Abstract
Background
Hantaviral antigens were originally reported more than 20 years ago in tissues of the Eurasian common shrew (Sorex araneus), captured in European and Siberian Russia. The recent discovery of Seewis virus (SWSV) in this soricid species in Switzerland provided an opportunity to investigate its genetic diversity and geographic distribution in Russia.
Methods
Lung tissues from 45 Eurasian common shrews, 4 Laxmann's shrews (Sorex caecutiens), 3 Siberian large-toothed shrews (Sorex daphaenodon), 9 pygmy shrews (Sorex minutus), 28 tundra shrews (Sorex tundrensis), and 6 Siberian shrews (Crocidura sibirica), captured in 11 localities in Western and Eastern Siberia during June 2007 to September 2008, were analyzed for hantavirus RNA by reverse transcription–polymerase chain reaction.
Results
Hantavirus L and S segment sequences, detected in 11 S. araneus, 2 S. tundrensis, and 2 S. daphaenodon, were closely related to SWSV, differing from the prototype mp70 strain by 16.3–20.2% at the nucleotide level and 1.4–1.7% at the amino acid level. Alignment and comparison of nucleotide and amino acid sequences showed an intrastrain difference of 0–11.0% and 0% for the L segment and 0.2–8.5% and 0% for the S segment, respectively. Phylogenetic analysis, using neighbor-joining, maximum-likelihood, and Bayesian methods, showed geographic-specific clustering of SWSV strains in Western and Eastern Siberia.
Conclusions
This is the first definitive report of shrew-borne hantaviruses in Siberia, and demonstrates the impressive distribution of SWSV among phylogenetically related Sorex species. Coevolution and local adaptation of SWSV genetic variants in specific chromosomal races of S. araneus may account for their geographic distribution.
Key Words: Field studies, Hantavirus, PCR
Introduction
Modern-day hantavirology is usually dated to 1976 when Hantaan virus was detected in the striped-field mouse (Apodemus agrarius) in Korea (Lee et al. 1978). This seminal discovery established the cause of hemorrhagic fever with renal syndrome (HFRS) in Eurasia (Yanagihara and Gajdusek 1988) and later facilitated the diagnosis of hantavirus cardiopulmonary syndrome (HCPS) in the Americas (Nichol et al. 1993, Duchin et al. 1994). Each hantavirus (family Bunyaviridae, genus Hantavirus) shares a long-standing relationship with a specific rodent species or closely related reservoir species in the family Muridae and Cricetidae (Plyusnin and Morzunov 2001, Vapalahti et al. 2003). Representative examples include Dobrava-Belgrade virus in the yellow-necked field mouse (Apodemus flavicollis) (Avsic-Zupanc et al. 1992, Gligic et al. 1992), Seoul virus in the brown rat (Rattus norvegicus) (Lee et al. 1982), Thailand virus in the bandicoot rat (Bandicota indica) (Elwell et al. 1985), Puumala virus in the bank vole (Myodes glareolus) (Brummer-Korvenkontio et al. 1980), Prospect Hill virus in the meadow vole (Microtus pennsylvanicus) (Lee et al. 1985), Tula virus in the European common vole (Microtus arvalis) (Plyusnin et al. 1994a), Muju virus in the royal vole (Myodes regulus) (Song et al. 2007), Sin Nombre virus in the deer mouse (Peromyscus maniculatus) (Nerurkar et al. 1993, 1994, Nichol et al. 1993), and Andes virus in the long-tailed rice rat (Oligoryzomys longicaudatus) (Levis et al. 1998).
Until recently, the single exception to the hantavirus–rodent association was Thottapalayam virus (TPMV), a long-unclassified virus isolated from the Asian house shrew (Suncus murinus), captured in 1964 near Vellore in Tamil Nadu, India (Carey et al. 1971, Zeller et al. 1989). However, whether or not TPMV was naturally harbored by a nonrodent host or represented spillover from a rodent reservoir was a continuing source of debate. Whole-genome sequence analysis of TPMV, showing that it forms a unique phylogenetic clade, has finally put this argument to rest (Song et al. 2007a, Yadav et al. 2007). Also, recent identification of genetically divergent hantaviruses in multiple species of shrews (Order Soricomorpha, Family Soricidae) from widely separated geographic regions provides convincing evidence that soricids serve as reservoir hosts (Arai et al. 2007, 2008a, Klempa et al. 2007, Song et al. 2007b, 2007c, 2009). Moreover, the natural host range has been further expanded with the detection of hantaviruses in moles (family Talpidae), suggesting that ancestral soricomorphs may have served as the early or original mammalian hosts of hantaviruses (Arai et al. 2008b, Kang et al. 2009a, 2009b).
Although hantaviral antigens have been previously reported in the Eurasian common shrew (Sorex araneus), pygmy shrew (Sorex minutus), and Eurasian water shrew (Neomys fodiens), captured in European Russia (Gavrilovskaya et al. 1983, Tkachenko et al. 1983) and Siberia (Miasnikov et al. 1992), definitive molecular evidence of soricid-borne hantaviruses from Russia is lacking. The recent detection of a hantavirus, named Seewis virus (SWSV), in the Eurasian common shrew from Switzerland (Song et al. 2007b) provided an opportunity to investigate the genetic diversity and geographic distribution of SWSV and other hantaviruses harbored by shrews in Western and Eastern Siberia. In this study, we demonstrate the geographic-specific genetic variation of SWSV strains in five administrative regions in Siberia.
Overall, the segregation of SWSV genetic variants according to their geographic locale resembles that of rodent-borne hantaviruses, suggesting long-standing phylogeographic relationships.
Materials and Methods
Trapping and sample collection
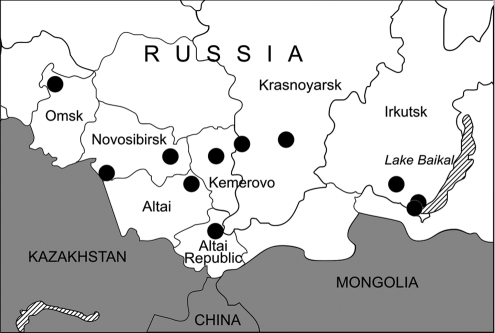
Multiple trapping expeditions were conducted in different ecotones, in the seven administrative regions of Western and Eastern Siberia (Altai Republic, Altai, and Krasnoyarsk Krais, and Novosibirsk, Omsk, Kemerovo, and Irkutsk Oblasts) between June 2007 and September 2008 (Table 1 and Fig. 1). Shrews were trapped and tissues were processed according to well-established protocols and safety recommendations (Mills et al. 1995). Sherman traps were set at 10-m intervals and baited with rye bread soaked with sunflower oil. Pitfall traps were spaced at approximately 5-m intervals along 50-m trap lines. All traps were checked twice daily. Species, sex, age class, body mass, and point of capture of each trapped shrew were recorded. Lung tissues were dissected aseptically and stored in liquid nitrogen or RNAlater RNA Stabilization Reagent (Qiagen), for subsequent analysis by reverse transcription–polymerase chain reaction (RT-PCR).
Table 1.
Field Expeditionary Sites for Trapping of Shrews
| Administrative region | Locality | Ecotope | Month and year | Shrew species |
|---|---|---|---|---|
| Altai Republic | Teletskoye | Dark coniferous forest | August–September 2007 | Sorex araneus (9) |
| Crocidura sibirica (6) | ||||
| Altai Krai | Khmelevka | Dark coniferous forest | June 2007 | S. araneus (2) |
| Sorex caecutiens (1) | ||||
| Krasnoyarsk Krai | East Sayan | Dark coniferous forest | August 2008 | S. araneus (2) |
| Parnaya | Light coniferous forest | August 2008 | S. araneus (3) | |
| Sorex tundrensis (3) | ||||
| Sorex minutus (3) | ||||
| Irkutsk Oblast | Zalary | Forest-steppe | October 2007 | S. tundrensis (7) |
| Sorex daphaenodon (1) | ||||
| Irkutsk | Coniferous forest | December 2007 | S. tundrensis (5) | |
| Irkutsk City | Mixed forest | December 2007 | S. araneus (2) | |
| S. tundrensis (2) | ||||
| S. daphaenodon (2) | ||||
| Kemerovo Oblast | Krapivino | Coniferous forest | June–July 2008 | S. araneus (6) |
| Novosibirsk Oblast | Karasuk | Steppe | June–July 2007 | S. araneus (6) |
| S. tundrensis (11) | ||||
| June 2008 | S. araneus (7) | |||
| S. minutus (4) | ||||
| Novosibirsk City | Forest-steppe | June–September 2008 | S. araneus (6) | |
| S. minutus (2) | ||||
| Omsk Oblast | Irtish River | Mixed forest | July 2007 | S. araneus (2) |
| S. caecutiens (3) |
FIG. 1.
Map of Western and Eastern Siberia showing the administrative regions where shrews were trapped between June 2007 and September 2008.
RT-PCR and DNA sequencing
Total RNA was extracted from tissues using the RNeasy MiniKit (Qiagen), and cDNA was synthesized using Expand reverse transcriptase (Roche) and universal primer 5′-TAGTAGTAGACTCC-3′. All samples were tested by nested RT-PCR (Arai et al. 2008a), using two sets of primers to recover partial large (L) and small (S) segment sequences: L (outer: 5′-ATGTAYGTBAGTGCWGATGC-3′ and 5′-AACCADTCWGTYCCRTCATC-3′; inner: 5′-TGCWGATGCHACIAARTGGTC-3′ and 5′-GCRTCRTCWGARTGRTGDGCA-3′); S (outer: 5′-TAGTAGTAKRCTCCCTAAARAG-3′; inner: 5′-GWGGHCARACWGCAGAYTGG-3′ and 5′-AGCTCAGGATCCATGTCATC-3′).
Sequence and phylogenetic analyses
Hantavirus nucleotide sequences were aligned and compared using ClustalW (Thompson et al. 1994). The distance-based neighbor-joining method supported by MEGA 4.1 (Tamura et al. 2007), as well as maximum-likelihood (ML) and Bayesian methods, implemented in PAUP* (Phylogenetic Analysis Using Parsimony, 4.0b10) (Swofford 2003), RAxML Blackbox web-server (Stamatakis et al. 2008), and MrBayes 3.1 (Ronquist and Huelsenbeck 2003), under the best-fit GTR+I+Γ model of evolution, were used for constructing phylogenetic trees. ML topologies were evaluated by bootstrap analysis of 1000 neighbor-joining iterations in PAUP* or 1000 ML iterations in RAxML. Bayesian analysis consisted of at least 2 million Markov Chain Monte Carlo generations to ensure convergence across two runs of four chains each, with average standard deviations of split frequencies less than 0.01 and effective sample sizes well over 100, resulting in consensus trees supported by posterior-node probabilities (Kang et al. 2009b). GenBank accession numbers for new hantaviral genomic sequences are shown in Table 2.
Table 2.
Seewis Virus Strains Detected in Sorex Shrews in Siberia, Russia
| Genus species | Capture site | Virus strain | GenBank no. |
|---|---|---|---|
| S. araneus | Altai, Teletskoye | Telet-Sa300 | GQ284578, EU424334 |
| Telet-Sa301 | GQ284577, EU424335 | ||
| Telet-Sa313 | GQ284579, EU424336 | ||
| Telet-Sa321 | GQ284575, EU424337 | ||
| Telet-Sa500 | GQ284576, EU424338 | ||
| Kemerovo, Krapivino | Kemerovo-Sa65 | GQ284582, GQ267812 | |
| Novosibirsk, Novosibirsk City | Novosib-Sa374 | GQ284581, GQ267804 | |
| Novosibirsk, Karasuk | Karasuk-Sa56 | GQ284586, GQ267809 | |
| Krasnoyarsk, East Sayan | Krasn-Sa5 | GQ284584, GQ267811 | |
| Krasn-Sa6 | GQ355616, GQ284583 | ||
| Krasnoyarsk, Parnaya | Parnaya-Sa1220 | GQ284580, GQ267810 | |
| S. daphaenodon | Irkutsk, Irkutsk City | Irkutsk-Sd456 | GQ284572, GQ267805 |
| Irkutsk-Sd475 | GQ284573, GQ267806 | ||
| S. tundrensis | Irkutsk, Irkutsk City | Irkutsk-St489 | GQ284574, GQ267807 |
| Novosibirsk, Karasuk | Karasuk-St2 | GQ284585, GQ267808 |
mtDNA sequencing and host phylogeny
To verify the taxonomic identity of shrews and to ascertain their phylogenetic relationships, genomic DNA was extracted from frozen lung tissue using the QIAamp DNA Mini Kit (Qiagen), and the partial 426-nucleotide region of the cytochrome b gene of mtDNA was amplified by PCR. Amplicons were gel purified with QIAquick Gel Extraction kit (Qiagen) and directly sequenced using ABI Prizm BigDye Terminator kit (PE Applied Biosystem) and an automatic ABI Prism 310 genetic analyzer.
Results and Discussion
Lung tissues from 95 shrews—45 Eurasian common shrews, 4 Laxmann's shrews (Sorex caecutiens), 3 Siberian large-toothed shrews (Sorex daphaenodon), 9 pygmy shrews (S. minutus), 28 tundra shrews (Sorex tundrensis), and 6 Siberian shrews (Crocidura sibirica)—were examined by nested RT-PCR. Hantaviral RNA was detected in 15 shrews captured in 7 of the 11 localities studied: Teletskoye Lake (Altai Republic), Krapivino (Kemerovo Oblast), Karasuk and the suburbs of Novosibirsk City (Novosibirsk Oblast), Parnaya and East Sayan (Krasnoyarsk Krai), and the suburbs of Irkutsk City (Irkutsk Oblast) (Fig. 1 and Table 2). Nucleotide sequence analysis of partial hantavirus L (positions 2968–3313) and S (positions 407–1243) segments from 11 S. araneus, 2 S. tundrensis, and 2 Siberian large-toothed shrew (Table 2) showed close similarity to SWSV, previously reported from S. araneus captured in Switzerland (Song et al. 2007b). The sequences appeared to be genetic variants of SWSV, differing from the prototype mp70 strain by 16.3–20.2% at the nucleotide level and 1.7% at the amino acid level for the L segment, and by 17.4–19.1% and 1.4% for the S segment, respectively. Pair-wise alignment and comparison of nucleotide and amino acid sequences showed an intrastrain difference of 0–11.0% and 0% for the L segment and 0.2–8.5% and 0% for the S segment, respectively. Nucleotide divergence from other previously known shrew- and rodent-borne hantavirus strains ranged from 24.5% to 35.6% and from 31.9% to 48.4%, respectively.
The new sequences showed geographic-specific clustering. Nucleotide sequences in the same locality demonstrated minimal differences even if they were recovered from different soricid hosts. For example, hantavirus sequences recovered from S. araneus and S. tundrensis captured near Karasuk village in Novosibirsk Oblast were identical for the L segment and showed 0.2% difference for the S segment. Similarly, S and L segment nucleotide sequences from S. daphaenodon and S. tundrensis captured in Irkutsk Oblast differed by 0.3–0.6% and 0.9–1.2%, respectively. By contrast, SWSV sequences from five S. araneus captured near Teletskoye Lake in Altai Republic formed two separate groups and showed high intergroup L segment nucleotide sequence divergence (6.4–7.7%). A lower divergence was demonstrated for S segment sequences (0.9–2.4%).
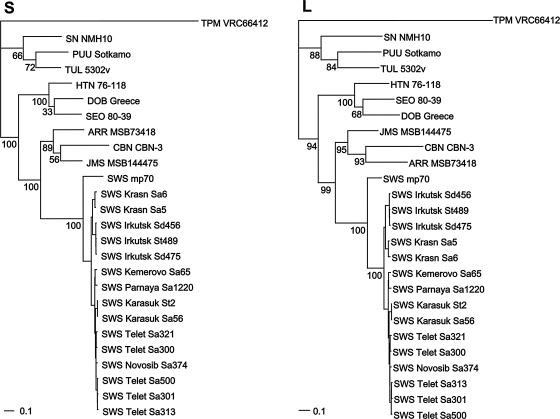
Phylogenetic analysis, based on 346 and 837 nucleotides of the L and S segments, respectively, using the ML method, implemented in RAxML Blackbox web-server showed geographic-specific clustering of SWSV strains (Fig. 2), not unlike that reported previously for rodent-borne hantaviruses, including Hantaan virus in the striped field mouse (Song et al. 2000), Puumala virus in the bank vole (Plyusnin et al. 1994b, 1995), Muju virus in the royal vole (Song et al. 2007), and Tula virus in the European common vole (Song et al. 2004). Similar topologies were evident using neighbor-joining and Bayesian methods. The correlation between hantavirus genetic variation and geographic origin of the reservoir species provided support for local host-specific adaptation through deep evolutionary time.
FIG. 2.
Phylogenetic trees, based on 837- and 346-nucleotide regions of the S and L segments, respectively, generated using the maximum-likelihood method, implemented in RAxML Blackbox web-server, under the best-fit GTR+I+Γ model of evolution, showing geographic-specific clustering of Seewis (SWS) virus strains. Bootstrap confidence limits were based on 1000 replicates. Phylogenetic positions of SWS virus strains from Siberia are shown in relationship to previously reported rodent- and soricid-hantaviruses, including SWS virus (SWS mp70, EF636024, EF636026), Ash River virus (ARR MSB73418, EF650086, EF619961), Jemez Springs virus (JMS MSB89332, FJ593499, FJ593501), Cao Bang virus (CBN CBN-3, EF543524, EF543525), Thottapalayam virus (TPM VRC66412, AY526097, EU001330), Hantaan virus (HTN 76-118, X55901, M14626), Dobrava virus (DOB Greece, AJ410619, AJ410617), Seoul virus (SEO 80-39, AY273791, X56492), Tula virus (TUL 5302v, NC_005227, AJ005637), Puumala virus (PUU Sotkamo, X61035, Z66548), and Sin Nombre virus (SN NMH10, L25784, L37902).
As verified by phylogenetic analysis of the partial 426-nucleotide region of the cytochrome b gene, SWSV-positive shrews were identified as S. araneus, S. tundrensis, and S. daphaenodon (GenBank accession numbers GQ355606–GQ355620). Unlike the hantavirus sequences, the host mtDNA sequences did not segregate into distinct lineages, according to the geographic locales or capture sites of the shrews.
Previous studies of shrew communities, conducted over multiple years in four of the five administrative regions, where hantavirus-positive shrews were detected, showed that S. araneus was the predominant species, comprising more then 30% of the shrew population: 49% in Teletskoye Lake, 32% in Karasuk, 71% in Novosibirsk, and 47% in Kemerovo. As determined by RT-PCR, the prevalence of hantavirus infection among Eurasian common shrews in each locality varied considerably, ranging from 2 of 2 (100%) in East Sayan, 5 of 9 (55.5%) in Teletskoye Lake, 1 of 3 (33.3%) in Parnaya, 1 of 6 (16.7%) in suburbs of Novosibirsk City and Krapivino, and 1 of 13 (7.7%) in Karasuk. We were unable to find a significant correlation between the infection rate and population density or male/female ratio in these sites. In our study, high S. araneus density was registered near Teletskoye Lake (13.6 individuals per 100 trap-days; male/female = 3/6), in Krapivino (10.7 individuals/100 trap-days; male/female = 2/4), and in Novosibirsk City (16.9 individuals/100 trap-days; male/female = 4/2), compared to low S. araneus density in Karasuk (3.1 individuals/100 trap-days; male/female = 5/2).
The Eurasian common shrew is among the most widely dispersed small mammal species in Eurasia. S. araneus has one of the most variable karyotypes among small mammals, displaying 70 chromosome races throughout its distribution in Europe and Siberia. The seven chromosome races in Siberia sequentially replaced each other in the latitudinal direction (Polyakov et al. 2001). Based on previous studies we suggest that at least four chromosomal races of the Eurasian common shrew are present in areas covered by our research: Novosibirsk race (Novosib-Sa374), Tomsk race (Kemerovo-Sa65 and Parnaya-Sa1220), Strelka race (Krasn-Sa5 and Krasn-Sa6), and Altai race (Telet-Sa300, Telet-Sa301, Telet-Sa313, Telet-Sa321, and Telet-Sa500). Our data demonstrated high nucleotide L-sequence divergence (up to 7.7%) among sequences from Teletskoye Lake. Based on the hypothesis of coevolution of hantaviruses and their hosts, we suggest that two separate SWSV sublineages had different evolution histories and, most probably, circulated not in a single Altai race, but rather in two different races. A second race may be a new race or the Seminsk race that was described previously in the Altai Republic in a site located 100 km away from the capture site near Teletskoye Lake (Polyakov et al. 2001).
This report represents the first molecular evidence of shrew-borne hantaviruses in Siberia. Our data corroborate earlier reports of hantaviral antigens in S. araneus captured in Russia (Gavrilovskaya et al. 1983, Tkachenko et al. 1983, Miasnikov et al. 1992). Also, together with recent findings of the geographic-specific genetic variation of SWSV in S. araneus captured in Finland and Hungary (H.J. Kang and R. Yanagihara, unpublished observations), as well as in Germany and Austria (R. Dürrwald and N. Nowotny, pers. comm.), our data establish the widespread circulation of SWSV throughout the geographic range of S. araneus, spanning across Northern Europe to Eastern Siberia. Moreover, detection of SWSV in S. araneus, S. tundrensis, and S. daphaenodon in Siberia is consistent with the shared ancestry of these closely related Sorex species within the S. araneus group (Searle and Wójcik 1998). The vast distribution of SWSV, therefore, may be a consequence of a primordial hantavirus infecting ancestral Sorex shrews, with subsequent host switching and local host adaptation through deep evolutionary time. Intensified studies, now underway, in search of SWSV and other genetically distinct hantaviruses in sympatric and syntopic shrew species across Siberia will provide additional important insights into the complex phylogeographic history of hantaviruses.
A pressing issue is whether nonrodent-borne hantaviruses cause infection or disease in humans. Although suggestive evidence of TPMV infection in humans has been reported (Okumura et al. 2007), neither TPMV nor any of the newly identified soricid- and talpid-borne hantaviruses has been etiologically linked to specific diseases or syndromes in humans. Nevertheless, it is still far too premature to label these viruses as being nonpathogenic. Instead, the realization that rodent-borne hantaviruses are capable of causing diseases as clinically disparate as HFRS and HCPS raises the possibility that still-orphan hantaviruses harbored by shrews and moles might similarly cause diseases that do not clinically resemble either HFRS or HCPS. Efforts are now underway to develop recombinant nucleocapsid proteins and other diagnostic reagents to test sera from human patients with various diseases for antibodies against these newfound hantaviruses. In addition, sera from mammalogists and field biologists having exposure to soricomorphs are being analyzed.
Acknowledgments
We thank our colleagues, Dr. Alexander Timoshenko, Dr. Dmitry Petrovski, and Dr. Alexander Pozdnyakov, for providing additional shrew tissues for testing. This work was supported in part by grants from the Biotechnology Engagement Program, through the International Science and Technology Center (#0805.2), and from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01AI075057).
Disclosure Statement
All authors have no competing financial interests. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- Arai S. Bennett SN. Sumibcay L. Cook JA, et al. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am J Trop Med Hyg. 2008a;78:348–351. [PMC free article] [PubMed] [Google Scholar]
- Arai S. Ohdachi SD. Asakawa M. Kang HJ, et al. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides) Proc Natl Acad Sci USA. 2008b;105:16296–16301. doi: 10.1073/pnas.0808942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S. Song J-W. Sumibcay L. Bennett SN, et al. Hantavirus in northern short-tailed shrew, United States. Emerg Infect Dis. 2007;13:1420–1423. doi: 10.3201/eid1309.070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avsic-Zupanc T. Xiao SY. Stojanovic R. Gligic A, et al. Characterization of Dobrava virus: a hantavirus from Slovenia, Yugoslavia. J Med Virol. 1992;38:132–137. doi: 10.1002/jmv.1890380211. [DOI] [PubMed] [Google Scholar]
- Brummer-Korvenkontio M. Vaheri A. Hovi T. von Bonsdorff CH, et al. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980;141:131–134. doi: 10.1093/infdis/141.2.131. [DOI] [PubMed] [Google Scholar]
- Carey DE. Reuben R. Panicker KN. Shope RE, et al. Thottapalayam virus: a presumptive arbovirus isolated from a shrew in India. Indian J Med Res. 1971;59:1758–1760. [PubMed] [Google Scholar]
- Duchin JS. Koster FT. Peters CJ. Simpson GL, et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- Elwell MR. Ward GS. Tingpalapong M. LeDuc JW. Serologic evidence of Hantaan-like virus in rodents and man in Thailand. Southeast Asian J Trop Med Public Health. 1985;16:349–354. [PubMed] [Google Scholar]
- Gavrilovskaya IN. Apekina NS. Miasnikov YA. Bernshtein AD, et al. Features of circulation of hemorrhagic fever with renal syndrome (HFRS) virus among small mammals in the European U.S.S.R. Arch Virol. 1983;75:313–316. doi: 10.1007/BF01314898. [DOI] [PubMed] [Google Scholar]
- Gligic A. Dimkovic N. Xiao S-Y. Buckle GJ, et al. Belgrade virus: a new hantavirus causing severe hemorrhagic fever with renal syndrome in Yugoslavia. J Infect Dis. 1992;166:113–120. doi: 10.1093/infdis/166.1.113. [DOI] [PubMed] [Google Scholar]
- Kang HJ. Bennett SN. Dizney L. Sumibcay L, et al. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii) Virology. 2009a;388:8–14. doi: 10.1016/j.virol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ. Bennett SN. Sumibcay L. Arai S, et al. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea) PLoS ONE. 2009b;4:e6149. doi: 10.1371/journal.pone.0006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempa B. Fichet-Calvet E. Lecompte E. Auste B, et al. Novel hantavirus sequences in shrew, Guinea. Emerg Infect Dis. 2007;13:520–552. doi: 10.3201/eid1303.061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW. Baek LJ. Johnson KM. Isolation of Hantaan virus, the etiologic agent of Korean hemorrhagic fever from wild urban rats. J Infect Dis. 1982;146:638–644. doi: 10.1093/infdis/146.5.638. [DOI] [PubMed] [Google Scholar]
- Lee HW. Lee P-W. Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- Lee P-W. Amyx HL. Yanagihara R. Gajdusek DC, et al. Partial characterization of Prospect Hill virus isolated from meadow voles in the United States. J Infect Dis. 1985;152:826–829. doi: 10.1093/infdis/152.4.826. [DOI] [PubMed] [Google Scholar]
- Levis S. Morzunov SP. Rowe JE. Enria D, et al. Genetic diversity and epidemiology of hantaviruses in Argentina. J Infect Dis. 1998;177:529–538. doi: 10.1086/514221. [DOI] [PubMed] [Google Scholar]
- Miasnikov YA. Apekina NS. Zuevskii AP. Khitrin AV, et al. The disposition of natural foci of haemorrhagic fever with renal syndrome in different landscape areas of Tiumen Province. Vopr Virusol. 1992;37:161–165. [PubMed] [Google Scholar]
- Mills JN. Childs JE. Ksiazek TG. Peters CJ. Methods for Trapping and Sampling Small Mammals for Virologic Testing. Atlanta: U.S. Department of Health and Human Services, Center for Disease Control and Prevention; 1995. [Google Scholar]
- Nerurkar VR. Song K-J. Gajdusek DC. Yanagihara R. Genetically distinct hantavirus in deer mice. Lancet. 1993;342:1058–1059. doi: 10.1016/0140-6736(93)92917-i. [DOI] [PubMed] [Google Scholar]
- Nerurkar VR. Song K-J. Song J-W. Nagle JW, et al. Genetic evidence for a hantavirus enzootic in deer mice (Peromyscus maniculatus) a decade before the recognition of hantavirus pulmonary syndrome. Virology. 1994;204:563–568. doi: 10.1006/viro.1994.1570. [DOI] [PubMed] [Google Scholar]
- Nichol ST. Spiropoulou CF. Morzunov S. Rollin PE, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Okumura M. Yoshimatsu K. Kumperasart S. Nakamura I, et al. Development of serological assays for Thottapalayam virus, an insectivore-borne hantavirus. Clin Vaccine Immunol. 2007;14:173–181. doi: 10.1128/CVI.00347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyusnin A. Morzunov S. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr Top Microbiol Immunol. 2001;256:47–75. doi: 10.1007/978-3-642-56753-7_4. [DOI] [PubMed] [Google Scholar]
- Plyusnin A. Vapalahti O. Lankinen H. Lehväslaiho H, et al. Tula virus: a newly detected hantavirus carried by European common voles. J Virol. 1994a;68:7833–7839. doi: 10.1128/jvi.68.12.7833-7839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyusnin A. Vapalahti O. Lehväslaiho H. Apekina N, et al. Genetic variation of wild Puumala viruses within the serotype, local rodent populations and individual animal. Virus Res. 1995;38:25–41. doi: 10.1016/0168-1702(95)00038-r. [DOI] [PubMed] [Google Scholar]
- Plyusnin A. Vapalahti O. Ulfves K. Lehvaslaiho H, et al. Sequences of wild Puumala virus genes show a correlation of genetic variation with geographic origin of the strains. J Gen Virol. 1994b;75:405–409. doi: 10.1099/0022-1317-75-2-405. [DOI] [PubMed] [Google Scholar]
- Polyakov AV. Panov VV. Ladygina TY. Bochkarev MN, et al. Chromosomal evolution of the common shrew Sorex araneus L from the southern Urals and Siberia in the postglacial period. Russ J Genet. 2001;37:351–357. [PubMed] [Google Scholar]
- Ronquist F. Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Searle JB. Wójcik JM. Chromosomal evolution: the case of Sorex araneus. In: Wójcik JM, editor; Wolsan M, editor. Evolution of Shrews. Bialowieza: Polish Academy of Sciences; 1998. pp. 219–268. [Google Scholar]
- Song J-W. Baek LJ. Kim SH. Kho EY, et al. Genetic diversity of Apodemus agrarius-borne Hantaan virus in Korea. Virus Genes. 2000;21:227–232. doi: 10.1023/a:1008199800011. [DOI] [PubMed] [Google Scholar]
- Song J-W. Baek LJ. Schmaljohn CS. Yanagihara R. Thottapalayam virus: a prototype shrew-borne hantavirus. Emerg Infect Dis. 2007a;13:980–985. doi: 10.3201/eid1307.070031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W. Baek LJ. Song K-J. Skrok A, et al. Characterization of Tula virus from common voles (Microtus arvalis) in Poland: evidence for geographic-specific phylogenetic clustering. Virus Genes. 2004;29:239–247. doi: 10.1023/B:VIRU.0000036384.50102.cf. [DOI] [PubMed] [Google Scholar]
- Song J-W. Gu SH. Bennett SN. Arai S, et al. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus) Virol J. 2007b;4:114. doi: 10.1186/1743-422X-4-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W. Kang HJ. Gu SH. Moon SS, et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura) J Virol. 2009;83:6184–6191. doi: 10.1128/JVI.00371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W. Kang HJ. Song K-J. Truong TT, et al. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerg Infect Dis. 2007c;13:1784–1787. doi: 10.3201/eid1311.070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K-J. Baek LJ. Moon SS. Ha SJ, et al. Muju virus, a newfound hantavirus harbored by the arvicolid rodent Myodes regulus in Korea. J Gen Virol. 2007;88:3121–3129. doi: 10.1099/vir.0.83139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. Hoover P. Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4. Sunderland, Massachusetts: Sinauer Associates; 2003. [Google Scholar]
- Tamura K. Dudley J. Nei M. Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD. Higgins DG. Gibson TJ. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko EA. Ivanov AP. Donets MA. Miasnikov YA, et al. Potential reservoir and vectors of haemorrhagic fever with renal syndrome (HFRS) in the U.S.S.R. Ann Soc Belg Med Trop. 1983;63:267–269. [PubMed] [Google Scholar]
- Vapalahti O. Mustonen J. Lundkvist A. Henttonen H, et al. Hantavirus infections in Europe. Lancet Infect Dis. 2003;3:653–661. doi: 10.1016/s1473-3099(03)00774-6. [DOI] [PubMed] [Google Scholar]
- Yadav PD. Vincent MJ. Nichol ST. Thottapalayam virus is genetically distant to the rodent-borne hantaviruses, consistent with its isolation from the Asian house shrew (Suncus murinus) Virol J. 2007;4:80. doi: 10.1186/1743-422X-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara R. Gajdusek DC. Hemorrhagic fever with renal syndrome: a historical perspective and review of recent advances. In: Gear JHS, editor. CRC Handbook of Viral and Rickettsial Hemorrhagic Fevers. Boca Raton, Florida: CRC Press, Inc.; 1988. pp. 151–188. [Google Scholar]
- Zeller HG. Karabatsos N. Calisher CH. Digoutte JP, et al. Electron microscopic and antigenic studies of uncharacterized viruses. II. Evidence suggesting the placement of viruses in the family Bunyaviridae. Arch Virol. 1989;108:211–227. doi: 10.1007/BF01310935. [DOI] [PubMed] [Google Scholar]