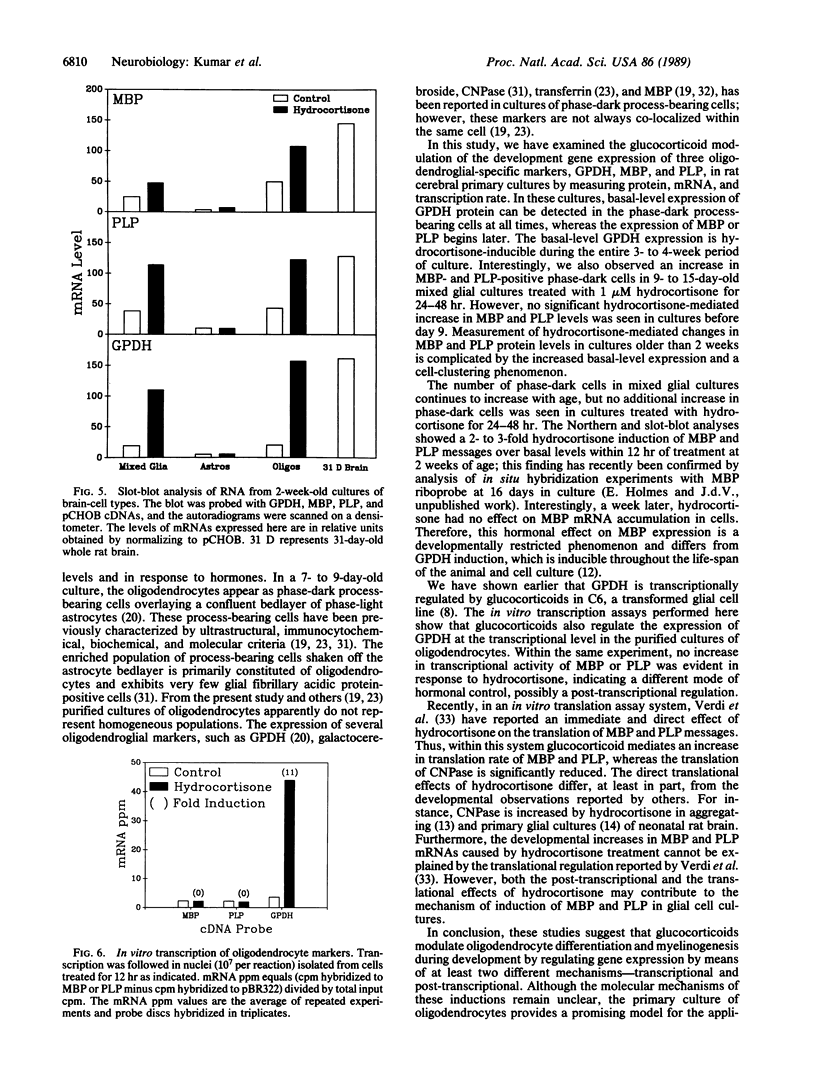
Abstract
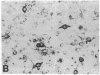
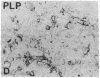
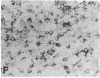
During neonatal development glucocorticoids potentiate oligodendrocyte differentiation and myelinogenesis by regulating the expression of myelin basic protein, proteolipid protein, and glycerol phosphate dehydrogenase (sn-glycerol-3-phosphate: NAD+ 2-oxidoreductase, EC 1.1.1.8). The actual locus at which hydrocortisone exerts its developmental influence on glial physiology is, however, not well understood. Glycerol phosphate dehydrogenase is glucocorticoid-inducible in oligodendrocytes at all stages of development both in vivo and in vitro. In newborn rat cerebral cultures, between 9 and 15 days in vitro, a 2- to 3-fold increase in myelin basic protein and proteolipid protein mRNA levels occurs in oligodendrocytes within 12 hr of hydrocortisone treatment. Immunostaining demonstrates that this increase in mRNAs is followed by a 2- to 3-fold increase in the protein levels within 24 hr. In vitro transcription assays performed with oligodendrocyte nuclei show an 11-fold increase in the transcriptional activity of glycerol phosphate dehydrogenase in response to hydrocortisone but no increase in transcription of myelin basic protein or proteolipid protein. These results indicate that during early myelinogenesis, glucocorticoids influence the expression of key oligodendroglial markers by different processes: The expression of glycerol phosphate dehydrogenase is regulated at the transcriptional level, whereas the expression of myelin basic protein and proteolipid protein is modulated via a different, yet uncharacterized, mechanism involving post-transcriptional regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almazan G., Honegger P., Du Pasquier P., Matthieu J. M. Dexamethasone stimulates the biochemical differentiation of fetal forebrain cells in reaggregating cultures. Dev Neurosci. 1986;8(1):14–23. doi: 10.1159/000112237. [DOI] [PubMed] [Google Scholar]
- Almazan G., Honegger P., Matthieu J. M., Guentert-Lauber B. Epidermal growth factor and bovine growth hormone stimulate differentiation and myelination of brain cell aggregates in culture. Brain Res. 1985 Aug;353(2):257–264. doi: 10.1016/0165-3806(85)90214-7. [DOI] [PubMed] [Google Scholar]
- BUNGE M. B., BUNGE R. P., PAPPAS G. D. Electron microscopic demonstration of connections between glia and myelin sheaths in the developing mammalian central nervous system. J Cell Biol. 1962 Feb;12:448–453. doi: 10.1083/jcb.12.2.448. [DOI] [PubMed] [Google Scholar]
- Barinaga M., Yamonoto G., Rivier C., Vale W., Evans R., Rosenfeld M. G. Transcriptional regulation of growth hormone gene expression by growth hormone-releasing factor. Nature. 1983 Nov 3;306(5938):84–85. doi: 10.1038/306084a0. [DOI] [PubMed] [Google Scholar]
- Bologa L., Aizenman Y., Chiappelli F., de Vellis J. Regulation of myelin basic protein in oligodendrocytes by a soluble neuronal factor. J Neurosci Res. 1986;15(4):521–528. doi: 10.1002/jnr.490150409. [DOI] [PubMed] [Google Scholar]
- Bologa L. Oligodendrocytes, key cells in myelination and target in demyelinating diseases. J Neurosci Res. 1985;14(1):1–20. doi: 10.1002/jnr.490140102. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Vellis J., Inglish D. Age-dependent changes in the regulation of glycerolphosphate dehydrogenase in the rat brain and in a glial cell line. Prog Brain Res. 1973;40(0):321–330. doi: 10.1016/S0079-6123(08)60697-4. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A., de Vellis J. Myelin basic protein and transferrin characterize different subpopulations of oligodendrocytes in rat primary glial cultures. J Neurosci Res. 1988 Oct-Dec;21(2-4):181–187. doi: 10.1002/jnr.490210210. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Holmes E., Hermanson G., Cole R., de Vellis J. Developmental expression of glial-specific mRNAs in primary cultures of rat brain visualized by in situ hybridization. J Neurosci Res. 1988 Apr;19(4):389-96, 458-65. doi: 10.1002/jnr.490190402. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic esterification of alpha-glycerophosphate by long chain fatty acids. J Biol Chem. 1953 Sep;204(1):345–357. [PubMed] [Google Scholar]
- Kitraki E., Alexis M. N., Stylianopoulou F. Glucocorticoid receptors in developing rat brain and liver. J Steroid Biochem. 1984 Jan;20(1):263–269. doi: 10.1016/0022-4731(84)90215-2. [DOI] [PubMed] [Google Scholar]
- Kumar S., Holmes E., Scully S., Birren B. W., Wilson R. H., de Vellis J. The hormonal regulation of gene expression of glial markers: glutamine synthetase and glycerol phosphate dehydrogenase in primary cultures of rat brain and in C6 cell line. J Neurosci Res. 1986;16(1):251–264. doi: 10.1002/jnr.490160122. [DOI] [PubMed] [Google Scholar]
- Kumar S., Sachar K., Huber J., Weingarten D. P., de Vellis J. Glucocorticoids regulate the transcription of glycerol phosphate dehydrogenase in cultured glial cells. J Biol Chem. 1985 Nov 25;260(27):14743–14747. [PubMed] [Google Scholar]
- LAATSCH R. H. Glycerol phosphate dehydrogenase activity of developing rat central nervous system. J Neurochem. 1962 Sep-Oct;9:487–492. doi: 10.1111/j.1471-4159.1962.tb04201.x. [DOI] [PubMed] [Google Scholar]
- Leveille P. J., McGinnis J. F., Maxwell D. S., de Vellis J. Immunocytochemical localization of glycerol-3-phosphate dehydrogenase in rat oligodendrocytes. Brain Res. 1980 Sep 8;196(2):287–305. doi: 10.1016/0006-8993(80)90397-2. [DOI] [PubMed] [Google Scholar]
- Macklin W. B., Weill C. L., Deininger P. L. Expression of myelin proteolipid and basic protein mRNAs in cultured cells. J Neurosci Res. 1986;16(1):203–217. doi: 10.1002/jnr.490160118. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis J. F., de Vellis J. Glucocorticoid regulation in rat brain cell cultures. Hydrocortisone increases the rate of synthesis of glycerol phosphate dehydrogenase in C6 glioma cells. J Biol Chem. 1978 Dec 10;253(23):8493–8492. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mithen F. A., Wood P. M., Agrawal H. C., Bunge R. P. Immunohistochemical study of myelin sheaths formed by oligodendrocytes interacting with dissociated dorsal root ganglion neurons in culture. Brain Res. 1983 Feb 28;262(1):63–69. doi: 10.1016/0006-8993(83)90469-9. [DOI] [PubMed] [Google Scholar]
- Naismith A. L., Hoffman-Chudzik E., Tsui L. C., Riordan J. R. Study of the expression of myelin proteolipid protein (lipophilin) using a cloned complementary DNA. Nucleic Acids Res. 1985 Oct 25;13(20):7413–7425. doi: 10.1093/nar/13.20.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum J. L., Nussbaum F., Gensburger C., Araceli Espinosa de los Monteros M. T., Roussel G., Labourdette G. Detection of Wolfgram W1 protein, myelin basic proteins and proteolipids in cultured oligodendrocytes by the electro-immunoblotting method. Neurosci Lett. 1983 Sep 30;40(2):111–117. doi: 10.1016/0304-3940(83)90288-4. [DOI] [PubMed] [Google Scholar]
- Pavlík A., Buresová M. The neonatal cerebellum: the highest level of glucocorticoid receptors in the brain. Brain Res. 1984 Jan;314(1):13–20. doi: 10.1016/0165-3806(84)90171-8. [DOI] [PubMed] [Google Scholar]
- Saneto R. P., de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proc Natl Acad Sci U S A. 1985 May;82(10):3509–3513. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki T., Noguchi T., Tsukada Y. Cerebral myelinogenesis in the Snell dwarf mouse: stimulatory effects of GH and T4 restricted to the first 20 days of postnatal life. Neurochem Res. 1985 Jun;10(6):767–778. doi: 10.1007/BF00964534. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Verdi J. M., Kampf K., Campagnoni A. T. Translational regulation of myelin protein synthesis by steroids. J Neurochem. 1989 Jan;52(1):321–324. doi: 10.1111/j.1471-4159.1989.tb10935.x. [DOI] [PubMed] [Google Scholar]
- Warringa R. A., Hoeben R. C., Koper J. W., Sykes J. E., van Golde L. M., Lopes-Cardozo M. Hydrocortisone stimulates the development of oligodendrocytes in primary glial cultures and affects glucose metabolism and lipid synthesis in these cultures. Brain Res. 1987 Jul;431(1):79–86. doi: 10.1016/0165-3806(87)90197-0. [DOI] [PubMed] [Google Scholar]
- de Ferra F., Engh H., Hudson L., Kamholz J., Puckett C., Molineaux S., Lazzarini R. A. Alternative splicing accounts for the four forms of myelin basic protein. Cell. 1985 Dec;43(3 Pt 2):721–727. doi: 10.1016/0092-8674(85)90245-4. [DOI] [PubMed] [Google Scholar]