Abstract
Background
This study was designed to determine whether pramlintide added to insulin therapy reduced the risks associated with extreme blood glucose (BG) fluctuations in patients with type 1 diabetes.
Methods
Self-monitored BG (SMBG) records were retrospectively analyzed from a randomized, double-blind, placebo-controlled study of the effects of pramlintide on intensively treated patients with type 1 diabetes. Two groups—pramlintide (n = 119), 30/60 μg administered subcutaneously at each meal, or placebo (n = 129)—were matched by age, gender, and baseline hemoglobin A1C. Using SMBG, daily BG profiles, BG rate of change, and low and high BG indices (LBGI and HBGI, respectively) measuring the risk for hypoglycemia and hyperglycemia were calculated.
Results
Compared with placebo, pramlintide significantly attenuated the pre- to postprandial BG rate of change (F = 83.8, P < 0.0001). Consequently, in pramlintide-treated patients, the average post-meal BG (8.4 vs. 9.7 mmol/L [151.2 vs. 174.6 mg/dL]) and postprandial HBGI were significantly lower than placebo (both P < 0.0001). Substantial daily BG variation was observed in placebo-treated patients, with most significant hyperglycemia occurring after breakfast and during the night; post-meal BG did not vary significantly throughout the day in pramlintide-treated patients. The reduction in postprandial hyperglycemia in pramlintide-treated patients occurred without increased risk for preprandial hypoglycemia as quantified by the LBGI.
Conclusions
Risk analysis of the effect of pramlintide treatment demonstrated risk-reduction effects independent of changes in average glycemia, most notably reduced rate and magnitude of postprandial BG fluctuations. These effects were not accompanied by an increased risk of hypoglycemia.
Introduction
Therapies to treat diabetes mellitus are evaluated primarily based on their ability to reduce hemoglobin A1C (A1C), a measure of long-term glycemia. For patients with type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)1 and patients with type 2 diabetes in the United Kingdom Prospective Diabetes Study (UKPDS),2 the risks of microvascular complications associated with diabetes were generally predicted by A1C. A recent report confirmed that variability in blood glucose (BG) around a patient's mean value has no influence on the development or progression of retinopathy or nephropathy.3 However, the conclusions of this report were confined to microvascular complications and were limited by methodological and data analysis factors: first, only 7-point glucose profiles taken at 3-month intervals were available for analysis of glucose variability; second, glucose variability was quantified using standard deviation, which has been shown to be a poor measure of variability-associated risks.4
By definition, measures of long-term average glycemia such as A1C are not designed to capture the rate and magnitude of acute BG fluctuations.5,6 As A1C often represents the only test of glycemic control routinely performed in clinical practice, the presence of marked diurnal BG fluctuations likely remains hidden for many patients. The emergence of evidence suggesting that A1C is insufficient for determining the risk for the full spectrum of complications associated with diabetes is therefore alarming. While the devastating effects of unpredictable episodes of severe hypoglycemia have been well documented,7–9 the same cannot be said of hyperglycemia. An increasing number of studies implicate acute hyperglycemic excursions as a factor contributing to morbidity associated with diabetes. Recently, mood and cognitive disturbances, known symptoms of hypoglycemia, have been demonstrated to occur in response to acute hyperglycemia.10–13 Several long-term epidemiological studies have also documented an association between 2-h post-meal BG level and an elevated risk for cardiovascular disease, a relationship that appears independent of other glycemic variables, including fasting BG and A1C.14–18 Finally, an increase in the magnitude of BG excursions, but not A1C or fasting plasma BG, has been shown to correspond with elevations in laboratory markers associated with cardiovascular risk, including measures of oxidative stress and inflammation.19 Taken together, this evidence highlights the need for therapies that lower A1C and minimize acute BG extremes. It also underscores the importance of developing standardized measures that quantify aspects of glycemic control not reflected by A1C.
Pramlintide is a novel, first-in-class therapy, that when used adjunctively with mealtime insulin in patients with type 1 and type 2 diabetes improves overall glycemic control and reduces BG fluctuations. The current study characterized the effects of pramlintide on diurnal BG variability via a retrospective analysis of pre- and post-meal self-monitored BG (SMBG) readings. The data were analyzed using previously validated20–22 measures of risk for hypoglycemia (the low BG index [LBGI]), hyperglycemia (the high BG index [HBGI]), and glucose instability (the BG rate of change). As reported elsewhere,23 mean A1C reductions at the end of the trial were nearly identical for pramlintide- versus placebo-treated subjects. Thus, the results of this post hoc analysis elucidate glycemic effects of pramlintide treatment, which are independent of A1C and not achievable with intensive insulin alone.
Subjects and Methods
Patients
A database containing 253,122 SMBG records from 295 patients with type 1 diabetes participating in a study to investigate the effects of pramlintide on metabolic control was analyzed. At baseline patients were randomized into two groups: pramlintide (n = 148) and placebo (n = 149). As previously reported, patients in the two groups were well matched with respect to age, weight, duration of diabetes, body mass index, and gender.23
Procedure
Throughout the study, all patients self-monitored their BG concentrations a minimum of six times per day: before and after breakfast, lunch, and dinner. Pramlintide was initiated at a 15 μg dose immediately before each meal and titrated in 15 μg increments to a final dose of 60 μg as tolerated.23 During the pramlintide initiation period (4 weeks), mealtime insulin doses were reduced by 30–50%. During the maintenance period (25 weeks), all patients adjusted insulin to achieve predetermined glycemic targets.
SMBG data for the current analysis included measurements from the maintenance period only. The 248 subjects who had 3 months of maintenance data with at least 180 readings total (e.g., two readings per day) were included in the analysis: 119 of these subjects were receiving pramlintide, and 129 were receiving placebo. Any data from the maintenance period taken less than 0.5 h apart or more than 4 h apart, as well as data from patients with less than 3 months of maintenance SMBG measurements, were excluded (<3% of all maintenance period readings). Statistical comparisons showed that baseline characteristics did not differ significantly across the two groups (all P > 0.5); thus, it was assumed that the initial study randomization was preserved during the selection of patients having sufficient for analysis SMBG data during their maintenance period (Table 1).
Table 1.
Baseline Demographics
| Demographics | Placebo (n = 129) | Pramlintide (n = 119) |
|---|---|---|
| Sex: male/female (%) | 42/58 | 46/54 |
| Age (years) | 42 ± 11 | 42 ± 14 |
| Weight (kg) | 80.6 ± 17.0 | 81.8 ± 17.4 |
| A1C (%) | 8.1 ± 0.8 | 8.0 ± 0.8 |
| Duration of diabetes (years) | 21 ± 12 | 20 ± 12 |
Dynamics of postprandial glucose excursions
Using pairs of pre- and postprandial SMBG readings and their exact timing, the pre- to postprandial BG rate of change was computed using the equation: (BGpost − BGpre)/(elapsed time), measured in mmol/L/h.20 The BG rates of change were compared between the two patient groups using a 2 × 3 repeated-measures analysis of variance (treatment group × meal [breakfast, lunch, dinner]).
Risk analysis of SMBG data
The risk analysis20 proceeded in three steps: First, the nonlinear transformation f(BG) = 1.794 × [(ln (BG))1.026 − 1.861] was applied to all SMBG readings. Second, a quadratic risk function, r(BG) = 10 × f(BG)2, was applied to the data. This procedure generates a parabola with a minimum value of 0 achieved at BG = 6.25 mmol/L (112.5 mg/dL), a safe euglycemic reading, and maximum values of 100 at the extreme ends of the BG scale. Thus, r(BG) can be interpreted as a measure of the risk associated with a certain BG level. The left branch of this parabola identifies the risk of hypoglycemia, while the right branch identifies the risk of hyperglycemia. Third, given a series of SMBG readings, 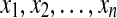 , the LBGI and HBGI are computed as:
, the LBGI and HBGI are computed as:
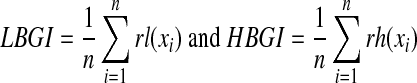 |
where
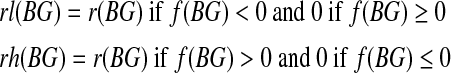 |
In other words, the LBGI is a non-negative quantity that increases when the number and/or extent of low BG readings increase; the LBGI has been shown to be predictive of severe hypoglycemia.22,24 Similarly, the HBGI increases with increased number and/or extent of high BG and is associated with A1C.21 The LBGI and the HBGI were analyzed using 2 × 2 × 3 repeated-measures analyses of variance (treatment group × pre-meal to post-meal × type of meal). Similar analysis was used to assess the interaction effect of preprandial and postprandial average BG increase by meal. All analyses included average BG as a covariate, which allowed for the evaluation of the risk-reducing effects of pramlintide independently of its effects on average glycemia.
Results
Average glycemia
Average glucose was reduced almost uniformly across both groups from the initiation to the maintenance period of the study: from 9.40 mmol/L (169.2 mg/dL) to 8.74 mmol/L (157.3 mg/dL) for pramlintide-treated patients and from 9.94 mmol/L (178.9 mg/dL) to 9.17 mmol/L (165.1 mg/dL) for placebo-treated patients (effect of time: F = 12.9, P < 0.005). During the maintenance period there was no further reduction in average glycemia (F = 1.0, difference not significant). The initial reduction in average glycemia is consistent with the previously reported overall across-group reduction in A1c during the study.23 To account for the generally lower average BG in the pramlintide group during the maintenance period, we used average BG as a covariate in all analyses.
Pre- to postprandial glucose variability
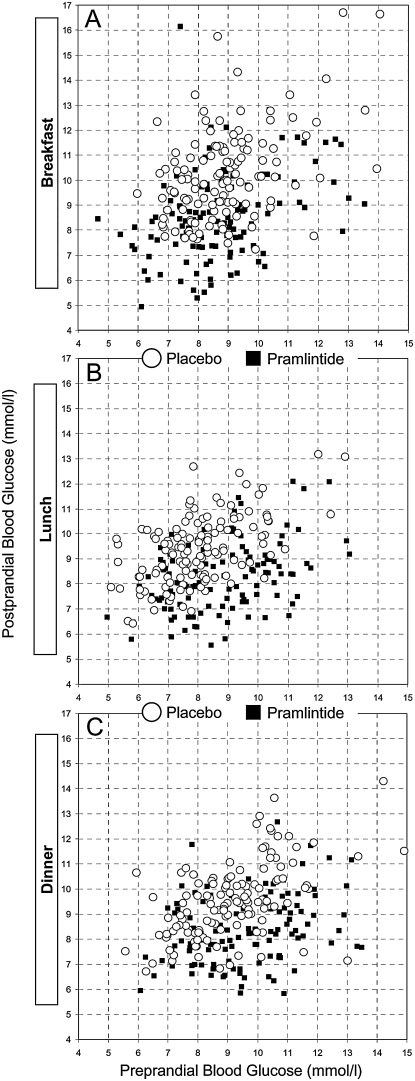
Figure 1 presents the pre- to postprandial glucose variability of all patients around breakfast (Fig. 1A), lunch (Fig. 1B), and dinner (Fig. 1C). The idea of the graphs is the following: for each meal the preprandial glucose of each person is plotted on the x-axis, while the postprandial glucose is plotted on the y-axis. This way, the difference of the y–x coordinates of each dot is the average pre- to postprandial excursion of a person observed throughout the study. A shift down of the group data cloud indicates reduced postprandial glucose values. A shift to the left indicates increased risk for hypoglycemia. Compared to placebo, the data of the pramlintide group appear shifted down at all meals and shifted left at breakfast. This visual impression is supported by the numerical results and the statistical tests presented in the following sections.
FIG. 1.
(A–C) Scatterplots of the pre- to postprandial glucose excursions observed during the maintenance phase of the study at breakfast, lunch, and dinner, respectively. The data of the pramlintide group (black squares) are generally below the data of the placebo group (circles), indicating lower postprandial glucose excursions on pramlintide. Except for breakfast, there is no apparent shift of the data to the left; therefore there is no increased risk for hypoglycemia on pramlintide.
Dynamics of postprandial glucose excursions
Overall, the median elapsed time between a preprandial SMBG reading and its corresponding postprandial reading was 1.5 h. In pramlintide-treated patients, the average (across all meals) pre- to postprandial BG rate of change was negative, −0.47 mmol/L/h (−8.46 mg/dL/h), which was mainly due to decreased BG post-dinner. In placebo-treated patients the pre- to postprandial BG rate of change was positive, 0.74 mmol/L/h (13.3 mg/dL/h). The effect of pramlintide on the BG rate of change was highly significant (F = 83.8, P < 0.0001) (Table 2A). Stratified by meal, the BG rate of change in pramlintide-treated patients treated was increasingly negative throughout the day. The BG rate of increase for placebo-treated patients diminished as well, but the difference between pramlintide and placebo held steady at approximately 1.2 mmol/L/h (21.6 mg/dL/h) throughout the day. As a result, there was no group by meal interaction effect (F = 2.3, difference not significant). Because of lower pre- to postprandial glucose rate of change, the average postprandial glucose with pramlintide was significantly lower than the average postprandial glucose with placebo (F = 80.6, P < 0.0001) (Table 2A).
Table 2.
Effects of Pramlintide on the Dynamics of Pre- to Postprandial BG Excursions and on the Risks of Hypoglycemia and Hyperglycemia
| |
|
Pramlintide |
Placebo |
|
||
|---|---|---|---|---|---|---|
| Meal | Pre-meal | Post-meal | Pre-meal | Post-meal | Group effect (F and P level)a | |
| A. Dynamics of postprandial glucose excursions | ||||||
| Pre- to postprandial BG | Breakfast | −0.12/ −2.2 | 1.02/18.4 | F = 83.3 | ||
| rate of change | Lunch | −0.41/−7.2 | 0.93/16.7 | P < 0.0001 | ||
| [(mmol/L/h)/(mg/dL/h)] | Dinner | −0.89/−16.0 | 0.27/4.9 | |||
| Average BG | Breakfast | 8.7/156.6 | 8.5/153.8 | 8.9/160.2 | 10.2/183.6 | F = 80.6 |
| [(mmol/L)/(mg/dL)] | Lunch | 8.8/158.4 | 8.3/149.4 | 8.0/144.0 | 9.3/167.4 | P < 0.0001 |
| Dinner | 9.6/172.8 | 8.4/151.2 | 9.1/163.8 | 9.5/171.0 | ||
| B. Risk analysis of pramlintide effect | ||||||
| LBGI (risk for hypoglycemia) | Breakfast | 3.1 | — | 2.7 | — | F = 10.3 |
| Lunch | 2.9 | — | 4.0 | — | P = 0.002 | |
| Dinner | 2.1 | — | 2.6 | — | ||
| HBGI (risk for hyperglycemia) | Breakfast | 5.4 | 5.1 | 5.6 | 8.2 | F = 73.1 |
| Lunch | 5.7 | 4.4 | 4.2 | 6.2 | P < 0.0001 | |
| Dinner | 7.5 | 4.7 | 6.4 | 6.7 | ||
All analyses include average BG as a covariate; thus the significance level is independent of average glycemia.
Risk of hypoglycemia
Throughout the day, the BG nadir was not significantly lower (t = 1.7, difference not significant) with pramlintide (2.1 ± 0.5 mmol/L [37.8 ± 9 mg/dL]) compared with placebo treatment (2.2 ± 0.5 mmol/L [39.6 ± 9 mg/dL]), indicating that pramlintide-treated patients were not exposed to lower BG levels than placebo-treated patients. The overall risk of preprandial hypoglycemia as quantified by the LBGI was marginally lower in pramlintide-treated patients (LBGI = 2.7 ± 1.5) versus placebo-treated patients (LBGI = 3.1 ± 1.9; F = 10.3, P = 0.002). This observation was confirmed by comparing the distributions of patients in moderate- (2.5 < LBGI < 5.0) and high-risk (LBGI >5.0) groups.22 Among pramlintide-treated patients, 36% were at moderate risk of preprandial hypoglycemia, and 9% were at high risk. Among placebo-treated patients, these percentages were 41% and 15%, respectively (Mann-Whitney Z = 1.8, P = 0.07).
Table 2B presents the pre-meal risk for hypoglycemia stratified by meal. In pramlintide-treated patients the risk for hypoglycemia was highest pre-breakfast, while in the placebo-treated patients, the risk was highest pre-lunch, which led to a significant group × meal interaction effect (F = 9.2, P = 0.001).
Risk of hyperglycemia post-meals
The overall postprandial HBGI indicated an almost 50% higher risk of postprandial hyperglycemia in placebo-treated patients compared with pramlintide-treated patients: 7.1 ± 3.2 versus 4.8 ± 2.6 (F = 73.1, P < 0.0001). Table 2B presents this risk stratified by meal: the risk of postprandial hyperglycemia in pramlintide-treated patients did not change substantially throughout the day (F = 3.7, P = 0.03), while a significant variation was observed among placebo-treated patients (F = 21.7, P < 0.001), with highest risk of hyperglycemia after breakfast (Table 2B).
Conclusions
This analysis demonstrated that pramlintide treatment reduced glucose variability, particularly in terms of reduced pre-to postprandial BG excursions. The variability-reduction effect of pramlintide was independent from, and substantially more significant than, any reduction in average glycemia. Indeed, reduction in average glycemia occurred concurrently in both the treatment and the placebo groups and was limited to the transition from initiation to maintenance periods. Thus, this reduction is likely a study effect rather than an effect of pramlintide. Because A1c is generally proportional to average glucose (with certain corrections for hypo- and hyperglycemia21), this result is consistent with the previously reported overall reduction in A1c.23 This result is also consistent with pramlintide's mechanisms of action: the primary effects of pramlintide that are likely to influence glycemic control include its ability to reduce the rate of gastric emptying, the reduction of inappropriately elevated glucagon levels in subjects with diabetes mellitus, and its modest but consistent effects to reduce appetite and increase satiety, the latter of which appear to persist beyond initial side effects of nausea.25–28 The blunting of postprandial hyperglycemia resulting from pramlintide treatment occurred at all meals, with relatively constant differences between BG rates of change in the pramlintide- versus placebo-treated patients at all three meals. Post hoc analysis indicated that the post-meal to pre-meal BG rate of change was also lower with pramlintide treatment (F = 65.3, P < 0.0001), suggesting that its postprandial therapeutic effect occurred concurrently with an overall decrease in glucose variability.
In previous studies, patients with type 1 and type 2 diabetes expressed symptoms such as sadness/feeling blue, irritability/frustration, nervousness/anxiety, etc., during large postprandial BG excursions.12 Pilot data suggest that the BG rate of change may be an important predictor of postprandial mood and cognitive symptoms.13 Postprandial hyperglycemia may also influence postprandial atherogenic risk factors, including quantitative and qualitative alterations in lipoproteins and markers of oxidative stress.29 Because a reduced rate of BG fluctuation may reduce negative cognitive and mood symptoms, a reduction in these postprandial symptoms upon pramlintide treatment would be a reasonable hypothesis to test in future studies. It may also be useful to determine whether the pharmacological effects of pramlintide influence atherogenic risk factors in the postprandial period.
A consequence of reducing hyperglycemia is often an increase in the risk of hypoglycemia. In some studies, pramlintide has caused an increased risk of hypoglycemia, occasionally including severe hypoglycemia. Reduced meal insulin doses are important during initiation and titration of this therapy to prevent this. However, this analysis showed that reducing hyperglycemia with pramlintide treatment marginally decreased the risk of hypoglycemia. In this study pramlintide-treated patients were not exposed to lower BG levels than placebo-treated patients. In keeping with this observation, the risk of preprandial hypoglycemia as measured by the LBGI was marginally lower in pramlintide- compared with placebo-treated patients. However, we need to emphasize that the mealtime insulin doses with pramlintide were reduced by 30–50%. Thus, in order to avoid potential hypoglycemia, appropriate insulin adjustment should be made with pramlintide treatment. We should also acknowledge that pramlintide treatment may present problems for certain patients. Among these is the initial nausea that appears most intense in amylin-deficient type 1 diabetes subjects. This side effect limits initial dosing and requires gradual dose titration.30 Also, because of its duration of action, pramlintide must be administered by a separate injection at each meal.
In conclusion, these results indicate that an important effect of pramlintide treatment in patients with type 1 diabetes is the reduction in the rate and magnitude of pre- to postprandial BG increase, resulting in decreased risk of postprandial hyperglycemia. This effect was independent of improved average glycemia and was not accompanied by increased risk of hypoglycemia. Therefore, it can be speculated that the improvement in average glycemia due to pramlintide therapy observed in this and other studies23,31 might be secondary to reduced BG variability, which results in reduced risk of hypoglycemia, thereby moderating a major obstacle to improved glycemic control.
Acknowledgments
The development of the analytical methods used in this study was supported by grant RO1 DK 51562 from the National Institutes of Health. The authors would like to thank Amylin Pharmaceuticals, Inc., San Diego, CA, for sharing their database. This study has been conducted under Clinical Trial Registry Number NCT00107107.
References
- 1.The Diabetes Control Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.Kilpatrick ES. Rigby AS. Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29:1486–1490. doi: 10.2337/dc06-0293. [DOI] [PubMed] [Google Scholar]
- 4.Kovatchev BP. Otto E. Cox DJ. Gonder-Frederick LA. Clarke WL. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch IB. Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178–181. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Derr R. Garrett E. Stacy GA. Saudek CD. Is A1C affected by glycemic instability? Diabetes Care. 2003;26:2728–2733. doi: 10.2337/diacare.26.10.2728. [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43:1378–1389. doi: 10.2337/diab.43.11.1378. [DOI] [PubMed] [Google Scholar]
- 8.Cryer PE. Hypoglycemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 9.Cryer PE. Davis SN. Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 10.Van der Does FE. De Neeling JN. Snoek FJ. Kostense PJ. Grootenhuis PA. Bouter LM. Heine RJ. Symptoms and well-being in relation to glycemic control in type II diabetes. Diabetes Care. 1996;19:204–210. doi: 10.2337/diacare.19.3.204. [DOI] [PubMed] [Google Scholar]
- 11.De Sonnaville JJ. Snoek FJ. Colly LP. Deville W. Wijkel D. Heine RJ. Well-being and symptoms in relation to insulin therapy in type 2 diabetes. Diabetes Care. 1998;21:919–924. doi: 10.2337/diacare.21.6.919. [DOI] [PubMed] [Google Scholar]
- 12.Cox DJ. Gonder-Frederick LA. McCall A. Kovatchev BP. Clarke WL. The effects of glucose fluctuation on cognitive function and QOL: the functional costs of hypoglycemia and hyperglycemia among adults with type 1 or type 2 diabetes. Int J Clin Pract. 2002;129(Suppl):20–26. [PubMed] [Google Scholar]
- 13.Kovatchev BP. Cox DJ. Summers KH. Gonder-Frederick LA. Clarke WL. Postprandial glucose dynamics and associated symptoms in Type 2 diabetes mellitus. J Appl Res. 2003;3:449–458. [Google Scholar]
- 14.Hanefeld M. Fisher S. Julius U. Risk factor for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577–1583. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM. Lehto S. Rönnemaa T. Pyörälä K. Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 16.Haffner S. The importance of postprandial hyperglycemia in development of cardiovascular disease in people with diabetes. Int J Clin Pract. 2001;(Suppl 123):24–26. [PubMed] [Google Scholar]
- 17.Hanefeld M. Postprandial hyperglycemia: noxious effects on the vessel wall. Int J Clin Pract. 2002;129(Suppl):45–50. [PubMed] [Google Scholar]
- 18.Esposito K. Giugliano D. Nappo F. Martella K Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–219. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 19.Quagliaro L. Piconi L. Assalone R. Martinelli L. Motz E. Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 20.Kovatchev BP. Cox DJ. Gonder-Frederick LA. Clarke WL. Methods for quantifying self-monitoring blood glucose profiles exemplified by an examination of blood glucose patterns in patients with type 1 and type 2 diabetes. Diabetes Technol Ther. 2002;4:295–303. doi: 10.1089/152091502760098438. [DOI] [PubMed] [Google Scholar]
- 21.Kovatchev BP. Cox DJ. Kumar A. Gonder-Frederick LA. Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose (SMBG) data. Diabetes Technol Ther. 2003;5:817–828. doi: 10.1089/152091503322527021. [DOI] [PubMed] [Google Scholar]
- 22.Kovatchev BP. Cox DJ. Gonder-Frederick LA. Young-Hyman D. Schlundt D. Clarke WL. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21:1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 23.Edelman S. Garg S. Frias J. Maggs D. Wang Y. Zhang B. Strobel S. Lutz K. Kolterman O. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006;29:2189–2195. doi: 10.2337/dc06-0042. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 25.Young AA. Amylin's physiology and its role in diabetes. Curr Opin Endocrinol Diabetes. 1997;4:282–290. [Google Scholar]
- 26.Whitehouse F. Kruger DF. Fineman M. Shen L. Ruggles JA. Maggs DG. Weyer C. Kolterman OG. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25:724–730. doi: 10.2337/diacare.25.4.724. [DOI] [PubMed] [Google Scholar]
- 27.Fineman M. Weyer C. Maggs DG. Strobel S. Kolterman OG. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–508. doi: 10.1055/s-2002-34790. [DOI] [PubMed] [Google Scholar]
- 28.Levetan C. Want LL. Weyer C. Strobel SA. Crean J. Wang Y. Maggs DG. Kolterman OG. Chandran M. Mudaliar SR. Henry RR. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26:1–8. doi: 10.2337/diacare.26.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163:1306–1316. doi: 10.1001/archinte.163.11.1306. [DOI] [PubMed] [Google Scholar]
- 30.Want LL. Ratner RE. Pramlintide: a new tool in diabetes management. Curr Diabetes Rep. 2006;6:344–349. doi: 10.1007/s11892-006-0004-0. [DOI] [PubMed] [Google Scholar]
- 31.Hollander PA. Levy P. Fineman MS. Maggs DG. Shen LZ. Strobel SA. Weyer C. Kolterman OG. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784–790. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]