Abstract
Recent studies suggest that older human immunodeficiency virus (HIV)-infected adults are at particular risk for HIV-associated neurocognitive disorders (HAND), including dementia. Deficits in attention/working memory are posited to play a central role in the development of HAND among older adults. The aim of the present study was to examine the possible protective benefits of spontaneous strategy use during a visual working memory task in 46 older and 42 younger adults infected with HIV. Results revealed a significant interaction between age and strategy use, with older adults who used a meta-cognitive strategy demonstrating superior working memory performance versus non-strategy users. This effect was not observed in the younger HIV-infected sample and was not better explained by possible confounding factors, such as education, comorbid medical conditions, or HIV disease severity. Within the older group, strategy use was associated with better executive functions and higher estimated verbal intelligence. Findings from this study suggest that working memory declines in older HIV-infected adults are moderated by the use of higher-level mnemonic strategies and may inform cognitive neurorehabilitation efforts to improve cognitive and everyday functioning outcomes in older persons living with HIV infection.
Keywords: Human immunodeficiency virus, Working memory, Aging, Strategies, Neuropsychology
Introduction
Infection with the human immunodeficiency virus (HIV) is commonly associated with mild-to-moderate deficits in working memory (Reger, Welsh, Razani, Martin, & Boone, 2002), which are evident in approximately one-third to one-half of infected individuals (e.g., Heaton et al., 2009; Rippeth et al., 2004). Of clinical relevance, HIV-associated working memory impairments can adversely affect higher-level cognitive abilities, such as decision-making (e.g., Martin et al., 2004), and are an established risk factor for declines in numerous aspects of everyday functioning (e.g., Heaton et al., 2004). Baddeley's (2003) influential multicomponent model proposes that working memory is comprised of a central executive that coordinates the activities of sensory slave components (i.e., a visuospatial sketchpad and phonological loop) and an episodic buffer, which enables access to recent and remote episodic memory. This complex series of interrelated processes is thought to facilitate higher-order cognitive abilities, most notably executive functions such as planning and reasoning (e.g., Martin et al., 2004). Considered in the context of this model, HIV-associated working memory impairment is suggestive of dysfunction in the central executive system (e.g., selection, planning, monitoring, and control of cognitive operations) and episodic buffer, rather than its sensory slave components (e.g., Hinkin et al., 2002). This is consistent with the neuropathogenesis of HIV disease, which predominately affects the cerebral white matter, basal ganglia, and frontal neocortex (see Ellis, Calero, & Stockin, 2009, for a review). In fact, neuroimaging studies show associations between deficits in working memory and dysregulation of prefrontal systems in HIV (e.g., Chang et al., 2001; Ernst, Chang, & Arnold, 2003), perhaps related to glial inflammation (e.g., Ernst et al., 2003).
The impact of aging on HIV-associated working memory deficits, however, is poorly understood. This is an issue of considerable relevance because there are a growing number of older adults living with HIV due to the effectiveness of antiretroviral therapies and subsequent decline in mortality rates (Centers for Disease Control and Prevention, 2007). Approximately 18% of the incident AIDS cases and 32% of AIDS-related deaths in the United States are among individuals aged 50 years and older (Centers for Disease Control and Prevention, 2007). Older HIV-infected adults tend to experience more rapid systemic disease progression (e.g., Goetz, Boscardin, Wiley, & Alkasspooles, 2001) and higher rates of mortality (Centers for Disease Control and Prevention, 2007). Independent of HIV disease severity, older adults are at increased risk of central nervous system (CNS) complications, including HIV-associated neurocognitive disorders (HAND; e.g., Valcour, Shikuma, Shiramizu, et al., 2004).
As such, aging may play an important role in the expression of HIV-associated deficits in working memory. It has long been known that healthy, seronegative adults experience a decline in working memory ability in later life (e.g., Bopp & Verhaeghen, 2005). Older age is associated with adverse changes in brain structure and function (e.g., Dekaban & Sadowsky, 1978), which may be particularly evident in the prefrontal cortex and neostriatum (e.g., Drachman, 2006) and therefore of direct relevance to working memory capacity (e.g., Rajah & D'Esposito, 2005). Akin to the above-described findings in HIV infection, dysfunction in the central executive has been cited as a possible mechanism for age-associated working memory declines (e.g., Meguro et al., 2000). Although several different mechanisms have been proposed (e.g., processing speed; Salthouse, 1994), evidence for the role of the central executive in age-related working memory deficits comes from studies of inhibition (e.g., Shimamura & Jurica, 1994), monitoring (e.g., Chaytor & Schmitter-Edgecombe, 2004; West, Ergis, Winocur, & Saint-Cyr, 1998), and strategy use (e.g., Brebion, Smith, & Ehrlich, 1997; cf. Bailey, Dunlosky, & Hertzog, 2009).
Thus, considering the independent effects of HIV disease and aging on similar aspects of working memory, older HIV-infected adults may be at particular risk for impairment in this domain. In fact, it has been posited that deficits in attention and working memory play a central role in the expression of HAND in older persons (Hardy & Vance, 2009). Cherner and colleagues (2004), for example, demonstrated that older HIV+ adults with more advanced CNS disease (viz., detectable viral load in the cerebrospinal fluid) were more likely than their younger seropositive counterparts to exhibit impairment on standard clinical measures of working memory. Yet, many questions remain regarding working memory impairments in older adults, including their nature, extent, and protective factors. The latter issue is particularly important because research now clearly demonstrates that not all older HIV-infected adults will develop an HAND, which suggests the presence of protective factors that might be systematically identified and exploited for prevention and/or remediation purposes.
One such protective factor is the deployment of higher-order meta-cognitive strategies (e.g., chunking). The cognitive neurorehabilitation literature suggests that strategy use is effective in normalizing working memory dysfunction in healthy older adults (e.g., Wegesin, Jacobs, Zubin, Ventura, & Stern, 2000) and in various clinical populations (e.g., mild traumatic brain injury [TBI]; Cicerone, 2002). In fact, even healthy adults who spontaneously deploy meta-cognitive strategies, such as chunking, during the performance of a complex working memory task reliably demonstrate fewer errors when compared with persons who do not use a strategy (e.g., Bryan & Luszcz, 2001). This phenomenon is also consistently reported in persons with CNS insults such as TBI (e.g., Schmitter-Edgecombe & Chaytor, 2003). It is theorized that the use of meta-cognitive strategies minimizes the complexity of the working memory task (e.g., concurrent processing) and allows for deeper levels of encoding, which in turn liberates cognitive resources that may be used to facilitate higher levels of overall performance.
Accordingly, the aim of the present study was to examine the possible protective benefits of spontaneous strategy use on visual working memory as measured by the Self-Ordered Pointing Test (SOPT; Petrides & Milner, 1982) in older and younger adults infected with HIV. The SOPT is a reliable and well-validated (e.g., Ross, Hanouskova, Giarla, Calhoun, & Tucker, 2007) experimental measure of working memory that involves the generation, monitoring, and maintenance of a response set to a series of novel complex visual stimuli. Considering the extensive literature on the adverse effects of aging on the SOPT (e.g., Chaytor & Schmitter-Edgecombe, 2004; Daigneault & Braun, 1993; Daigneault, Braun, & Whitaker, 1992; Shimamura & Jurica, 1994; West et al., 1998), we hypothesized that the older HIV+ adults would make more errors than their younger counterparts. Also drawing from prior literature in healthy adults (e.g., Bryan & Luszcz, 2001; Daigneault & Braun, 1993) and clinical populations (e.g., Roth et al., 2004; Schmitter-Edgecombe & Chaytor, 2003), we expected that individuals who spontaneously used a mnemonic strategy would commit fewer errors when compared with those who did not use such strategies. Finally, we aimed to determine whether the beneficial effects of strategy use on working memory performance differed between older and younger HIV-infected adults.
Materials and Methods
Participants
Participants were selected from among 166 HIV-infected subjects enrolled in an National Institutes of Mental Health (NIMH)-funded grant on prospective memory in HIV disease. Eligibility criteria for the current substudy included: (a) HIV infection as determined by enzyme-linked immunosorbent assays and confirmed by a Western blot test; and (b) current age <40 years (younger) or >50 years old (older). Although this classification approach yielded a group of “older” adults who were fairly young by the standard of the cognitive aging literature, it is nevertheless reflective of the HIV epidemic in the United States (Centers for Disease Control and Prevention, 2007) and is recommended by the NIMH guidelines for neuroAIDS research (Stoff, 2004). We excluded individuals with severe psychiatric (e.g., psychotic disorders), neurological (e.g., seizure disorders, closed head injuries with loss of consciousness >15 min, stroke, non-HIV-associated dementias, and active CNS opportunistic infections), or medical (e.g., advanced liver disease) conditions known to affect cognitive functioning. We also excluded individuals who met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) criteria for substance abuse or dependence within 6 months of evaluation or who provided a positive urine toxicology screen for illicit drugs (other than cannabis) on the day of evaluation. Finally, we excluded persons with estimated verbal IQ (VIQ) scores below 70, as measured by the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001). Four potential participants (2%) were excluded due to CNS (e.g., closed head injury) or psychiatric (e.g., current methamphetamine dependence) confounds and 74 (45%) were excluded based on their age at the time of assessment.
Thus, eligible study participants included 88 individuals comprised of 42 younger and 46 older HIV+ adults. The demographic, psychiatric, general medical, HIV disease, and treatment characteristics of the two study samples are displayed in Table 1. There were no significant between-groups differences in education, sex, ethnic identity, or estimated premorbid VIQ (ps > .10). Likewise, the groups were comparable in the prevalence of current and lifetime DSM-IV diagnoses of major depressive, generalized anxiety, and substance use disorders (ps > .10) as determined by the Composite International Diagnostic Interview (CIDI version 2.1; World Health Organization, 1998); however, the younger group reported slightly higher levels of recent overall affective distress (p < .10) on the Profile of Mood States (McNair, Lorr, & Droppleman, 1992). As might be expected, the older group had a longer estimated duration of HIV infection, lower nadir CD4 counts, as well as greater rates of AIDS diagnoses and hepatitis C co-infection (ps < .05). The two groups did not differ in current HIV disease markers, including CD4 count, viral burden, or the proportion prescribed antiretroviral therapies (ps > .10).
Table 1.
Demographic and psychiatric characteristics of the study samples
| Younger HIV+ (n = 42) | Older HIV+ (n = 46) | p-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 35.5 (4.8) | 55.7 (6.5) | <.001 |
| Education (years) | 13.3 (2.9) | 13.7 (2.8) | .603 |
| Sex (% women) | 14.3% | 21.7% | .365 |
| Ethnicity (% Caucasian) | 45.2% | 60.9% | .142 |
| WTAR verbal IQ | 102.4 (13.0) | 102.1 (14.2) | .920 |
| Psychiatric characteristics | |||
| Major depressive disorder | |||
| Current | 4.8% | 13.0% | .270 |
| Lifetime | 45.2% | 54.4% | .393 |
| Generalized anxiety | 5.7% | 8.5% | .610 |
| Current | 0.0% | 0.0% | — |
| Lifetime | 4.8% | 8.9% | .677 |
| Substance dependencea | |||
| Any | 54.8% | 47.8% | .516 |
| Alcohol | 33.3% | 28.3% | .606 |
| Marijuana | 7.1% | 6.5% | 1.000 |
| Stimulants | 38.1% | 41.3% | .759 |
| Opioids | 7.1% | 13.0% | .489 |
| Profile of Mood States | |||
| Total | 60.2 (34.9) | 47.2 (25.2) | .051 |
| Tension/anxiety | 10.2 (7.2) | 7.5 (5.3) | .054 |
| Depression/dejection | 10.1 (10.5) | 6.9 (6.3) | .085 |
| Anger/hostility | 7.0 (7.5) | 3.8 (4.1) | .018 |
| Vigor/activation | 15.9 (6.7) | 16.9 (6.9) | .460 |
| Fatigue/inertia | 9.2 (6.2) | 7.5 (6.7) | .227 |
| Confusion/bewilderment | 7.5 (4.8) | 6.4 (4.8) | .304 |
Notes: WTAR = Wechsler Test of Adult Reading; HIV = human immunodeficiency virus.
aDenotes a lifetime diagnosis.
Materials and Procedure
After providing written, informed consent, all participants completed the SOPT (Shimamura & Jurica, 1994) as part of a comprehensive medical, psychiatric, and neuropsychological evaluation, which also included the WTAR, California Verbal Learning Test-Second Edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), Trail-Making Test (TMT; Reitan & Wolfson, 1985), Digit Span subtest of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Psychological Corporation, 1997), and the Tower of London–Drexel (ToL; Culbertson & Zillmer, 2001). Details regarding this specific version of the SOPT, including examples of the test stimuli, can be found in Morgan and colleagues (2009). In brief, participants were presented a series of stimulus pages that included 12 computer-generated visual designs. Each of the 12 designs appeared in a 3 × 4 array on every stimulus page, but was located in different, randomly selected position in the array on each page. Participants were instructed to select a different design on each of the 12 stimulus pages; that is, on each page, they were asked to point to a design to which they had not pointed previously. The task was untimed and examiner did not provide feedback on the accuracy of responses. Participants underwent two separate, consecutive trials of this task in a randomized order using different sets of designs. The total number of errors across the two trials was the primary variable of interest. Immediately after the SOPT, the examiner queried the participants as to whether they used any strategy during the task. Participants who reported using a meta-cognitive strategy were classified as “strategy users” (n = 26; 30%). All strategy users noted that they grouped or “chunked” the designs based on the visual characteristics of the stimuli (e.g., solids, stripes, etc.). The proportion of strategy users did not differ between the old (15 of 46, 32.6%) and young (11 of 42, 26.2%) samples (p > .10).
Results
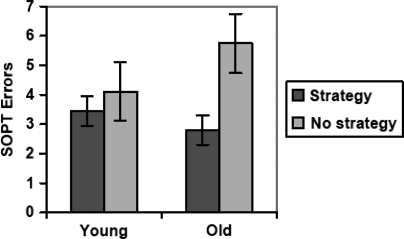
Although the dependent variable (i.e., SOPT errors) had a slightly non-normal distribution (Shapiro–Wilk test, p < .05), the results did not change using a non-parametric approach (i.e., Wilcoxon rank-sums) and therefore all analyses were conducted using standard parametric statistics for ease of interpretation and presentation. A two-way ANOVA indicated a main effect of efficient strategy use whereby strategy users performed significantly better than strategy non-users (p < .001; partial η2 = 0.157) but there was no main effect of age group (p > .10; partial η2 = 0.014). However, there was an interaction between strategy use and age—F(3,84) = 9.66, p < .001; partial η2 = 0.071. Planned post hoc analyses indicated that strategy use was associated with better SOPT performance in older adults (Cohen's d = 1.42; p < .001), but not in the young cohort (Cohen's d = 0.38; p > .10). The older strategy users (OSUs) performed comparably to younger strategy users (YSUs; Cohen's d = 0.39, p > .10), and superior to younger strategy non-users (YSNUs; Cohen's d = 0.83, p < .05), who in turn performed better than the older strategy non-users (OSNUs; Cohen's d = 0.81, p < .05). These data are displayed graphically in Fig. 1. Post hoc analyses showed that there was no main effect of trial order, nor any interaction between trial, age, and strategy use (p > .10).
Fig. 1.
Bar graph displaying the interaction between age group and strategy use on SOPT performance.
Despite the relative demographic comparability of the young and old samples, it is additionally important to consider the potentially confounding effects of factors that may have differed across the strategy users and non-users. Across the collapsed older and younger samples (N = 88), we observed that spontaneous strategy users (n = 26) had attained more years of education (p = .003, d = 0.73) and displayed higher estimated VIQs (p = .006, d = 0.63) when compared with non-strategy users (n = 62), but did not differ on any other variable listed in Tables 1 and 2 (ps > .05). Subsequent analyses across the four groups (i.e., OSU, YSU, OSNU, and YSNU) revealed a number of “conservative” biases (i.e., increasing risk of type II, rather than type I error). Specifically, the OSU group had a longer duration of HIV infection (vs. all other groups; p < .001), “lower” nadir CD4 counts (vs. YSNU; p < .05), and a higher proportion of AIDS (vs. all other groups; p < .05) and cholesterolemia (vs. OSNU; p < .05). Nevertheless, these analyses also revealed a series of potentially confounding biases (i.e., risk of type I error), indicating that the OSU group was more educated (vs. OSNU and YSNU; p < .05), had higher estimated VIQs (vs. OSNU and YSNU, p < .05), and had lower lifetime rates of stimulant dependence (vs. OSNU; p < .05). There were no differences in the OSU group versus the other cohorts on any of other demographic, psychiatric, or disease factors displayed in Tables 1 and 2 (all ps > .10). A follow-up linear regression analysis was then conducted to determine whether the OSU group effect persisted as a predictor SOPT total errors when considered alongside all of these factors (i.e., duration, nadir CD4, AIDS, cholesterolemia, education, VIQ, and lifetime stimulant dependence). The overall model was significant (adjusted R2 = 0.35, p < .001), but the only variables that contributed to the model were age/strategy use (p = .002) and VIQ (p < .001).
Table 2.
HIV disease and medical characteristics of the study samples
| Younger HIV+ (n = 42) | Older HIV+ (n = 46) | p-value | |
|---|---|---|---|
| Hepatitis C | 7.1% | 26.1% | .023 |
| Hypertension | 7.1% | 10.9% | .716 |
| Hypercholesterolemia | 2.4% | 6.5% | .618 |
| Diabetes mellitus | 2.4% | 6.5% | .618 |
| HIV disease characteristics | |||
| HIV duration (years) | 8.6 (5.4) | 13.1 (6.4) | <.001 |
| AIDS (%) | 45.2% | 69.6% | .021 |
| HAART (%) | 76.2% | 78.3% | .974 |
| Nadir CD4a (cells/μl) | 235 (112, 304) | 102 (28, 266) | .018 |
| Current CD4a (cells/μl) | 550 (326, 823) | 512 (253, 694) | .434 |
| Plasma HIV RNAa (log10) | 1.7 (1.7, 3.8) | 1.7 (1.7, 1.9) | .148 |
| CSF HIV RNAa,b (log10) | 1.7 (1.7, 1.8) | 1.7 (1.7, 1.7) | .454 |
Note: HIV = human immunodeficiency virus.
aData represent medians with interquartile ranges in parentheses.
bO+ n = 31 and Y+ n = 25.
Finally, we wished to examine whether working memory strategy use generalized to a variety of other cognitive ability areas. Table 3 shows that, when compared with the OSNU group (n = 31), the OSU cohort (n = 15) achieved significantly higher scores on the WTAR VIQ, CVLT-II semantic clustering index, and ToL total move variable (ps < .05), along with a trend-level finding on TMT Part B (p < .10). In contrast, there were no significant group differences on measures of simple verbal attention/working memory (i.e., WAIS-III Digit Span) or information processing speed (i.e., TMT A and ToL Problem-Solving Time;ps > .10). Similarly, there was no effect of SOPT strategy use on any of these other cognitive variables within the younger HIV+ cohort (ps > .10).
Table 3.
Associations between visual working memory strategy use and other cognitive functions among older HIV+ adults
| Strategy users (n = 15) | Non-strategy users (n = 31) | p-value | d | |
|---|---|---|---|---|
| General cognitive functions | ||||
| WTAR verbal IQ | 111.3 (10.2) | 97.6 (13.9) | .001 | 1.05 |
| Executive functions | ||||
| CVLT-II semantic clustering (z) | 1.8 (1.6) | 0.2 (1.9) | .006 | 0.87 |
| ToL total moves (standard) | 111.6 (13.6) | 100.6 (16.9) | .025 | 0.68 |
| TMT B (T) | 59.3 (11.9) | 51.9 (12.6) | .064 | 0.59 |
| Working memory | ||||
| WAIS-III Digit Span (T) | 48.9 (10.3) | 48.3 (7.8) | .832 | 0.07 |
| Information processing speed | ||||
| ToL time (standard) | 91.2 (14.5) | 92.1 (14.1) | .845 | −0.06 |
| TMT A (T) | 57.1 (13.8) | 53.0 (9.7) | .321 | 0.36 |
Notes: CVLT-II = California Verbal Learning Test (2nd ed.); ToL = Tower of London–Drexel; TMT = Trail-Making Test; WAIS-III = Wechsler Adult Intelligence Scale (3rd ed.); WTAR = Wechsler Test of Adult Reading; HIV = human immunodeficiency virus.
Discussion
HIV-associated neurocognitive deficits, particularly in the area of working memory, are highly prevalent in older adults (e.g., Valcour, Shikuma, Watters, et al., 2004), suggesting a need to identify protective factors that may inform rehabilitation efforts in this growing subset of the HIV-infected population. Therefore, this study sought to examine whether spontaneous strategy use protects against working memory deficits in older adults living with HIV. Consistent with prior research (e.g., Wegesin et al., 2000), we observed a main effect of strategy use, such that individuals who used a mnemonic strategy during the SOPT performed better than those who did not. However, this main effect was tempered by an interaction between strategy use and age; i.e., older adults with HIV who used a mnemonic strategy made fewer errors than their older seronegative counterparts who did not employ strategies, but there was no effect of strategy use in the young HIV+ adults. In other words, older HIV+ adults who grouped designs by visual details were better able to correctly identify novel targets on the SOPT, which requires an individual to generate, monitor, and maintain a response set to a series of repeated complex visual stimuli. In fact, older adults who implemented a strategy performed comparably to YSUs and significantly better than younger adults who did not use a strategy. Importantly, this finding was associated with a large effect size and remained significant after considering various demographic and disease characteristics that might otherwise have confounded the analyses (e.g., education). As such, these findings suggest that working memory declines in older HIV-infected adults may be moderated by the use of higher-level mnemonic strategies.
This study provides the first glimpse into the cognitive mechanisms of working memory impairment in older adults with HIV by suggesting that such deficits may be driven, at least in part, by deficiencies in strategy deployment. Our results are consistent with the literature on HIV-associated decrements in the strategic aspects of cognition (e.g., Gongvatana, Woods, Taylor, Vigil, & Grant, 2007; Woods, Dawson, Weber, Grant, & The HNRC Group, 2010), as well as frontostriatal neural injury in HIV infection (e.g., Chang et al., 2003) and aging (Drachman, 2006), which are brain regions integral to the strategic aspects of working memory (Collette & Van der Linden, 2002). Indeed, it has been hypothesized that HIV-associated working memory deficits are secondary to dysregulation of the central executive (cf. sensory slave systems), as outlined by Baddeley's model of working memory (Hinkin et al., 2002). NeuroAIDS imaging research also supports the link of frontal systems impairment by the pattern of abnormal blood oxygenation level-dependent signals in frontoparietal pathways during high cognitive load working memory tasks, which arguably require greater involvement of the central executive (Ernst et al., 2003). Similar patterns of increased levels of prefrontal activation are seen in healthy older adults during tasks involving greater working memory demands (e.g., Emery, Heaven, Paxton, & Braver, 2008), which suggests that the strategic aspects of working memory may be differentially affected in older adults (Bopp & Verhaeghen, 2005). In fact, older HIV+ adults are at increased risk for fronto-striato-thalamo-cortical circuitry dysfunction (Chang et al., 2008), including impairment in working memory (Cherner et al., 2004) and the strategic aspects of prospective memory (Woods et al., 2010).
Concurrent validity of this interpretation is supported by analysis of measures of executive functions and estimated verbal intelligence that were conducted within the older cohort. Specifically, OSUs demonstrated higher estimated premorbid VIQs, made significantly fewer moves during a novel problem-solving task, were more likely to spontaneously deploy a semantic clustering strategy on the CVLT-II, and were slightly faster in completing the TMT B. Together, these data suggest that the observed effects of this meta-cognitive approach may be generalizable from visual working memory because spontaneous strategy users were also more effective in navigating the demands of other complex cognitive tasks. In contrast, there was no effect of strategy use on measures of basic verbal working memory or information processing speed. Whether strategy use also generalizes to better daily functioning outcomes (e.g., employment, medication management, etc.) remains to be determined. Such efforts may shed some additional light on the cognitive aging paradox whereby a subset of older adults demonstrates superior everyday functioning (e.g., medication adherence) on semi-naturalistic assessments despite poorer performance on cognitive tasks in the laboratory (e.g., Rendell & Craik, 2000).
Non-cognitive factors might also play a role in determining whether one develops, deploys, and benefits from using a strategy during working memory tasks; in other words, individuals who implement strategies may be inherently different from those who do not. Indeed, across the entire cohort, strategy use was associated with higher cognitive reserve, including more educational attainment and higher estimated VIQ. This is consistent with prior studies showing that cognitive reserve may play an important role in the expression of executive dysfunction in HAND (Basso & Bornstein, 2000). Specific to the older group, who benefited most from spontaneously deploying mnemonic aids, strategy users were better educated, had higher estimated VIQs, and were less likely to have histories of stimulant dependence. Nevertheless, even when these potentially confounding factors were considered in the statistical model, the effects strategy use and age group remained a significant predictor of working memory performance.
Unexpectedly, the OSUs also had more advanced HIV disease, including longer duration of infection and higher rates of AIDS diagnoses, which represent a conservative bias. This is particularly interesting given the compelling research on historic immune compromise and risk of HAND (e.g., Valcour, Shikuma, Shiramizu, et al., 2004; Valcour, Shikuma, Watters, & Sacktor, 2004). Although this counterintuitive finding may be spurious and/or reflect a survival bias, it is also possible that OSUs have greater cognitive reserve, as supported by the independent effects of VIQ in the final regression model. There may be, however, more subtle and unexplored differences in CNS (e.g., brain reserve) or psychosocial (e.g., wisdom, social support) factors, which are important in the successful cognitive aging literature, that further explain age-related differences in the efficacy of strategy use and warrant further study.
Having ruled out the influence of several possible confounding factors, these results then raise questions about how and why older HIV+ adults may spontaneously generate and utilize meta-cognitive strategies. Considering the cognitive findings reported above, it may the decision that a strategy would be necessary for optimal performance may be controlled by an individual's insight into their cognitive deficits, as well as appropriate assessment of the nature and complexity of the task at hand. Second, the generation and selection of a strategy requires that an individual has learned and can activate the necessary tools to devise appropriate strategies for given situations. Next, the individual must be able to effectively deploy the decided-upon strategy, drawing upon available cognitive and environmental resources. Elements of cognitive control/flexibility may also be required to modify the selected strategy if it is determined that the deployed technique is ineffective. Finally, after task completion, one must evaluate the effectiveness of a strategy with the ability to make an unbiased assessment of task performance. The complex array of cognitive skills required for successful spontaneous strategy implementation suggests that not only is strategy use ability multidetermined, but that there may be various avenues through which to intervene.
The study sample and methods have several limitations that are worth consideration. First and foremost, we did not have access to a group of demographically comparable seronegative adults with SOPT data. As such, these findings do not directly speak to an HIV effect on working memory, but rather interpretations are restricted to age effects among HIV+ individuals. In addition, the relatively small samples of strategy users within the old and young groups may have increased our risk of type II error for some analyses. For example, although there were no significant SOPT differences based on strategy use in the younger group, we may have been underpowered to detect this effect, which was associated with a medium effect size. Another major limitation of the present study is that we measured strategy use with a single self-report item that was administered after the completion of the SOPT and did not include a post-test validation check. Although this is a commonly used and well-validated methodology (e.g., Roth et al., 2004), future investigators may wish to prospectively examine the specific component processes of strategy generation and use. Finally, we did not consider the possible role of psychotropic medications (e.g., Letendre et al., 2007), smoking (e.g., Durazzo et al., 2007), or recent substance use, which may have influenced our findings (we excluded individuals with substance abuse or dependence within 6 months of evaluation).
Although the present results suggest that spontaneous strategy use may be protective against working memory impairment, they also raise the question of whether didactic administration of working memory strategies through cognitive rehabilitation in this cohort would be equally effective. Recent attempts to provide explicit intensive cognitive training have been largely successful in remediating working memory and executive functioning deficits across several conditions, including multiple sclerosis (e.g., Flavia, Stampatori, Zanotti, Parrinello, & Capra, 2010) and TBI (e.g., Vallat-Azouvi, Pradat-Diehl, & Azouvi, 2009). Examining the specific strategy use components as proposed would be helpful in determining whether it is “strategy generation or strategy instruction” that could be protective against working memory deficits, as other studies in neurologic populations have noted normal performance by patients in the generation condition but worse performance when instructed to use a particular strategy (Goebel, Mehdorn, & Leplow, 2010). In order to better address the question at hand (as related to our study limitations), it would be beneficial for future research to examine this issue prospectively in a randomized fashion, and with attention paid to cognitive characteristics within the sample (e.g., working memory impairment) as well as strategy generalizability.
Despite the theoretical evidence to support our findings, existing literature on strategy use in older adults to improve working memory have produced mixed results. For example, Duverne, Lemaire, and Vandierendonck (2008) demonstrated that older adults might not benefit from the use of strategies in arithmetic-based working memory tasks due to the overall impact of cognitive slowing on task performance. Similarly, Bailey and colleagues (2009) found that performance on reading and operation span tasks were related to differences in processing speed between younger and older adults as opposed to differential strategy use. Other studies have indicated that both younger and older adults benefit equally from strategy use training on a working memory task and that there is no differential benefit (or deficit to the remediated) between groups (Carretti, Borella, & De Beni, 2007). More basically, there is some controversy over whether it is the strategic aspects (cf. automatic components) of working memory that impede performance in older adults, which may then help explain why strategy-based interventions are not differentially beneficial in this group (e.g., Rose, Bowman, Radziewicz, Lewis, & O'Toole, 2009). To our knowledge, strategy-based rehabilitation of working memory deficits (or other cognitive impairments) have yet to be explored in the context of aging and HIV infection, but our results suggest that such efforts may be worthwhile.
Indeed, it is surprising that cognitive rehabilitation approaches have not yet been developed for persons with HAND. In general, the mild-to-moderate cognitive deficits in memory and executive functions associated with HIV-related impairment lend themselves well to established intervention strategies (e.g., self-monitoring, external reminders). For example, while numerous studies (e.g., Gongvatana et al., 2007) have demonstrated spontaneous strategy use deficiency during learning and memory tasks in individuals with HIV, it has not been explored whether implementation of a strategy can remedy such deficits. Importantly, the current classification and treatment of HIV as a chronic illness provides a large window for intervention possibilities and long-term use of rehabilitation gains. Numerous studies in other disorders have established that cognitive rehabilitation may help reduce the risk of functional declines (e.g., acquired brain injury; Spikman, Boelen, Lamberts, Brouwer, & Fasotti, 2010), which is highly relevant in HIV considering the importance of daily tasks (e.g., antiretroviral adherence) to health outcomes. Clinical deployment of such cognitive neurorehabilitation strategies with older HIV+ adults, however, is not yet indicated and awaits a demonstration of effectiveness through careful, prospective research.
Funding
This research was supported by National Institutes of Health grants R01-MH073419 to SPW and P30-MH62512 to IG.
Conflict of Interest
None declared.
Acknowledgements
The HIV Neurobehavioral Research Center (HNRC) Group is affiliated with the University of California–San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, MD; Co-Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and J. Allen McCutchan, MD; Center Manager: Thomas D. Marcotte, PhD; Naval Hospital San Diego: Braden R. Hale, MD, MPH (PI); Neuromedical Component: Ronald J. Ellis, MD, PhD (PI), J. Allen McCutchan, MD, Scott Letendre, MD, Edmund Capparelli, PharmD, Rachel Schrier, PhD; Neurobehavioral Component: Robert K. Heaton, PhD (PI), Mariana Cherner, PhD, David J. Moore, PhD, Steven Paul Woods, PsyD; Neuroimaging Component: Terry Jernigan, PhD (PI), Christine Fennema-Notestine, PhD, Sarah L. Archibald, MA, John Hesselink, MD, Jacopo Annese, PhD, Michael J. Taylor, PhD; Neurobiology Component: Eliezer Masliah, MD (PI), Ian Everall, FRCPsych., FRCPath., PhD, T. Dianne Langford, PhD; Neurovirology Component: Douglas Richman, MD (PI), David M. Smith, MD; International Component: J. Allen McCutchan, MD (PI); Developmental Component: Ian Everall, FRCPsych., FRCPath., PhD (PI), Stuart Lipton, MD, PhD; Clinical Trials Component: J. Allen McCutchan, MD, J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, Scott Letendre, MD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI), Rodney von Jaeger, MPH; Data Management Unit: Anthony C. Gamst, PhD (PI), Clint Cushman, BA (Data Systems Manager), Daniel R. Masys, MD (Senior Consultant); Statistics Unit: Ian Abramson, PhD (PI), Christopher Ake, PhD, Florin Vaida, PhD. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank Dr Catherine L. Carey, Lisa Moran, Ofilio Vigil, Sarah Gibson, and Patricia Riggs for their help with study management.
References
- American Psychological Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bailey H., Dunlosky J., Hertzog C. Does differential strategy use account for age-related deficits in working-memory performance? Psychology and Aging. 2009;24:82–92. doi: 10.1037/a0014078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso M. R., Bornstein R. A. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. Journal of Clinical and Experimental Neuropsychology. 2000;22:208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- Bopp K. L., Verhaeghen P. Aging and verbal memory span: A meta-analysis. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2005;60:P223–P233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Brebion G., Smith M. J., Ehrlich M. F. Working memory and aging: Deficit or strategy differences? Aging Neuropsychology and Cognition. 1997;4:58–73. [Google Scholar]
- Bryan J., Luszcz M. A. Adult age differences in self-ordered pointing task performance: Contributions from working memory, executive function and speed of information processing. Journal of Clinical and Experimental Neuropsychology. 2001;23:608–619. doi: 10.1076/jcen.23.5.608.1250. [DOI] [PubMed] [Google Scholar]
- Carretti B., Borella E., De Beni R. Does strategic memory training improve the working memory performance of younger and older adults? Experimental Psychology. 2007;54:311–320. doi: 10.1027/1618-3169.54.4.311. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. Atlanta, GA: U.S. Department of Health and Human Services, Center for Disease Control and Prevention; 2007. [Google Scholar]
- Chang L., Ernst T., Witt M. D., Ames N., Walot I., Jovicich J., et al. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antiviral Therapy. 2003;8:17–26. [PubMed] [Google Scholar]
- Chang L., Speck O., Miller E. N., Braun J., Jovicich J., Koch C., et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L., Wong V., Nakama H., Watters M., Ramones D., Miller E. N., et al. Greater than age-related changes in brain diffusion of HIV patients after 1 year. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor N., Schmitter-Edgecombe M. Working memory and aging: A cross-sectional and longitudinal analysis using a self-ordered pointing task. Journal of the International Neuropsychological Society. 2004;10:489–503. doi: 10.1017/S1355617704104013. [DOI] [PubMed] [Google Scholar]
- Cherner M., Ellis R. J., Lazzaretto D., Young C., Mindt M. R., Atkinson J. H., et al. Effects of HIV-1 infection and aging on neurobehavioral functioning: Preliminary findings. AIDS. 2004;18:S27–S34. [PubMed] [Google Scholar]
- Cicerone K. D. Remediation of ‘working attention’ in mild traumatic brain injury. Brain Injury. 2002;16:185–195. doi: 10.1080/02699050110103959. [DOI] [PubMed] [Google Scholar]
- Collette F., Van der Linden M. Brain imaging of the central executive component of working memory. Neuroscience and Biobehavioral Reviews. 2002;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Culbertson W. C., Zillmer E. A. The tower of London DX (TOL-DX) manual. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- Daigneault S., Braun C. M. Working memory and the self-ordered pointing task: Further evidence of early prefrontal decline in normal aging. Journal of Clinical and Experimental Neuropsychology. 1993;15:881–895. doi: 10.1080/01688639308402605. [DOI] [PubMed] [Google Scholar]
- Daigneault S., Braun C. M. J., Whitaker H. A. Early effects of normal aging on perseverative and non-perseverative prefrontal measures. Developmental Neuropsychology. 1992;8:99–114. [Google Scholar]
- Dekaban A. S., Sadowsky D. Changes in brain weights during span of human life—relation of brain weights to body heights and body weights. Annals of Neurology. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. The California Verbal Learning Test. 2nd ed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Drachman D. A. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67:1340–1352. doi: 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- Durazzo T. C., Rothlind J. C., Cardenas V. A., Studholme C., Weiner M. W., Meyerhoff D. J. Chronic cigarette smoking and heavy drinking in human immunodeficiency virus: Consequences for neurocognition and brain morphology. Alcohol. 2007;41:489–501. doi: 10.1016/j.alcohol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S., Lemaire P., Vandierendonck A. Do working-memory executive components mediate the effects of age on strategy selection or on strategy execution? Insights from arithmetic problem solving. Psychological Research. 2008;72:27–38. doi: 10.1007/s00426-006-0071-5. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Calero P., Stockin M. D. HIV infection and the central nervous system: A primer. Neuropsychology Review. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery L., Heaven T. J., Paxton J. L., Braver T. S. Age-related changes in neural activity during performance matched working memory manipulation. NeuroImage. 2008;42:1577–1586. doi: 10.1016/j.neuroimage.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T., Chang L., Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. NeuroImage. 2003;19:1686–1693. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- Flavia M., Stampatori C., Zanotti D., Parrinello G., Capra R. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. Journal of Neurological Sciences. 2010;288:101–105. doi: 10.1016/j.jns.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Goebel S., Mehdorn H. M., Leplow B. Strategy instruction in Parkinson's disease: Influence on cognitive performance. Neuropsychologia. 2010;48:574–580. doi: 10.1016/j.neuropsychologia.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Goetz M. B., Boscardin W. J., Wiley D., Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS. 2001;15:1576–1579. doi: 10.1097/00002030-200108170-00017. [DOI] [PubMed] [Google Scholar]
- Gongvatana A., Woods S. P., Taylor M. J., Vigil O., Grant I. Semantic clustering inefficiency in HIV-associated dementia. The Journal of Neuropsychiatry Clinical Neurosciences. 2007;19:36–42. doi: 10.1176/jnp.2007.19.1.36. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Vance D. E. The neuropsychology of HIV/AIDS in older adults. Neuropsychology Review. 2009;19:263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- Heaton R. K., Franklin D. R., Jr., Clifford D., Woods S. P., Mindt M. R., Vigil O. R., et al. Persistence and progression of HIV-associated neurocognitive impairment (NCI) in the era of combination antiretroviral therapy (CART) and the role of comorbidities: The CHARTER Study [Abstract] Journal of Neurovirology. 2009;15(Suppl. 1):34–35. [Google Scholar]
- Heaton R. K., Marcotte T. D., Mindt M. R., Sadek J., Moore D. J., Bentley H., et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Hinkin C. H., Hardy D. J., Mason K. I., Castellon S. A., Lam M. N., Stefaniak M., et al. Verbal and spatial working memory performance among HIV-infected adults. Journal of the International Neuropsychological Society. 2002;8:532–538. doi: 10.1017/s1355617702814278. [DOI] [PubMed] [Google Scholar]
- Letendre S. L., Marquie-Beck J., Woods S. P., Best B., Clifford D. B., Collier A. C., et al. The role of cohort studies in drug development: Clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. Journal of Neuroimmune Pharmacology. 2007;2:120–127. doi: 10.1007/s11481-006-9054-y. [DOI] [PubMed] [Google Scholar]
- Martin E. M., Pitrak D. L., Weddington W., Rains N. A., Nunnally G., Nixon H., et al. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- McNair D., Lorr M., Droppleman L. Profile of mood states (POMS) manual. San Diego, CA: Educational and Industrial Testing Services; 1992. [Google Scholar]
- Meguro Y., Fugii T., Yamadori A., Tsukiura T., Suzuki K., Okuda J., et al. The nature of age-related decline on the reading span task. Journal of Clinical and Experimental Neuropsychology. 2000;22:391–398. doi: 10.1076/1380-3395(200006)22:3;1-V;FT391. [DOI] [PubMed] [Google Scholar]
- Morgan E. E., Woods S. P., Weber E., Dawson M. S., Carey C. L., Moran L. M., et al. HIV-associated episodic memory impairment: Evidence of a possible differential deficit in source memory for complex visual stimuli. The Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21:189–198. doi: 10.1176/appi.neuropsych.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Milner B. Deficits on subject-ordered tasks after frontal-lobe and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Author; 1997. [Google Scholar]
- Psychological Corporation. Wechsler Test of Adult Reading manual. San Antonio, TX: Author; 2001. [Google Scholar]
- Rajah M. N., D'Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Reger M., Welsh R., Razani J., Martin D. J., Boone K. B. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Reitan R. M., Wolfson D. The Halstead–Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Rendell P. G., Craik F. I. M. Virtual week and actual week: Age-related differences in prospective memory. Applied Cognitive Psychology. 2000;14:43–62. [Google Scholar]
- Rippeth J. D., Heaton R. K., Carey C. L., Marcotte T. D., Moore D. J., Gonzalez R., et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Rose J. H., Bowman K. F., Radziewicz R. M., Lewis S. A., O'Toole E. E. Predictors of engagement in a coping and communication support intervention for older patients with advanced cancer. Journal of the American Geriatrics Society. 2009;57:S296–S299. doi: 10.1111/j.1532-5415.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- Ross T. P., Hanouskova E., Giarla K., Calhoun E., Tucker M. The reliability and validity of the self-ordered pointing task. Archives of Clinical Neuropsychology. 2007;22:449–458. doi: 10.1016/j.acn.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Roth R. M., Wishart H. A., Flashman L. A., Riordan H. J., Huey L., Saykin A. J. Contribution of organizational strategy to verbal learning and memory in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:78–84. doi: 10.1037/0894-4105.18.1.78. [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. The nature of the influence of speed on adult age-differences in cognition. Developmental Psychology. 1994;30:240–259. doi: 10.1037//0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M., Chaytor N. S. Self-ordered pointing performance following severe closed-head injury. Journal of Clinical and Experimental Neuropsychology. 2003;25:918–932. doi: 10.1076/jcen.25.7.918.16484. [DOI] [PubMed] [Google Scholar]
- Shimamura A. P., Jurica P. J. Memory interference effects and aging: Findings from a test of frontal lobe function. Neuropsychology. 1994;8:408–412. [Google Scholar]
- Spikman J. M., Boelen D. H., Lamberts K. F., Brouwer W. H., Fasotti L. Effects of a multifaceted treatment program for executive dysfunction after acquired brain injury on indications of executive functioning in daily life. Journal of the International Neuropsychological Society. 2010;16:118–129. doi: 10.1017/S1355617709991020. [DOI] [PubMed] [Google Scholar]
- Stoff D. M. Mental health research in HIV/AIDS and aging: Problems and prospects. AIDS. 2004;18:S3–S10. [PubMed] [Google Scholar]
- Valcour V., Shikuma C., Shiramizu B., Watters M., Poeff P., Selnes O., et al. Higher frequency of dementia in older HIV-1 individuals: The Hawaii aging with HIV-1 cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V. G., Shikuma C. M., Watters M. R., Sacktor N. C. Cognitive impairment in older HIV-1-seropositive individuals: Prevalence and potential mechanisms. AIDS. 2004;18(Suppl. 1):S79–S86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat-Azouvi C., Pradat-Diehl P., Azouvi P. Rehabilitation of the central executive of working memory after severe traumatic brain injury: Two single-case studies. Brain Injury. 2009;23:585–594. doi: 10.1080/02699050902970711. [DOI] [PubMed] [Google Scholar]
- Wegesin D. J., Jacobs D. M., Zubin N. R., Ventura P. R., Stern Y. Source memory and encoding strategy in normal aging. Journal of Clinical and Experimental Neuropsychology. 2000;22:455–464. doi: 10.1076/1380-3395(200008)22:4;1-0;FT455. [DOI] [PubMed] [Google Scholar]
- West R., Ergis A. M., Winocur G., Saint-Cyr J. The contribution of impaired working memory monitoring to performance of the self-ordered pointing task in normal aging and Parkinson's disease. Neuropsychology. 1998;12:546–554. doi: 10.1037//0894-4105.12.4.546. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Dawson M. D., Weber E., Grant I. The HIV Neurobehavioral Research Center Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, Version 2.1) Geneva, Switzerland: Author; 1998. [Google Scholar]