Abstract
In the title compound, C13H12ClN3·H2O, the organic molecule is almost planar, with a dihedral angle of 3.22 (10)° between the benzene and pyridine rings. The crystal structure is stabilized by O—H⋯N and C—H⋯O hydrogen bonding and π–π stacking interactions [centroid–centroid distances = 3.630 (1) and 3.701 (1) Å].
Related literature
For the synthesis and pharmacological activity of (benzylidene-hydrazono)-1,4-dihydropyridine derivatives, see: Douglas et al. (1977 ▶); Alptüzün et al. (2010 ▶); Savini et al. (2002 ▶); Pandey et al. (2002 ▶); Salgın-Gökşen et al. (2007 ▶); Silva et al. (2004 ▶); Vicini et al. (2009 ▶). For bond-length data, see: Allen et al. (1987 ▶); Diao et al. (2008 ▶); Odabaşoğlu et al. (2003 ▶). For quantum-chemical calculations, see: Pople & Beveridge (1970 ▶).
Experimental
Crystal data
C13H12ClN3·H2O
M r = 263.72
Monoclinic,

a = 5.8492 (4) Å
b = 20.3101 (10) Å
c = 12.2035 (7) Å
β = 113.855 (4)°
V = 1325.90 (14) Å3
Z = 4
Mo Kα radiation
μ = 0.28 mm−1
T = 296 K
0.60 × 0.30 × 0.04 mm
Data collection
Stoe IPDS 2 diffractometer
Absorption correction: integration (X-RED32; Stoe & Cie, 2002 ▶) T min = 0.905, T max = 0.989
14028 measured reflections
2759 independent reflections
1746 reflections with I > 2σ(I)
R int = 0.064
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.095
S = 0.95
2759 reflections
170 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.16 e Å−3
Δρmin = −0.18 e Å−3
Data collection: X-AREA (Stoe & Cie, 2002 ▶); cell refinement: X-AREA; data reduction: X-RED32 (Stoe & Cie, 2002 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810015709/sj2782sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810015709/sj2782Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯N1 | 0.84 (3) | 2.26 (3) | 3.089 (3) | 173 (3) |
| O1—H1B⋯N2i | 0.91 (4) | 1.95 (4) | 2.859 (3) | 174 (2) |
| C3—H3⋯O1ii | 0.93 | 2.58 | 3.421 (3) | 150 |
| C11—H11⋯O1iii | 0.93 | 2.49 | 3.378 (3) | 159 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
The authors acknowledge the Faculty of Arts and Sciences, Ondokuz Mayıs University, Turkey, for the use of the Stoe IPDS 2 diffractometer (purchased under grant F.279 of the University Research Fund).
supplementary crystallographic information
Comment
Hydrazones, a special group of compounds in the class of the schiff bases, are known to show significant biological activities including antimicrobial, antitubercular, anticancer, analgesic, anti-inflammatory, antiplatelet and antiviral activities (Savini et al., 2002; Pandey et al., 2002; Salgın-Gökşen et al., 2007; Silva et al., 2004; Vicini et al., 2009). In addition, (benzylidene-hydrazono)-1,4-dihydropyridine derivatives have anticoccidial activity (Douglas et al., 1977) and also display anti-Alzheimer's activity by inhibiting ABeta fibril formation and acetylcholinesterase (Alptüzün et al., 2010).
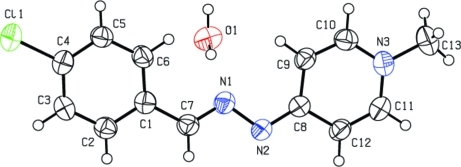
The title molecule (I), Fig. 1, crystallized as a monohydrate in the monoclinic space group P21/c. All bond lengths are as expected (Allen et al., 1987). The Cl1—C4, and N1—N2 bond lengths are 1.742 (2) Å, and 1.388 (2) Å, respectively. The Cl1—C4—C5 and N2—N1—C7 bond angles are 119.88 (18) ° and 113.95 (19) °, respectively. The bond lengths and the bond angles of (I) are comparable to those observed in related structures (Diao et al., 2008; Odabaşoğlu et al., 2003).
The main molecule is almost planar, except the methyl H atoms, forming a dihedral angle of 3.22 (10)° between the benzene (C1–C6) and dihydropyridine (N3/C8–C12) rings.
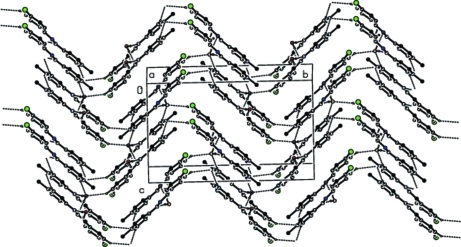
The crystal structure is stabilized by O—H···N and C—H···O hydrogen bonding (Table 1, Fig. 2) and π-π stacking interactions [Cg1···Cg1(-x, 1-y, -z) = 3.630 (1) Å and Cg1···Cg2(1-x, 1-y, 1-z) = 3.701 (1) Å, Cg1 and Cg2 are the centroids of the pyridine and benzene rings, respectively].
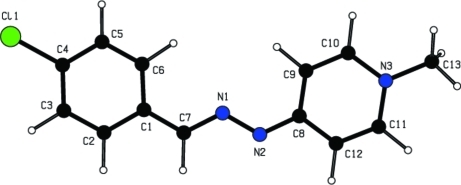
We have also carried out the quantum mechanical calculations using the CNDO (Pople et al., 1970) approximation. The spatial view of the single molecule considered in a vacuum, is shown in Fig.3. According to the theoretical CNDO and experimental X-rays results, the values of the geometric parameters of (I) are closely comparable within the observed experimental errors. The calculated dipole moment of (I) is about 11.481 Debye. The HOMO and LUMO energy levels are -8.3484 and 1.3565 eV, respectively.
Experimental
4-Hydrazinylpyridine (1.09 g, 0.01 mol) and 4-chlorobenzaldehyde (1.41 g, 0.01 mol) were stirred in ethanol (30 ml) at room temperature for 5-10 h. The precipitate was filtered and washed with cool ethanol and crystallized from ethanol. A mixture of 4-[(4-Chlorobenzylidene)hydrazinyl] pyridine (0.232 g, 0.001 mol) and methyl iodide (0.141 g, 0.002 mol) was refluxed in ethanol (20 ml) for 20 h. The mixture was cooled to room temperature and the resulting precipitate was filtered and washed with cool ethanol. The crude products were crystallized from ethanol to give the compound 4-(2-(4-Chlorobenzylidenehydrazinyl)-1-methylpyridinium iodide. This product (0.374 g, 0.001 mol) was partitioned between CH2Cl2 (50 ml) and 2 M NaOH (50 ml). The organic layer was evaporated to dryness and the residue recrystallized from ethanol-water.
Yield 88%, yellow needles, mp 407-409 K (lit. (Douglas et al. , 1977) 403-405 K). IR (KBr) vmax 1654, 1517, 1492, 1203, 824 cm-1. 1H-NMR (DMSO-d6): δ ppm 3.29 (3H, s, N—CH3), 6.12 (1H, dd, J=2.4/8.0 Hz, H-3 or H-5), 6.98 (1H, dd, J=2.0/7.8 Hz, H-3 or H-5), 7.23 (2H, td, J=7.2/2.0 Hz, H-2, H-6 ), 7.39 (2H, d, J=8.4 Hz, H-2', H-6'), 7.69 (2H, d, J=8.4 Hz, H-3', H-5'), 8.16 (1H, s, N=CH). 13 C NMR (CH3OH- d4): δ ppm 43.18 (q), 107.78 (d), 112.11 (d), 129.35 (d), 129.79 (d), 135.59 (s), 136.58 (s), 140.15 (d), 140.75 (d), 148.84 (d), 162.25 (s). EI—MS m/z (% relative intensity): 247 (M+2, 14), 246 (M+1, 28), 245 (M+, 43), 181 (25), 93 (100), 92 (24), 66 (30), 42 (18). C13H12N3Cl.H2O. C, H, N combustion analysis: Calc. (%) C 59.21, H 5.36, N 15.93; found (%) C 59.45, H 5.33, N 15.72.
Refinement
The H atoms of the water molecule were found from a difference Fourier map and their isotropic thermal parameters were refined by using a riding model with Uiso(H) = 1.5Ueq(O). Their positional parameters are refined freely [d(O–H) = 0.84 (3) and 0.91 (4) Å]. The remaining H atoms were positioned geometrically and refined using a riding model with C—H = 0.93 and 0.96 Å, and Uiso(H) = 1.2 or 1.5Ueq(C).
Figures
Fig. 1.
An ORTEP View of the title molecule with the atom numbering scheme. Displacement ellipsoids for non-H atoms are drawn at the 50% probability level.
Fig. 2.
The packing and hydrogen bonding interactions of (I) down the a-axis. H atoms not participating in hydrogen bonding have been omitted for clarity.
Fig. 3.
The spatial view of the title molecule (I), calculated by the CNDO aproximation.
Crystal data
| C13H12ClN3·H2O | F(000) = 552 |
| Mr = 263.72 | Dx = 1.321 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 11448 reflections |
| a = 5.8492 (4) Å | θ = 1.8–27.3° |
| b = 20.3101 (10) Å | µ = 0.28 mm−1 |
| c = 12.2035 (7) Å | T = 296 K |
| β = 113.855 (4)° | Needle, yellow |
| V = 1325.90 (14) Å3 | 0.60 × 0.30 × 0.04 mm |
| Z = 4 |
Data collection
| Stoe IPDS 2 diffractometer | 2759 independent reflections |
| Radiation source: sealed X-ray tube, 12 x 0.4 mm long-fine focus | 1746 reflections with I > 2σ(I) |
| plane graphite | Rint = 0.064 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 26.5°, θmin = 2.0° |
| ω scans | h = −7→7 |
| Absorption correction: integration (X-RED32; Stoe & Cie, 2002) | k = −25→25 |
| Tmin = 0.905, Tmax = 0.989 | l = −15→15 |
| 14028 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.095 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.95 | w = 1/[σ2(Fo2) + (0.0426P)2] where P = (Fo2 + 2Fc2)/3 |
| 2759 reflections | (Δ/σ)max < 0.001 |
| 170 parameters | Δρmax = 0.16 e Å−3 |
| 0 restraints | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell esds are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 1.22894 (12) | 0.72508 (3) | 0.80784 (5) | 0.0626 (2) | |
| N1 | 0.3123 (3) | 0.56701 (10) | 0.35465 (13) | 0.0453 (6) | |
| N2 | 0.0774 (3) | 0.54841 (9) | 0.27204 (13) | 0.0441 (6) | |
| N3 | 0.0599 (4) | 0.39257 (9) | 0.05147 (14) | 0.0478 (6) | |
| C1 | 0.5371 (4) | 0.63831 (11) | 0.52012 (15) | 0.0414 (7) | |
| C2 | 0.5222 (4) | 0.69371 (12) | 0.58483 (16) | 0.0452 (7) | |
| C3 | 0.7336 (4) | 0.72021 (11) | 0.67306 (16) | 0.0466 (7) | |
| C4 | 0.9614 (4) | 0.69105 (12) | 0.69752 (16) | 0.0449 (7) | |
| C5 | 0.9807 (4) | 0.63606 (12) | 0.63540 (16) | 0.0460 (8) | |
| C6 | 0.7697 (4) | 0.60996 (12) | 0.54740 (16) | 0.0460 (7) | |
| C7 | 0.3079 (4) | 0.61193 (11) | 0.42743 (16) | 0.0435 (7) | |
| C8 | 0.0859 (4) | 0.49832 (11) | 0.20400 (15) | 0.0396 (7) | |
| C9 | 0.3019 (4) | 0.46263 (11) | 0.21152 (16) | 0.0437 (7) | |
| C10 | 0.2819 (4) | 0.41223 (12) | 0.13694 (18) | 0.0480 (7) | |
| C11 | −0.1504 (4) | 0.42521 (12) | 0.04046 (17) | 0.0473 (7) | |
| C12 | −0.1430 (4) | 0.47578 (12) | 0.11261 (16) | 0.0449 (7) | |
| C13 | 0.0497 (5) | 0.33651 (13) | −0.0272 (2) | 0.0668 (10) | |
| O1 | 0.6224 (4) | 0.61903 (10) | 0.21954 (15) | 0.0611 (7) | |
| H2 | 0.36750 | 0.71300 | 0.56810 | 0.0540* | |
| H3 | 0.72240 | 0.75730 | 0.71540 | 0.0560* | |
| H5 | 1.13590 | 0.61680 | 0.65300 | 0.0550* | |
| H6 | 0.78280 | 0.57290 | 0.50560 | 0.0550* | |
| H7 | 0.15450 | 0.62820 | 0.42110 | 0.0520* | |
| H9 | 0.45830 | 0.47440 | 0.26870 | 0.0520* | |
| H10 | 0.42620 | 0.38990 | 0.14420 | 0.0580* | |
| H11 | −0.30330 | 0.41230 | −0.01840 | 0.0570* | |
| H12 | −0.29140 | 0.49680 | 0.10280 | 0.0540* | |
| H13A | 0.18000 | 0.34090 | −0.05550 | 0.1000* | |
| H13B | −0.10970 | 0.33600 | −0.09420 | 0.1000* | |
| H13C | 0.07200 | 0.29620 | 0.01700 | 0.1000* | |
| H1A | 0.551 (6) | 0.6046 (17) | 0.262 (2) | 0.0920* | |
| H1B | 0.768 (6) | 0.5959 (17) | 0.242 (2) | 0.0920* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0551 (4) | 0.0646 (4) | 0.0574 (3) | −0.0114 (3) | 0.0116 (2) | −0.0153 (3) |
| N1 | 0.0411 (10) | 0.0499 (12) | 0.0415 (8) | −0.0024 (9) | 0.0133 (7) | −0.0039 (8) |
| N2 | 0.0365 (10) | 0.0484 (12) | 0.0446 (8) | −0.0022 (9) | 0.0135 (7) | −0.0053 (8) |
| N3 | 0.0558 (12) | 0.0433 (12) | 0.0455 (9) | −0.0060 (10) | 0.0216 (8) | −0.0054 (8) |
| C1 | 0.0461 (13) | 0.0438 (13) | 0.0351 (9) | −0.0015 (11) | 0.0173 (9) | 0.0023 (8) |
| C2 | 0.0453 (13) | 0.0457 (14) | 0.0446 (10) | 0.0042 (11) | 0.0182 (9) | 0.0017 (9) |
| C3 | 0.0577 (14) | 0.0395 (13) | 0.0434 (10) | 0.0004 (12) | 0.0212 (10) | −0.0043 (9) |
| C4 | 0.0472 (13) | 0.0469 (14) | 0.0387 (10) | −0.0069 (11) | 0.0154 (9) | 0.0011 (9) |
| C5 | 0.0431 (13) | 0.0481 (15) | 0.0456 (11) | 0.0046 (11) | 0.0167 (10) | −0.0020 (9) |
| C6 | 0.0485 (14) | 0.0474 (14) | 0.0406 (10) | 0.0035 (11) | 0.0164 (9) | −0.0065 (9) |
| C7 | 0.0422 (12) | 0.0473 (14) | 0.0429 (10) | 0.0003 (11) | 0.0191 (9) | −0.0014 (9) |
| C8 | 0.0393 (12) | 0.0407 (13) | 0.0388 (9) | −0.0004 (10) | 0.0158 (8) | 0.0028 (9) |
| C9 | 0.0374 (12) | 0.0458 (14) | 0.0428 (10) | −0.0013 (10) | 0.0110 (9) | 0.0001 (9) |
| C10 | 0.0456 (13) | 0.0460 (14) | 0.0545 (11) | 0.0041 (11) | 0.0223 (10) | 0.0025 (10) |
| C11 | 0.0424 (13) | 0.0503 (15) | 0.0437 (10) | −0.0070 (12) | 0.0118 (9) | −0.0004 (10) |
| C12 | 0.0357 (12) | 0.0511 (15) | 0.0439 (10) | −0.0021 (11) | 0.0120 (9) | −0.0015 (9) |
| C13 | 0.084 (2) | 0.0570 (17) | 0.0615 (14) | −0.0065 (15) | 0.0316 (14) | −0.0174 (12) |
| O1 | 0.0514 (11) | 0.0661 (13) | 0.0668 (10) | 0.0032 (9) | 0.0250 (8) | 0.0088 (8) |
Geometric parameters (Å, °)
| Cl1—C4 | 1.742 (2) | C8—C9 | 1.427 (3) |
| O1—H1B | 0.91 (4) | C8—C12 | 1.428 (3) |
| O1—H1A | 0.84 (3) | C9—C10 | 1.343 (3) |
| N1—N2 | 1.388 (2) | C11—C12 | 1.342 (3) |
| N1—C7 | 1.281 (3) | C2—H2 | 0.9300 |
| N2—C8 | 1.327 (3) | C3—H3 | 0.9300 |
| N3—C10 | 1.356 (3) | C5—H5 | 0.9300 |
| N3—C11 | 1.355 (3) | C6—H6 | 0.9300 |
| N3—C13 | 1.475 (3) | C7—H7 | 0.9300 |
| C1—C2 | 1.398 (3) | C9—H9 | 0.9300 |
| C1—C7 | 1.462 (3) | C10—H10 | 0.9300 |
| C1—C6 | 1.388 (3) | C11—H11 | 0.9300 |
| C2—C3 | 1.379 (3) | C12—H12 | 0.9300 |
| C3—C4 | 1.376 (3) | C13—H13C | 0.9600 |
| C4—C5 | 1.380 (3) | C13—H13A | 0.9600 |
| C5—C6 | 1.373 (3) | C13—H13B | 0.9600 |
| Cl1···C2i | 3.521 (2) | C11···C8iv | 3.516 (3) |
| Cl1···C7i | 3.572 (2) | C11···O1iv | 3.378 (3) |
| Cl1···H10ii | 2.9800 | C13···C4xi | 3.598 (3) |
| O1···N2iii | 2.859 (3) | C13···C3xi | 3.490 (4) |
| O1···C11iv | 3.378 (3) | C3···H13Ax | 2.9800 |
| O1···N1 | 3.089 (3) | C7···H1Bvii | 3.07 (3) |
| O1···H13Biv | 2.9100 | C7···H1A | 2.91 (3) |
| O1···H3v | 2.5800 | C8···H1Bvii | 2.88 (4) |
| O1···H11iv | 2.4900 | C12···H1Bvii | 3.06 (3) |
| O1···H13Avi | 2.8100 | H1A···N1 | 2.26 (3) |
| N1···O1 | 3.089 (3) | H1A···C7 | 2.91 (3) |
| N2···O1vii | 2.859 (3) | H1B···H12iii | 2.5700 |
| N1···H1A | 2.26 (3) | H1B···N2iii | 1.95 (4) |
| N1···H6 | 2.6200 | H1B···C7iii | 3.07 (3) |
| N1···H9 | 2.4700 | H1B···C8iii | 2.88 (4) |
| N2···H1Bvii | 1.95 (4) | H1B···C12iii | 3.06 (3) |
| C2···Cl1viii | 3.521 (2) | H1B···H7iii | 2.5100 |
| C3···C10ix | 3.576 (3) | H2···H7 | 2.4300 |
| C3···C13x | 3.490 (4) | H3···O1xii | 2.5800 |
| C4···C13x | 3.598 (3) | H6···N1 | 2.6200 |
| C4···C10ix | 3.582 (3) | H7···H1Bvii | 2.5100 |
| C5···C8ix | 3.473 (3) | H7···H2 | 2.4300 |
| C5···C9ix | 3.569 (3) | H9···N1 | 2.4700 |
| C6···C9ix | 3.464 (3) | H10···H13A | 2.4800 |
| C6···C8ix | 3.561 (3) | H10···Cl1ii | 2.9800 |
| C7···Cl1viii | 3.572 (2) | H11···H13B | 2.3200 |
| C8···C11iv | 3.516 (3) | H11···O1iv | 2.4900 |
| C8···C6ix | 3.561 (3) | H12···H1Bvii | 2.5700 |
| C8···C5ix | 3.473 (3) | H13A···H10 | 2.4800 |
| C9···C5ix | 3.569 (3) | H13A···C3xi | 2.9800 |
| C9···C6ix | 3.464 (3) | H13A···O1vi | 2.8100 |
| C10···C4ix | 3.582 (3) | H13B···H11 | 2.3200 |
| C10···C3ix | 3.576 (3) | H13B···O1iv | 2.9100 |
| H1A—O1—H1B | 106 (3) | C1—C2—H2 | 120.00 |
| N2—N1—C7 | 113.95 (19) | C3—C2—H2 | 120.00 |
| N1—N2—C8 | 112.75 (18) | C4—C3—H3 | 120.00 |
| C10—N3—C13 | 120.1 (2) | C2—C3—H3 | 120.00 |
| C11—N3—C13 | 121.1 (2) | C4—C5—H5 | 120.00 |
| C10—N3—C11 | 118.73 (19) | C6—C5—H5 | 120.00 |
| C2—C1—C6 | 118.52 (19) | C5—C6—H6 | 120.00 |
| C6—C1—C7 | 122.5 (2) | C1—C6—H6 | 120.00 |
| C2—C1—C7 | 119.0 (2) | N1—C7—H7 | 119.00 |
| C1—C2—C3 | 121.0 (2) | C1—C7—H7 | 119.00 |
| C2—C3—C4 | 119.0 (2) | C10—C9—H9 | 120.00 |
| Cl1—C4—C5 | 119.88 (18) | C8—C9—H9 | 120.00 |
| C3—C4—C5 | 121.1 (2) | N3—C10—H10 | 119.00 |
| Cl1—C4—C3 | 119.03 (17) | C9—C10—H10 | 119.00 |
| C4—C5—C6 | 119.7 (2) | C12—C11—H11 | 119.00 |
| C1—C6—C5 | 120.7 (2) | N3—C11—H11 | 119.00 |
| N1—C7—C1 | 121.9 (2) | C8—C12—H12 | 119.00 |
| N2—C8—C12 | 118.3 (2) | C11—C12—H12 | 119.00 |
| C9—C8—C12 | 114.43 (19) | N3—C13—H13B | 109.00 |
| N2—C8—C9 | 127.27 (19) | N3—C13—H13C | 109.00 |
| C8—C9—C10 | 120.8 (2) | N3—C13—H13A | 109.00 |
| N3—C10—C9 | 122.7 (2) | H13A—C13—H13C | 110.00 |
| N3—C11—C12 | 121.5 (2) | H13B—C13—H13C | 110.00 |
| C8—C12—C11 | 122.0 (2) | H13A—C13—H13B | 109.00 |
| C7—N1—N2—C8 | −174.99 (18) | C6—C1—C7—N1 | −10.1 (3) |
| N2—N1—C7—C1 | −179.43 (18) | C1—C2—C3—C4 | −0.4 (3) |
| N1—N2—C8—C12 | −178.00 (18) | C2—C3—C4—C5 | 0.0 (3) |
| N1—N2—C8—C9 | 2.6 (3) | C2—C3—C4—Cl1 | 179.29 (17) |
| C13—N3—C11—C12 | 179.2 (2) | C3—C4—C5—C6 | 0.2 (3) |
| C10—N3—C11—C12 | −0.6 (3) | Cl1—C4—C5—C6 | −179.11 (17) |
| C11—N3—C10—C9 | 0.3 (3) | C4—C5—C6—C1 | 0.1 (3) |
| C13—N3—C10—C9 | −179.5 (2) | N2—C8—C9—C10 | 179.3 (2) |
| C2—C1—C6—C5 | −0.5 (3) | N2—C8—C12—C11 | −179.7 (2) |
| C7—C1—C2—C3 | 180.0 (2) | C9—C8—C12—C11 | −0.2 (3) |
| C7—C1—C6—C5 | −179.8 (2) | C12—C8—C9—C10 | −0.2 (3) |
| C2—C1—C7—N1 | 170.6 (2) | C8—C9—C10—N3 | 0.1 (3) |
| C6—C1—C2—C3 | 0.7 (3) | N3—C11—C12—C8 | 0.5 (3) |
Symmetry codes: (i) x+1, −y+3/2, z+1/2; (ii) −x+2, −y+1, −z+1; (iii) x+1, y, z; (iv) −x, −y+1, −z; (v) x, −y+3/2, z−1/2; (vi) −x+1, −y+1, −z; (vii) x−1, y, z; (viii) x−1, −y+3/2, z−1/2; (ix) −x+1, −y+1, −z+1; (x) −x+1, y+1/2, −z+1/2; (xi) −x+1, y−1/2, −z+1/2; (xii) x, −y+3/2, z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···N1 | 0.84 (3) | 2.26 (3) | 3.089 (3) | 173 (3) |
| O1—H1B···N2iii | 0.91 (4) | 1.95 (4) | 2.859 (3) | 174 (2) |
| C3—H3···O1xii | 0.93 | 2.58 | 3.421 (3) | 150 |
| C11—H11···O1iv | 0.93 | 2.49 | 3.378 (3) | 159 |
Symmetry codes: (iii) x+1, y, z; (xii) x, −y+3/2, z+1/2; (iv) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ2782).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Alptüzün, V., Prinz, M., Hörr, V., Scheiber, J., Radacki, K., Fallarero, A., Vuorela, P., Engels, B., Braunschweig, H., Erciyas, E. & Holzgrabe, U. (2010). Bioorg. Med. Chem.18, 2049–2059. [DOI] [PubMed]
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Diao, Y.-P., Huang, S.-S., Kong, Y., Xia, M.-Y. & Meng, D.-L. (2008). Z. Kristallogr. New Cryst. Struct.223, 285–286.
- Douglas, W. A., Fishinger, J. J., Gund, P., Haris, E. E., Olson, G., Patchett, A. A. & Ruyle, V. W. (1977). J. Med. Chem.20, 939–943. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Odabaşoğlu, M., Albayrak, Ç., Büyükgüngör, O. & Goesmann, H. (2003). Acta Cryst. C59, o234–o236. [DOI] [PubMed]
- Pandey, J., Pal, R., Dwivedi, A. & Hajela, K. (2002). Arzneimittelforschung, 52, 39–44. [DOI] [PubMed]
- Pople, J. A. & Beveridge, D. L. (1970). In Approximate Molecular Orbital Theory New York: McGraw–Hill.
- Salgın-Gökşen, U., Gökhan-Kelekçi, N., Göktaş, Ö., Köysal, Y., Kılıç, E., İşık, Ş., Aktay, G. & Özalp, M. (2007). Bioorg. Med. Chem.15, 5738–5751. [DOI] [PubMed]
- Savini, L., Chiasserini, L., Gaeta, A. & Pellerano, C. (2002). Bioorg. Med. Chem.10, 2193–2198. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Silva, G. A., Costa, L. M. M., Brito, F. C. F., Miranda, A. L. P., Barreiro, E. J. & Fraga, C. A. M. (2004). Bioorg. Med. Chem.12, 3149–3158. [DOI] [PubMed]
- Stoe & Cie (2002). X-AREA and X-RED32 Stoe & Cie, Darmstadt, Germany.
- Vicini, P., Incerti, M., La Colla, P. & Loddo, R. (2009). Eur. J. Med. Chem.44, 1801–1807. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810015709/sj2782sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810015709/sj2782Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report