results
The baseline demographics were similar in the two groups (Table 1). Since the patients were not stratified at randomization based on any clinical parameters, the subjects in the control group had a mean weight of 182.2 pounds as compared to 169.1 pounds in the experimental group (P = 0.1302; Table 1), and this weight difference, though not significant, was reflected in baseline insulin dosages (see below).
Table 1.
Baseline Demographics
| Control (n = 60) | Experimental (n = 61) | P value | |
|---|---|---|---|
| Age (years) | 32.5 | 33.0 | 0.7933 |
| Female/male | 31/29 | 34/27 | 0.6534 |
| Weight (lbs)a | 182.2 | 169.1 | 0.1302 |
| Body mass indexa | 27.2 | 26.2 | 0.3661 |
| Diabetes' duration (years) | 17.3 | 17.1 | 0.7410 |
| Race | |||
| Caucasian | 56 (93.3) | 56 (91.8) | 0.7484 |
| other | 4 (6.7) | 5 (8.2) | |
Two patients screen-failed at baseline. All baseline demographics were similar in the two treatment groups. The χ2 test of independence was used for categorical variables.
The Wilcoxon Rank Sum test was used for continuous variables.
All of the results reported below are for subjects who completed the study. However, data were analyzed as Intent to Treat (last observation carried forward), and the conclusions and results of that data analysis were similar with minor changes in the P values.
Discontinued subjects
There were 22 and 19 subjects who discontinued the study in the control and experimental groups, respectively (P = 0.565). Sixteen subjects discontinued because of scheduling conflicts. Other reasons for discontinuing the study included change in insulin regimen or a change in schedule not supported by the Advisor software, pregnancy, and abnormal lab test results. The mean (± SD) baseline A1c value for discontinued subjects was 8.84 ± 1.1% and 8.87 ± 1.1% for the control and experimental groups, respectively (P = 0.8). However, mean A1c levels in the discontinued subjects were significantly higher at baseline (8.83 ± 1.02%) as compared to subjects who completed the study (8.32 ± 0.76%) (P = 0.0067).
A1c
The A1c was plotted over time for both groups (Fig. 2). The plot of the data indicated that the curves for A1c for the two groups were not linear over time and suggested that the experimental group might be modeled quadratically, while the control group might require a 3rd or 4th degree polynomial. A series of mixed models were considered where for each group the mean curve was modeled using either a 1st, 2nd, 3rd, or 4th degree polynomial. Individual patient curves were modeled using 0–4th degree polynomials. The models were compared via successive differences in −2Log Likelihoods as well as by the smallest A1C. The best model included a 4th degree polynomial for the fixed effects and a 3rd polynomial for the random effects with weight included as a covariate. The two population average HbA1c curves are
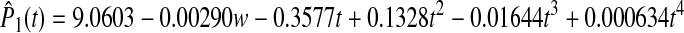 |
FIG. 2.
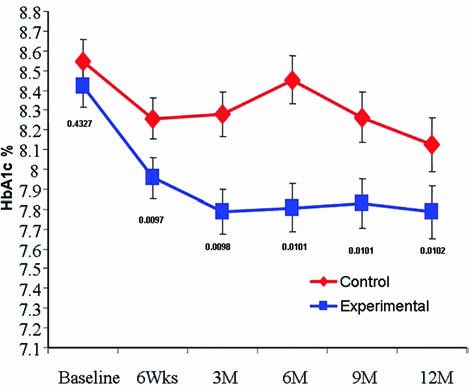
Least square mean A1c adjusted for weight significantly improved in the experimental group (squares) as compared to the control group (diamonds) from 3 to 12 months.
for the control group and
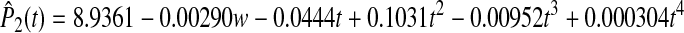 |
for the experimental group.
The tests of the fixed effects for the main effect of group and all of the interactions were highly significant (P < 0.001). Additionally, preplanned contrasts demonstrated that the groups were significantly different at 3 months (P = 0.0098), 6 months (P = 0.0101), 9 months (P = 0.0101), and 12 months (P = 0.0102) even though the control group tended to improve by 12 months. Despite the improvement in the control group, the experimental group maintained a 0.64% reduction in HbA1c. Least square means (adjusted for weight) illustrate that A1c was significantly lower in the experimental group from 3 to 12 months compared to the control group (Fig. 2). The number of subjects achieving A1c values <7.5% was significantly higher in the experimental group at 6 and 9 months (P = 0.0008 and P = 0.0076, respectively; Table 2). Seven patients in the control group had significant improvement in A1c between 9 and12 months. In contrast, only one patient had a similar decrease of 0.5% in A1c in the experimental group. Therefore, at 12 months there was no significant difference in subjects reaching target A1c values between the control and experimental groups.
Table 2.
Percentage of Patients Achieving Target A1c Values and Basal Insulin Dose in the Two Treatment Groups
| Control | Experimental | P value | |
|---|---|---|---|
| Number of patients achieving target A1c (%) valuesa | |||
| 6 months | |||
| <7.5 | 4 | 17 | 0.0008 |
| <7.0 | 1 | 1 | 1.0000 |
| <6.5 | 1 | 1 | 1.0000 |
| 9 months | |||
| <7.5 | 6 | 19 | 0.0076 |
| <7.0 | 4 | 6 | 0.7409 |
| <6.5 | 0 | 1 | 1.0000 |
| 12 months | |||
| <7.5 | 12 | 18 | 0.3574 |
| <7.0 | 1 | 4 | 0.3609 |
| <6.5 | 1 | 1 | 1.0000 |
| Basal insulin dose (mean ± SEM)b | |||
| Baseline | 43 ± 2 | 36 ± 2 | 0.0202 |
| 6 weeks | 42 ± 2 | 36 ± 2 | 0.0695 |
| 3 months | 42 ± 2 | 37 ± 2 | 0.1003 |
| 6 months | 43 ± 2 | 38 ± 2 | 0.1298 |
| 9 months | 44 ± 2 | 39 ± 2 | 0.1077 |
| 12 months | 46 ± 2 | 39 ± 2 | 0.0528 |
The number of patients achieving target A1c values <7.5% was significantly higher in the experimental group at 6 and 9 months.
There was no difference in total, basal, or bolus insulin dose throughout the study. However, basal insulin dose was higher in the control group at baseline.
Glucose target ranges
Mixed model repeated-measures analysis of glucose data was performed using measurements WTR (70–150 mg/dL [3.89–8.33 mmol/L]), BTR, and ATR. At 3, 6, and 9 months the mean percentage of glucose readings WTR was significantly greater in the experimental group than in the control group (Fig. 3a), while ATR was significantly higher in the control group at 3, 6, 9, and 12 months (Fig. 3a). The percentage of BTR was also higher in the experimental group at 3, 6, 9, and 12 months than in the control group (Fig. 3b).
FIG. 3.
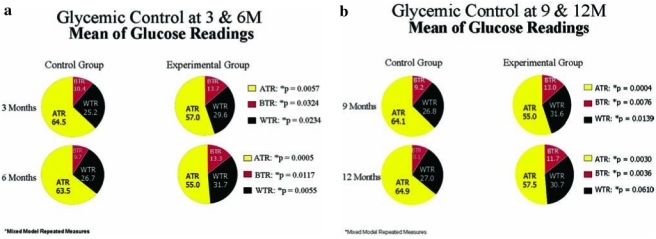
Glycemic control at (a) 3 and 6 and (b) 9 and 12 months. The ATR was significantly lower and BTR was higher in the experimental group throughout the study. However, WTR was higher in the experimental group at 3, 6, and 9 months and was nearly significant at 12 months.
Hypoglycemia
The number of nocturnal hypoglycemic events was not different between the two groups. However, the experimental group had a significant increase in severe hypoglycemic events (n = 26) as compared to the control group (n = 8) (P = 0.040). However, eight of the 26 severe hypoglycemic events occurred in two patients in the experimental group. There was no death, coma, or residual neurological deficits from any of the severe hypoglycemic events in either group.
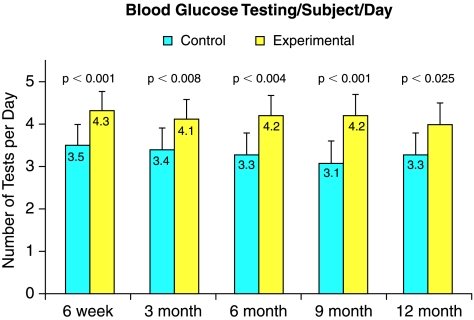
Blood glucose testing
All subjects were provided with an unlimited amount of blood glucose testing strips as part of their participation in the study. Subjects in the experimental group showed a significant increase in number of tests per subject per day as compared to the control group at 6 weeks, 3, 6, 9, and 12 months (Fig. 4).
FIG. 4.
Glucose testing per subject per day was significantly increased throughout the study in the experimental group (yellow columns) as compared to the control group (blue columns).
Insulin dose
Mean (± SE) basal insulin dose was 43 ± 2 units and 36 ± 2 units at baseline in the control and experimental group, respectively (P = 0.020). There was no difference between treatment arms at any other time point in basal, bolus, and total insulin dose (Table 2).
Body weight
There was a mean (± SD) increase in weight gain from baseline to 12 months of 5.30 ± 7.81 pounds and 3.44 ± 10.59 pounds for the control and experimental group, respectively (P = 0.376) despite improved glucose control in the experimental group.
The control group weighed 13 pounds more than the experimental group at baseline. Both groups gained weight during the study. The change in body mass index was small but significant in both groups; however, there was no difference in change of body mass index between the two groups.
Conclusions
This is the first study that documents improved glucose control in patients with type 1 diabetes with no weight gain or change in insulin dose from baseline to 12 months with use of insulin guidance software.
Improvement in A1c was not associated with changes in insulin dose, body weight, or addition of any new medical treatment. The change in A1c could thus be explained on the advice provided by the Advisor regarding insulin dose and possibly because of behavior changes.
The experimental group had a lower mean percentage of glucose readings ATR and a higher mean percentage BTR than the control group at 3, 6, 9, and 12 months. Additionally, WTR glucose readings in the experimental group were significantly higher at 3, 6, and 9 months and nearly significantly higher at 12 months than in the control group. There was a higher percentage of BTR readings from 3 to 12 months in addition to a significant increase in severe hypoglycemia in the experimental group. We feel that Advisor did not support hypoglycemia well, and this should be reviewed in the future development of the Advisor. However, eight of the 26 hypoglycemic events in the experimental group occurred in two patients. We do not know if use of the Advisor resulted in more hypoglycemic episodes.
There were a higher number of SMBG tests per subject per day in the experimental group despite unlimited supply of test strips in both groups. It has been shown that increasing frequency of SMBG testing results in improving glucose control with intensive insulin therapy.1,22 It is possible that increased frequency of SMBG testing may have resulted in better A1c values in the experimental group. However, we feel that increased frequency of testing was due to the fact that subjects were reminded to test and given insulin dosing suggestions by the PDA and supported subjects' ongoing diabetes management.
We conclude that the use of Advisor can improve glucose control in adult subjects with type 1 diabetes without any further weight gain or change in insulin dose, but with an increase of severe hypoglycemic events. Future developments on such tools must support hypoglycemia better.
Acknowledgments
This study was sponsored in part by grant 08 FLA 00250 from the State of Colorado Public Health and Environment; grant P30 DK575616 from the Diabetes Endocrine Research Center, National Institutes of Health; grant M01 RR0069 from the General Clinical Research Centers Program, National Institutes of Health; and grants R01 HL61753, RO1 HL079611, and RO1 DK32493 from the Children's Diabetes Foundation (Denver, CO).
Author Disclosure Statement
This protocol was written and developed by Satish K. Garg, M.D. at the Barbara Davis Center for Childhood Diabetes at the University of Colorado at Denver Health Sciences Center, Aurora, CO. Funding for this research was provided directly to the University of Colorado at Denver Health Sciences Center by Roche Diagnostics Corp., Indianapolis, IN. There are no other conflicts of interest for any of the authors involved in this study.
References
- 1.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development, progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner RC. Cull CA. Frighi V. Holman RR the UK Prospective Diabetes Study (UKPDS Group) Glycaemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. JAMA. 1998;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 6.Gray A. Raikou M. McGuire A. Fenn P. Stevens R. Cull C. Stratton I. Adler A. Holman R the United Kingdom Prospective Diabetes Study Group. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomized controlled trial (UKPDS 41) BMJ. 2000;320:1373–1378. doi: 10.1136/bmj.320.7246.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group. Resource utilization and costs of care in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18:1468–1478. doi: 10.2337/diacare.18.11.1468. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease and Control Prevention. National Diabetes Fact Sheet. General Information National Estimates on Diabetes in the United States, 2005. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 9.Centers for Disease Control and Prevention. National Diabetes Fact Sheet. General Information National Estimates on Diabetes in the United States 2003. rev. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2004. [Google Scholar]
- 10.Diabetes Epidemiology Research International Group: Secular trends in incidence of childhood IDDM in 10 countries. Diabetes Epidemiology Research International Group. Diabetes. 1990;39:858–864. [PubMed] [Google Scholar]
- 11.Onkamo P. Vaananen S. Karvonen M. Tuomilehto J. Worldwide increase in incidence of type 1 diabetes—the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–1403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 12.Vehik K. Hamman RF. Lezotte D. Norris JM. Klingensmith J. Bloch C. Rewers M. Dabelea D. Increasing incidence of type 1 diabetes mellitus in 0–17 year old Colorado youth. Diabetes Care. 2007;30:503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 13.Rizza R. Vigersky R. Rodbard H. Ladenson P. Young W. Surks M. Kahn R. Hogan P. A model to determine workforce needs for endocrinologists in the United States until 2020. Diabetes Care. 2003;26:1545–1552. doi: 10.2337/diacare.26.5.1545. [DOI] [PubMed] [Google Scholar]
- 14.Garg SK. Schwartz S. Edelman SV. Improved glucose excursions using an implantable real-time continuous implanted glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27:734–738. doi: 10.2337/diacare.27.3.734. [DOI] [PubMed] [Google Scholar]
- 15.Garg SK. Zisser H. Schwartz S. Bailey T. Kaplan R. Ellis S. Jovanovic L. Improvement in glycaemic control with a transcutaneous, real-time continuous glucose sensor: randomized controlled trial. Diabetes Care. 2006;29:44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 16.Garg S. Jovanovic L. Clinical trial of a seven-day continuous glucose sensor demonstrates the importance of fasting hyperglycemia and the dawn phenomenon. Diabetes Care. 2007;29:2644–2649. [Google Scholar]
- 17.Bailey TS. Zisser H. Garg SK. Reduction in hemoglobin A1c with real-time continuous glucose monitoring: results from a 12-week Study. Diabetes Technol Ther. 2007;9:203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 18.Garg SK. Kelly WC. Voelmle MK. Ritchie PJ. Gottlieb PA. McFann KK. Ellis SL. Improved glycemic control with real-life use of continuous glucose sensors in adult subjects with type 1 diabetes. Diabetes Care. 2007;30:3023–3025. doi: 10.2337/dc07-1436. [DOI] [PubMed] [Google Scholar]
- 19.Chase HP. Saib SZ. MacKenzie T. Hansen MM. Garg SK. Postprandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19:317–321. doi: 10.1046/j.1464-5491.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 20.Cnaan A. Laird NM. Slasor P. Tutorial in biostatistics, using the general linear model to analyze unbalanced repeated measures and longitudinal data. Statistics Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Brewer KW. Owen S. Garg SK. Chase HP. Slicing the pie: correlating hemoglobin A1c (HbA1c) values with average blood glucose (ABG) values in a pie chart form. Diabetes Care. 1998;21:209–212. doi: 10.2337/diacare.21.2.209. [DOI] [PubMed] [Google Scholar]
- 22.Schiffrin A. Belmonte M. Diabetes multiple daily self glucose monitoring: its essential role in long term glucose control in insulin dependent diabetic patients treated with pump and multiple subcutaneous injections. Diabetes Care. 1982;5:479–484. doi: 10.2337/diacare.5.5.479. [DOI] [PubMed] [Google Scholar]