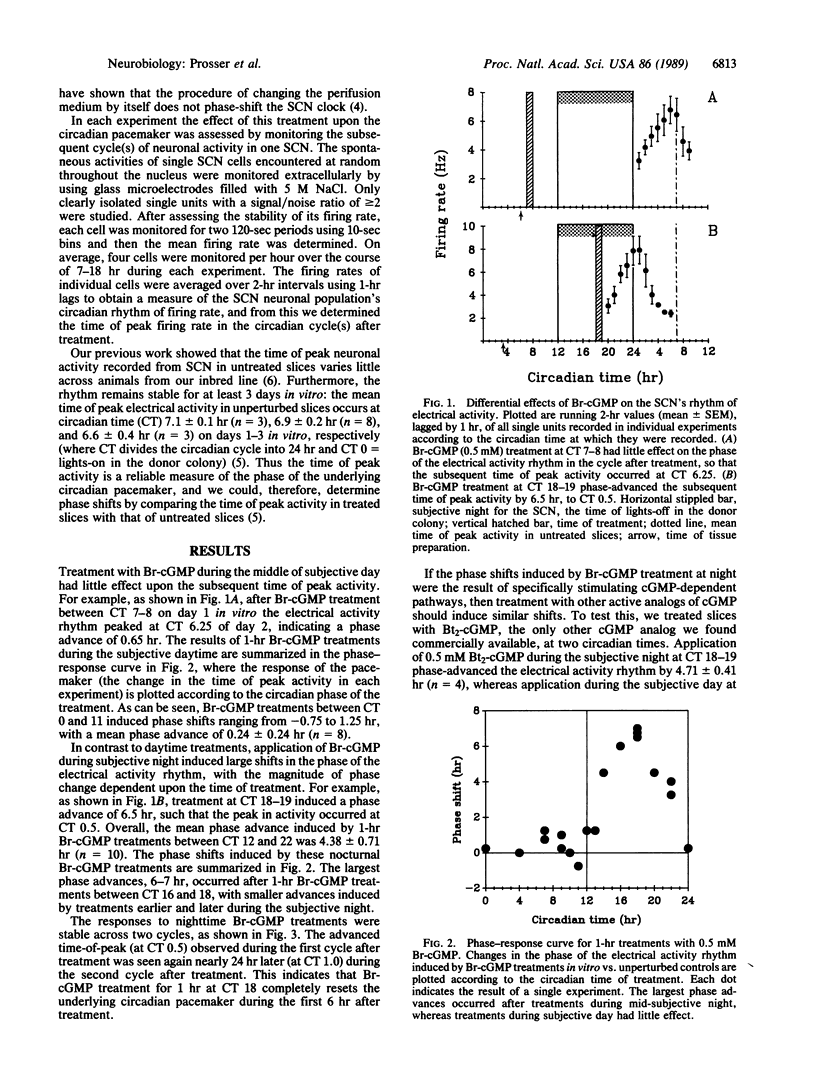
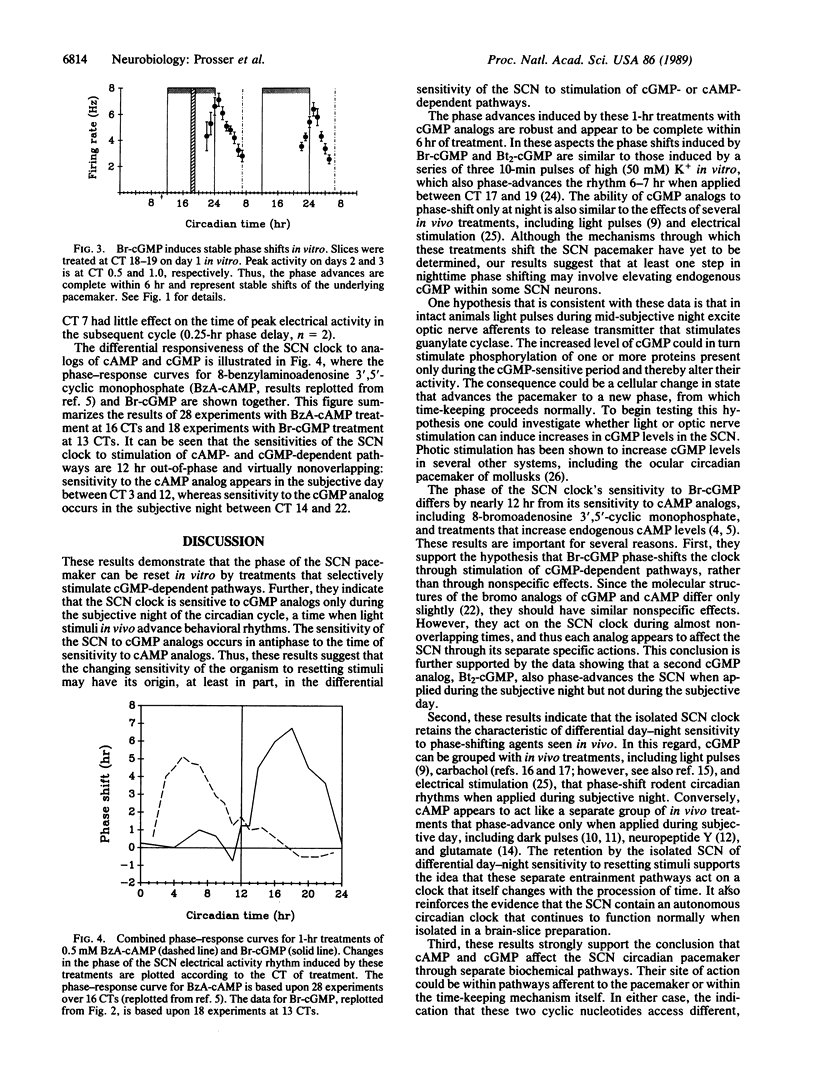
Abstract
The suprachiasmatic nuclei (SCN) of mammals contain a circadian clock that synchronizes behavioral and physiological rhythms to the daily cycle of light and darkness. We have been probing the biochemical substrates of this endogenous pacemaker by examining the ability of treatments affecting cyclic nucleotide-dependent pathways to induce changes in the phase of oscillation in electrical activity of rat SCN isolated in brain slices. Our previous work has shown that daytime treatments that stimulate cAMP-dependent pathways induce phase shifts of the SCN pacemaker in vitro but treatments during the subjective night are without effect. In this study we report that the phase of SCN oscillation is reset by treatments that stimulate cGMP-dependent pathways, but only during the subjective night. Thus, the nocturnal period of SCN sensitivity to cGMP is in antiphase to the diurnal period of sensitivity to cAMP. These results suggest that cAMP and cGMP affect the SCN pacemaker through separate biochemical pathways intrinsic to the SCN. These studies provide evidence that changing biochemical substrates within the SCN circadian clock may underlie some aspects of differential temporal sensitivity of mammals to resetting stimuli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers H. E., Ferris C. F. Neuropeptide Y: role in light-dark cycle entrainment of hamster circadian rhythms. Neurosci Lett. 1984 Sep 7;50(1-3):163–168. doi: 10.1016/0304-3940(84)90480-4. [DOI] [PubMed] [Google Scholar]
- DECOURSEY P. J. FUNCTION OF A LIGHT RESPONSE RHYTHM IN HAMSTERS. J Cell Physiol. 1964 Apr;63:189–196. doi: 10.1002/jcp.1030630208. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The cAMP cascade in the nervous system: molecular sites of action and possible relevance to neuronal plasticity. CRC Crit Rev Biochem. 1987;22(3):221–281. doi: 10.3109/10409238709101484. [DOI] [PubMed] [Google Scholar]
- Earnest D. J., Turek F. W. Neurochemical basis for the photic control of circadian rhythms and seasonal reproductive cycles: role for acetylcholine. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4277–4281. doi: 10.1073/pnas.82.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis G. B., McKlveen R. E., Turek F. W. Dark pulses affect the circadian rhythm of activity in hamsters kept in constant light. Am J Physiol. 1982 Jan;242(1):R44–R50. doi: 10.1152/ajpregu.1982.242.1.R44. [DOI] [PubMed] [Google Scholar]
- Eskin A., Corrent G., Lin C. Y., McAdoo D. J. Mechanism for shifting the phase of a circadian rhythm by serotonin: involvement of cAMP. Proc Natl Acad Sci U S A. 1982 Jan;79(2):660–664. doi: 10.1073/pnas.79.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin A., Takahashi J. S., Zatz M., Block G. D. Cyclic guanosine 3':5'-monophosphate mimics the effects of light on a circadian pacemaker in the eye of aplysia. J Neurosci. 1984 Oct;4(10):2466–2471. doi: 10.1523/JNEUROSCI.04-10-02466.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette M. U., Prosser R. A. Circadian rhythm of the rat suprachiasmatic brain slice is rapidly reset by daytime application of cAMP analogs. Brain Res. 1988 Dec 6;474(2):348–352. doi: 10.1016/0006-8993(88)90449-0. [DOI] [PubMed] [Google Scholar]
- Gillette M. U. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res. 1986 Jul 30;379(1):176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- Hatton G. I., Doran A. D., Salm A. K., Tweedle C. D. Brain slice preparation: hypothalamus. Brain Res Bull. 1980 Jul-Aug;5(4):405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Inouye S. T., Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic "island" containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou S. Y., Shibata S., Shiratsuchi A., Ueki S. Effects of dibutyryl cyclic adenosine monophosphate and dibutyryl cyclic guanosine monophosphate on neuron activity of suprachiasmatic nucleus in rat hypothalamic slice preparation. Neurosci Lett. 1986 Jun 30;67(3):339–343. doi: 10.1016/0304-3940(86)90333-2. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., van der Zee E. A., Dietz M. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci Lett. 1988 Mar 31;86(2):177–183. doi: 10.1016/0304-3940(88)90567-8. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., van der Zee E., Dietz M. The effects of intraventricular carbachol injections on the free-running activity rhythm of the hamster. J Biol Rhythms. 1988 Winter;3(4):333–348. doi: 10.1177/074873048800300403. [DOI] [PubMed] [Google Scholar]
- Meyer R. B., Jr, Miller J. P. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. Life Sci. 1974 Mar 16;14(6):1019–1040. doi: 10.1016/0024-3205(74)90228-8. [DOI] [PubMed] [Google Scholar]
- Prosser R. A., Gillette M. U. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989 Mar;9(3):1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusak B., Groos G. Suprachiasmatic stimulation phase shifts rodent circadian rhythms. Science. 1982 Mar 12;215(4538):1407–1409. doi: 10.1126/science.7063851. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Simon L. N., Shuman D. A., Robins R. K. The chemistry and biological properties of nucleotides related to nucleoside 3',5'-cyclic phosphates. Adv Cyclic Nucleotide Res. 1973;3:225–353. [PubMed] [Google Scholar]
- Turek F. W. Circadian neural rhythms in mammals. Annu Rev Physiol. 1985;47:49–64. doi: 10.1146/annurev.ph.47.030185.000405. [DOI] [PubMed] [Google Scholar]
- Valases C., Wright S. J., Jr, Catravas G. N. Diurnal changes in cyclic nucleotide levels in the hypothalamus of the rat. Exp Brain Res. 1980;40(3):261–264. doi: 10.1007/BF00237790. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Zatz M., Brownstein M. J. Intraventricular carbachol mimics the effects of light on the circadian rhythm in the rat pineal gland. Science. 1979 Jan 26;203(4378):358–361. doi: 10.1126/science.32619. [DOI] [PubMed] [Google Scholar]
- Zatz M., Herkenham M. A. Intraventricular carbachol mimics the phase-shifting effect of light on the circadian rhythm of wheel-running activity. Brain Res. 1981 May 11;212(1):234–238. doi: 10.1016/0006-8993(81)90059-7. [DOI] [PubMed] [Google Scholar]



