Abstract
The remarkable decline in cardiovascular disease (CVD) experienced in developed countries over the last 40 years appears to have abated. Currently, many CVD patients continue to show cardiac events despite optimal treatment of traditional risk factors. This evidence suggests that additional interventions, particularly those aimed at nontraditional factors, might be useful for continuing the decline. Psychosocial stress is a newly recognized (nontraditional) risk factor that appears to contribute to all recognized mechanisms underlying cardiac events, specifically, (a) clustering of traditional cardiovascular risk factors, (b) endothelial dysfunction, (c) myocardial ischemia, (d) plaque rupture, (e) thrombosis, and (f) malignant arrhythmias. A better understanding of the behavioral and physiologic associations between psychosocial stress and CVD will assist researchers in identifying effective approaches for reducing or reversing the damaging effects of stress and may lead to further reductions of CVD morbidity and mortality.
Index Terms: cardiovascular disease, psychosocial interventions, psychosocial stress, review
Cardiovascular disease (CVD) remains the largest contributor to morbidity and mortality in developed countries.1 Advances in prevention and treatment in the last 40 years have resulted in a 40% decline in mortality from this disease. 1 Despite this remarkable success, it is sobering to note that the decline has abated recently,2 paradoxically despite continued reductions in traditional risk factors.3 Up to 50% of patients with established coronary artery disease have recurrent cardiac events, such as myocardial infarction and cardiac death, even with aggressive management of traditional risk factors.4 From these observations, it is clear that additional therapies aimed at managing nontraditional risk factors might be useful.
Recently, many studies have demonstrated that psychosocial stress is a risk factor for cardiovascular disease in both patients with established disease5,6 and nondiseased individuals.7 Although most of these studies appear to support a simple, direct relationship between psychosocial stress and CVD, the magnitude of risk varies considerably across studies,5–8 and not all have demonstrated such positive findings.9,10 Given the complex nature of human behavior, it is likely that the relationship between psychosocial stress and CVD is mediated at many points along the sequence of pathophysiologic steps and conditions thought to be responsible for CVD events.
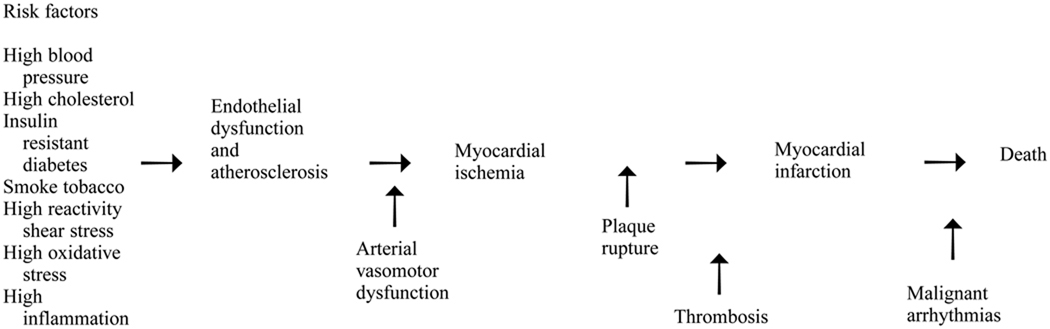
A model of the recognized steps leading to the different events characterizing CVD is shown in Figure 1. This model generalizes from many studies. All these mechanisms are not necessarily at work in each individual with fatal CVD. At the beginning are the traditional risk factors, many of which are thought to contribute directly to endothelial dysfunction and atherosclerosis. Next in line is myocardial ischemia, which can be enhanced by arterial vasomotor dysfunction. Plaque rupture and thrombosis contribute to or trigger myocardial infarction, and the presence of malignant arrythmias can mean the difference between survival and death. As reviewed here, evidence now exists that links each of these steps to psychosocial stress in a way that may help to explain the lack of consistency among studies relating traditional risk factors to CVD. The results reviewed also point to a need for continued mechanistic understanding as an aid to identifying effective therapies in the psychosocial arena.
Figure 1.
A model of recognized steps leading to events that characterize cardiovascular disease.
In the remainder of this article, we review the literature on pathophysiological mechanisms linking psychosocial stress and CVD events and introduce evidence suggesting new therapies that can be effective in further lowering CVD morbidity and mortality.
Pathophysiological Mechanisms Linking Psychosocial Stress and CVD Events
Psychosocial Stress and Clustering of Traditional Cardiovascular Risk Factors
Traditional cardiovascular risk factors, as outlined in the Framingham Study, include cigarette smoking, hypertension, diabetes mellitus, dyslipidemia, family history of premature coronary disease, and sedentary lifestyle.11 It is clear that psychosocial stress, assessed by a variety of measures, directly correlates with a higher prevalence and clustering of traditional cardiovascular risk factors (see Williams et al12 and Siegler et al13 for reviews). For example, although Type A behavior personality is no longer considered a traditional cardiovascular risk factor, it correlates with increases in blood pressure, cardiac reactivity, blood cholesterol, and cigarette smoking, as well as poorer diet and exercise habits.14 Indeed, the strength of the association between Type A behavior personality and the clustering of traditional risk factors probably explains the variable penetrance of Type A as a risk factor for CVD, depending on the completeness and accuracy of collection and statistical covariance analyses of traditional risk-factor data. Researchers found similar clustering when they considered psychosocial stress measures such as hostility,15 depression,16 and low socioeconomic status/social support.17 Indeed, adverse psychosocial variables are likely to cluster among themselves (eg, hostility, depression, lack of social support) as and also predicting clustering of traditional CVD risk factors.18
Psychosocial Stress and Atherosclerosis
Not surprisingly, most of the above-mentioned psychosocial variables have also been demonstrated to correlate with measures of atherosclerosis. Carefully controlled studies in cynomolgus monkeys have demonstrated the roles of psychosocial stress,19 including social distress20 created by varying cage rotations/restrictions, in atherosclerosis as measured at necropsy. It is noteworthy that the dominant male monkeys appear most susceptible to the stress-induced atherosclerosis, 21 suggesting a parallel to the research on human Type A behavior.
Type A behavior personality,14 hostility,22 depression/hopelessness,23 and job stress24 have all been found to correlate with atherosclerosis in humans when researchers use measures such as coronary angiography or carotid artery intimamedia thickness. In general, these human studies have adjusted for the traditional cardiovascular risk factors, suggesting that the psychosocial stress variables provide additional independent risks. Pathophysiologic links between psychosocial stress and atherosclerosis that are independent of traditional cardiovascular risk factors may include inflammation-induced low-density lipoprotein (LDL)-cholesterol oxidation, elevated shear stress, and adverse catecholamine and reproductive hormonal changes that lead to increased endothelial damage.25
Psychosocial Stress and Endothelial Function
There is evidence that endothelial dysfunction is one of the earliest signs of atherosclerosis so that the balance of local arterial mediators results in a loss of functional dilation. 26 Recent evidence also suggests that arterial vasomotor dysfunction plays an important role in acute cardiac events and death. Inappropriate vasoconstriction, or a lack of coronary artery dilation in response to an increased demand, is present in stable coronary heart disease.27,28 These symptoms can be the result of the underlying atherosclerosis and can contribute to the genesis of myocardial ischemia. Arterial vasomotor function reflects both endothelial function and the function of other local regulators, as well as systemic activity of the autonomic nervous system.
Preliminary work in both animals and humans suggests that psychosocial variables influence endothelial function. Psychosocial stress created by frequent cage rotation produced endothelial dysfunction in a primate model, even in the absence of diet-induced atherosclerosis,29 with a notable gender difference. In males, the dominant monkeys had the greatest endothelial dysfunction,29 whereas in females the subordinates demonstrated the greatest abnormalities,30 suggesting that behavioral and physiological gender differences may have implications for CVD.
One human angiographic study has demonstrated a relationship between reported anger and coronary endothelial dysfunction.31 Previous work evaluating a possible surrogate of endothelial function, peripheral vascular reactivity, has demonstrated links to psychosocial stress in monkeys,29 hostility in humans,32 and progression of atherosclerosis in humans.33 Pathophysiologic links between psychosocial stress and endothelial function may involve direct endothelial damage because of catecholamine and blood pressure surges, resulting in intimal damage, inflammatory-induced free radicals blocking nitric oxide synthesis, and increased endothelial reactivity triggered by activated platelets.34
Psychosocial Variables and Myocardial Ischemia
In the setting of atherosclerotic CVD, myocardial ischemia results when the demand for myocardial blood flow outstrips the supply. Mechanistically, this is triggered by increases in demand mediated by increases in heart rate and blood pressure and in reduced supply mediated by coronary artery vasoconstriction, typically during physical and/or mental exertion. 35 Prolonged myocardial ischemia results in myocardial infarction, most typically from obstructive thrombus formation. It is well documented now that laboratory mental stress triggers increased blood pressure demand,36 coronary artery vasoconstriction, and reduced blood flow37 with resultant myocardial ischemia.35 Parallel ambulatory studies in coronary artery disease patients in daily life show similar results38 and have been extended to demonstrate that both the intensity of mental effort and the presence of negative mood correlate with myocardial ischemia. In addition, both ambulatory38 and laboratory studies39 have demonstrated correlations between hostility and ischemia in humans.
Psychosocial Stress and Plaque Rupture
Current pathophysiologic understanding suggests that acute cardiovascular events such as myocardial infarction and unstable angina are precipitated by atherosclerotic rupture, in which the cholesterol crystals and cellular debris are extruded into the lumen of the coronary artery and the subendothelial collagen components are exposed to circulating blood, promoting intracoronary thrombus.40 Although it is possible that plaque rupture is serendipitous, increasing lines of evidence suggest it is more likely triggered by both internal and external events.41 Specifically, it has long been noted that these acute cardiovascular events occur in a circadian rhythm that matches the rhythm of sympathetic nervous system activity.42 Moreover, anger and other mentally stressful experiences have been identified as triggers in a carefully designed study of the behaviors preceding myocardial infarction.41 Pathophysiologic links between psychosocial stress and plaque rupture may include surges in heart rate, blood pressure, and sympathetic nervous system activity, as well as weakening of the collagenous plaque cap from inflammatory processes, all of which may contribute to plaque instability.43
Psychosocial Stress and Thrombosis
It is likely that atherosclerotic plaques rupture relatively frequently and that the propensity for thrombus formation is a determining factor in the progression to an acute cardiovascular event. Traditional risk factors clearly play a role in promoting thrombus formation. Research data demonstrate that dyslipidemia,44 diabetes,45 and cigarette smoking46 promote thrombus formation, probably through mechanisms mediated by platelets and some mechanisms not mediated by platelets. Preliminary studies suggest that psychosocial stress also plays a role in thrombus promotion. Pathophysiologic links appear to include increases in platelet aggregation or reactivity associated with anger expression47 and hostility48; between Type A/hostility and reduced bleeding time, as well as increased prostacyclin formation49; and between enhanced platelet aggregation and mental stress related to increases in catecholamines and sympathetic nervous system activity.50
Psychosocial Stress and Lethal Arrhythmias
Approximately half of the cardiovascular deaths experienced in the United States annually occur suddenly because of lethal ventricular arrhythmia (ventricular fibrillation), as the culmination of atherosclerotic plaque rupture/ischemia/infarction1 (Figure 1). We currently have no treatment for these patients other than the autonomic defibrillators implanted in the few who survive.
Electrical stability and ventricular fibrillation thresholds are influenced by many factors, including the autonomic nervous system. It is clear that lowered levels of parasympathetic nervous system tone and increased levels of sympathetic nervous system tone promote ventricular fibrillation in the myocardial substrate at risk. Work in dogs demonstrates that anger, produced by restraining the dog, lowers ventricular fibrillatory thresholds measured directly by electrophysiologic testing.51
More indirect measures are needed for human work. Heart rate variability, obtained from electrocardiographic recordings52 and baroreflex testing,53 are noninvasive correlates of autonomic nervous system tone that are responsive to both acute54 and chronic55 stress conditions. Multiple lines of evidence indicate that psychosocial stress, including mental stress,54 depression,56 and anxiety,57 influences the autonomic nervous system, as measured by heart rate variability and baroreflex testing. Because both heart rate variability and baroreflex measures have been demonstrated to predict future cardiovascular events,58,59 these tools provide a particularly rich insight into the interplay between psychosocial stress and CVD.
Trials of Intervention Approaches for Psychosocial Stress
As we have indicated in this review, psychosocial stress contributes to CVD in multiple ways and may well be the decisive factor determining survival or death in the majority of cases. It is reasonable, therefore, to ask whether interventions on the psychosocial level can reduce traditional risk factors for CVD or directly reduce CVD morbidity and death. A number of studies have addressed this question in clinical trials, with encouraging results. For example, 3 meta-analyses of stress management trials found reductions of recurrent cardiac events and death of 50% to 70%.60–62 However, the review by Linden and associates61 noted that these effects did not persist over the long term. Moreover, recent studies with larger groups and improved trial designs have given less impressive results,63,64 and a review of meta-analyses has highlighted the importance of choice of a stress-reduction approach in producing desired clinical outcomes.65
In regard to several of the risk factors that contribute to CVD, meta-analyses and direct comparisons indicate that traditional approaches to stress reduction, such as the Transcendental Meditation (TM) technique, are more effective than the clinically derived techniques modeled after them.65 Part 2 of this three-part series explores the issues surrounding the effectiveness of such interventions and reviews research on the TM technique as an example of an effective stress-reduction approach for preventing or reversing effects of psychosocial stress on pathophysiological mechanisms leading to morbidity and mortality from CVD.
Summary
It is clear that variables related to psychosocial stress contribute to CVD morbidity and mortality through complex interactions along a chain of pathophysiological events. Trials of interventions for psychosocial stress suggest that selected interventions may reduce CVD risk by affecting multiple sites along this chain and that the magnitude of reduction is comparable to that of other therapies, such as lipid lowering, antiplatelet or beta-blocker medications, and revascularization procedures. Continued efforts to understand behavioral and physiologic associations between psychosocial stress and CVD appear worthwhile and may lead to more widespread clinical use of new therapies for decreasing the toll taken by this disease.
ACKNOWLEDGMENT
This work was funded in part by grants from the John D. and Catherine T. MacArthur Foundation, the National Heart, Lung and Blood Institutes (232HL07380 and HL49910), and the National Center for Complementary and Alternative Medicine (Center Grant IP50AT00082-01).
Footnotes
This is the first of three articles on psychosocial stress and cardiovascular disease that will appear in future issues of Behavioral Medicine.
REFERENCES
- 1.Sytkowski PA, Kannel WB, Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med. 1990;322:1635–1641. doi: 10.1056/NEJM199006073222304. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987–1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Thom TJ. Death rates from coronary disease—progress and a puzzling paradox. N Engl J Med. 1998;229:915–917. doi: 10.1056/NEJM199809243391309. [DOI] [PubMed] [Google Scholar]
- 4.Haskell WL, Alderman EL, Fair JM, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease: The Stanford Coronary Risk Intervention Project (SCRIP) Circulation. 1994;89:975–990. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- 5.Frasure–Smith N. In-hospital symptoms of psychological stress as predictors of long-term outcome after acute myocardial infarction in men. Am J Cardiol. 1991;67:121–127. doi: 10.1016/0002-9149(91)90432-k. [DOI] [PubMed] [Google Scholar]
- 6.Ruberman W, Weinblatt E, Goldberg JD, et al. Psychosocial influences on mortality after myocardial infarction. N Engl J Med. 1987;311:552–559. doi: 10.1056/NEJM198408303110902. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren A, Tibblin G, Wilhelmsen L. Self-perceived psychological stress and incidence of coronary artery disease in middle-aged men. Am J Cardiol. 1991;68:1171–1175. doi: 10.1016/0002-9149(91)90189-r. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JV, Stewart W, Hall EM, Fredlund P, Theorell T. Long-term psychosocial work environment and cardiovascular mortality among Swedish men. Am J Public Health. 1996;86:324–331. doi: 10.2105/ajph.86.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed DM, LaCroix AZ, Karasek RA, Miller D, MacLean CA. Occupational strain and the incidence of coronary heart disease. Am J Epidemiol. 1989;129:495–502. doi: 10.1093/oxfordjournals.aje.a115160. [DOI] [PubMed] [Google Scholar]
- 10.Hlatky MA, Lam LC, Lee KL, et al. Job strain and the prevalence and outcome of coronary artery disease. Circulation. 1995;92:327–333. doi: 10.1161/01.cir.92.3.327. [DOI] [PubMed] [Google Scholar]
- 11.Forrester JS, Bairey Merz CN, Bush TL, et al. Task Force 4. Efficacy of risk factor management, from the 27th Bethesda Conference. J Am Coll Cardiol. 1996;27:991–1006. doi: 10.1016/0735-1097(96)87732-1. [DOI] [PubMed] [Google Scholar]
- 12.Barnes V, Schneider R, Alexander C, Staggers F. Stress, stress reduction, and hypertension in African Americans: an updated review. J Natl Med Assoc. 1997;89:464–476. [PMC free article] [PubMed] [Google Scholar]
- 13.Calderon R, Schneider RH, Alexander CN, Myers HF, Nidich SI, Haney C. Stress, stress reduction and hypercholesterolemia in African Americans: A review. Ethn Dis. 1999;9:451–462. [PubMed] [Google Scholar]
- 14.Williams RB, Barefoot JC, Haney TL, et al. Type A behavior and angiographically documented coronary atherosclerosis in a sample of 2,289 patients. Psychosom Med. 1988;50:139–152. doi: 10.1097/00006842-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Siegler IC, Peterson BL, Barefoot JC. Hostility during late adolescence predicts coronary risk factors at mid-life. Am J Epidemiol. 1992;136:146–154. doi: 10.1093/oxfordjournals.aje.a116481. [DOI] [PubMed] [Google Scholar]
- 16.Anda RF, Williamson DF, Escobdedo LG, et al. Depression and the dynamics of smoking. JAMA. 1990;264:1541–1546. [PubMed] [Google Scholar]
- 17.Williams RB, Barefoot JC, Califf RM, et al. Prognostic importance of social and economic resources among medically treated patients with angiographically documented coronary artery disease. JAMA. 1992;267:520–524. [PubMed] [Google Scholar]
- 18.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- 20.Shively CA, Clarkson TB, Kaplan JR. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis. 1989;77:69–76. doi: 10.1016/0021-9150(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan JR, Manuck SS, Clarkson TB, Lusso FM, Taub DM, Miller EW. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–735. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- 22.Julkunen J, Salonen R, Kaplan GA, et al. Hostility and the progression of carotid atherosclerosis. Psychosom Med. 1994;56:519–525. doi: 10.1097/00006842-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Everson S, Kaplan G, Goldberg D, Salonen R, Salonen J. Hopelessness and the 4-year progression of carotid atherosclerosis. The Kuopio Heart Disease Risk Factor Study. Arteriosclerosis, Thromb, and Vasc Biol. 1997;17:1490–1495. doi: 10.1161/01.atv.17.8.1490. [DOI] [PubMed] [Google Scholar]
- 24.Lynch J, Krause N, Kaplan GA, Salonen R, Salonen JT. Workplace demands, economic reward, and progression of carotid atherosclerosis. Circulation. 1997;96:302–307. doi: 10.1161/01.cir.96.1.302. [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ. A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation. 1990;82:II38–II46. [PubMed] [Google Scholar]
- 26.Furchgott RF, Zawadski D. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–378. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 27.Maseri A, L’Abbate A, Baroldi G, et al. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of preinfarction angina. N Engl J Med. 1978;99:1271–1277. doi: 10.1056/NEJM197812072992303. [DOI] [PubMed] [Google Scholar]
- 28.Maseri A. Coronary vasoconstriction: Visible and invisible. Editorial. N Engl J Med. 1991;325:1579–1580. doi: 10.1056/NEJM199111283252210. [DOI] [PubMed] [Google Scholar]
- 29.Williams JK, Vita JA, Manuck SB, Selwyn AP, Kaplan JR. Psychosocial factors impair vascular responses of coronary arteries. Circulation. 1991;84:2146–2153. doi: 10.1161/01.cir.84.5.2146. [DOI] [PubMed] [Google Scholar]
- 30.Williams JK, Shively CA, Clarkson TB. Determinants of coronary artery reactivity in premenopausal female cynomolgus monkeys with diet-induced atherosclerosis. Circulation. 1994;90:983–987. doi: 10.1161/01.cir.90.2.983. [DOI] [PubMed] [Google Scholar]
- 31.Boltwood MD, Taylor CB, Burke MD, et al. Anger report predicts coronary artery vasomotor response to mental stress in atherosclerotic segments. Am J Cardiol. 1993;72:1361–1365. doi: 10.1016/0002-9149(93)90180-k. [DOI] [PubMed] [Google Scholar]
- 32.Helmers KF, Krantz DS, Bairey Merz CN, et al. Defensive hostility: Relationships to multiple markers of cardiac ischemia in patients with coronary disease. Health Psychol. 1995;14:202–209. doi: 10.1037//0278-6133.14.3.202. [DOI] [PubMed] [Google Scholar]
- 33.Kamarck TW, Jennings JR, Manuck SB, et al. Cardiovascular reactivity is associated with carotid artery atherosclerosis in Finnish men. Ann Behav Med. 1997;19 suppl:S073. [Google Scholar]
- 34.Glasser SP, Selwyn AP, Ganz P. Atherosclerosis: Risk factors and the vascular endothelium. Am Heart J. 1996;131:379–384. doi: 10.1016/s0002-8703(96)90370-1. [DOI] [PubMed] [Google Scholar]
- 35.Rozanski AR, Bairey CN, Krantz DS, et al. Mental stress and silent myocardial ischemia in coronary artery disease patients. N Engl J Med. 1988;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 36.Krantz DS, Helmers KF, Bairey CN, Nebel LE, Hedges SM, Rozanski A. Psychophysiologic reactivity and determinants of myocardial oxygen demand in mental stress induced myocardial ischemia. Psychosom Med. 1990;53:1–12. doi: 10.1097/00006842-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Yeung A, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 38.Gabbay FH, Krantz DS, Kop WJ, et al. Triggers of myocardial ischemia during daily life in patients with coronary artery disease: Physical and mental activities, anger and smoking. J Am Coll Cardiol. 1996;27:585–592. doi: 10.1016/0735-1097(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 39.Helmers KF, Krantz DS, Howell RH, Klein J, Bairey CN, Rozanski A. Hostility and myocardial ischemia in coronary artery disease patients: evaluation by gender and ischemic index. Psychosom Med. 1993;55:29–36. doi: 10.1097/00006842-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Constantinides P. Plaque hemorrhages, their genesis and their role in supraplaque thrombosis and atherogenesis. In: Glagov S, Newman WP, Schaffer SA, editors. Pathobiology of the Human Atherosclerotic Plaque. New York: Springer–Verlag; 1990. pp. 393–411. [Google Scholar]
- 41.Bairey CN, Krantz DS, Rozanski A. Mental stress as an acute trigger of left ventricular dysfunction and blood pressure elevation in coronary patients. Am J Card. 1991;66:28G–31G. doi: 10.1016/0002-9149(90)90391-d. [DOI] [PubMed] [Google Scholar]
- 42.Muller JE, Stone PH, Turi SZ, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 43.Mittleman MA, Maclure M, Sherwood JB, et al. Triggering of acute myocardial infarction onset by episodes of anger. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 44.Shechter M, Bairey Merz CN, Paul-Labrador M, Shah PK, Kaul S. Plasma apolipoprotein B levels predict platelet–dependent thrombosis in patients with coronary artery disease. Cardiology. doi: 10.1159/000006964. In press. [DOI] [PubMed] [Google Scholar]
- 45.Shechter M, Bairey Merz CN, Paul-Labrador M, Kaul S. Blood glucose and platelet-dependent thrombosis in coronary artery disease patients. J Am Coll Cardiol. doi: 10.1016/s0735-1097(99)00545-8. In press. [DOI] [PubMed] [Google Scholar]
- 46.Ockene JK, Kuller LH, Svendsen KH, Meilahn E. The relationship of smoking cessation to coronary heart disease and lung cancer in the Multiple Risk Factor Intervention Trial (MRFIT) Am J Public Health. 1990;80:954–958. doi: 10.2105/ajph.80.8.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wenneberg SR, Schneider RH, Walton KG, et al. Anger expression correlates with platelet aggregation. Behav Med. 1997;22:174–177. doi: 10.1080/08964289.1997.10543551. [DOI] [PubMed] [Google Scholar]
- 48.Markovitz JH, Matthews KA, Kiss J, Smitherman TC. Effects of hostility on platelet reactivity to psychological stress in coronary heart disease patients and in healthy controls. Psychosom Med. 1996;58:143–149. doi: 10.1097/00006842-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Schonwetter DF, Dion PR, Ready AE, Dyck DG, Gerrard JM. The interactive effect of Type A behavior and hostility on bleeding time, thromboxane, and prostacyclin formation. J Psychosom Res. 1991;35:645–650. doi: 10.1016/0022-3999(91)90114-4. [DOI] [PubMed] [Google Scholar]
- 50.Levine SP, Towell BL, Surarez AM, Knieriem LK, Harris MM, George JN. Platelet activation and secretion associated with emotional stress. Circulation. 1985;71:1129–1134. doi: 10.1161/01.cir.71.6.1129. [DOI] [PubMed] [Google Scholar]
- 51.Verrier RL. Mechanisms of behaviorally induced arrhythmias. Circulation. 1987;76 Suppl. I:148–156. [PubMed] [Google Scholar]
- 52.Akselrod S, Gordon D, Ubel FA, et al. Power spectral analysis of heart rate fluctuation: A quantitative probe of beat–to–beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 53.La Rovere MT, Specchia G, Mortara A, et al. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction: A prospective study. Circulation. 1988;78:816–824. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 54.Sloan RP, Korten JB, Myers MM. Components of heart rate reactivity during mental arithmetic with and without speaking. Physiol & Behav. 1991;50:1039–1045. doi: 10.1016/0031-9384(91)90434-p. [DOI] [PubMed] [Google Scholar]
- 55.Pardo J, Bairey Merz CN, Paul-Labrador M, et al. Reproducibility and stability of 24-hour heart rate variability in coronary artery disease patients with daily life ischemia. Am J Cardiol. 1996;78:866–870. doi: 10.1016/s0002-9149(96)00458-4. [DOI] [PubMed] [Google Scholar]
- 56.Carney RM, Saunders RD, Freedland KE, et al. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–564. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 57.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Decreased heart rate variability in men with phobic anxiety (data from the Normative Aging Study) Am J Cardiol. 1995;75:882–885. doi: 10.1016/s0002-9149(99)80680-8. [DOI] [PubMed] [Google Scholar]
- 58.La Rovere MT, Bigger JT, Marcus FI, et al. Baroreflex sensitivity and heart–rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 59.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 60.Nunes EV, Frank KA, Kornfeld DS. Psychologic treatment for Type A behavior pattern and for coronary artery disease: A meta-analysis of the literature. Psychosom Med. 1987;49:159–173. doi: 10.1097/00006842-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Linden W, Stossel C, Maurice J. Psychosocial interventions for patients with coronary artery disease: A meta-analysis. Arch Intern Med. 1996;156:745–752. [PubMed] [Google Scholar]
- 62.Bairey Merz CN, Subramanian R. Efficacy of psychosocial interventions and stress management for reduction of coronary artery disease events. Preventive Cardiology. 1999;1:1–6. [Google Scholar]
- 63.Frasure-Smith N, Lesperance F, Prince RH, et al. Randomized trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet. 1997;350:473–479. doi: 10.1016/S0140-6736(97)02142-9. [DOI] [PubMed] [Google Scholar]
- 64.Jones DA, West RR. Psychological rehabilitation after myocardial infarction: Multicentre randomized controlled trial. BMJ. 1996;313:1517–1521. doi: 10.1136/bmj.313.7071.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orme-Johnson DW, Walton KG. All approaches to preventing or reversing effects of stress are not the same. Am J Health Promot. 1998;12:297–299. doi: 10.4278/0890-1171-12.5.297. [DOI] [PubMed] [Google Scholar]