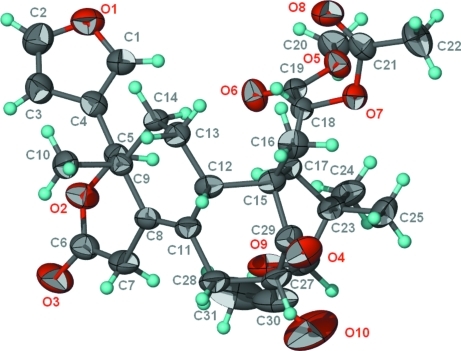
Abstract
The title compound, C31H38O10 [systematic name: (αR,4R,4aR,6aS,7R,8S,10R,11S)-methyl α,10-diacetoxy-4-(3-furyl)-4a,7,9,9-tetramethyl-2,13-dioxo-1,4,4a,5,6,6a,7,8,9,10,11,12-dodecahydro-7,11-methano-2H-cycloocta[f][2]benzopyran-8-acetate], was isolated from the seeds of Swietenia macrophylla. The molecule contains four six-membered rings connected together in the shape of a bowl; one of the inner rings adopts a twisted chair conformation owing to the carbon–carbon double bond. The furyl substitutent is connected to an outer ring, and it points away from the bowl cavity.
Related literature
For the isolation, spectroscopic characterization and absolute structure of the title compound, see: Chan et al. (1976 ▶); Connolly & Labbe (1980 ▶); Connolly et al. (1965 ▶); Govindachari et al. (1999 ▶); Kadota, Marpaung et al. (1990 ▶); Kadota, Yanagawa et al. (1990 ▶); Mootoo et al. (1999 ▶); Narender et al. (2008 ▶); Schefer et al. (2006 ▶); Taylor & Taylor (1983 ▶); Yuan et al. (2010 ▶).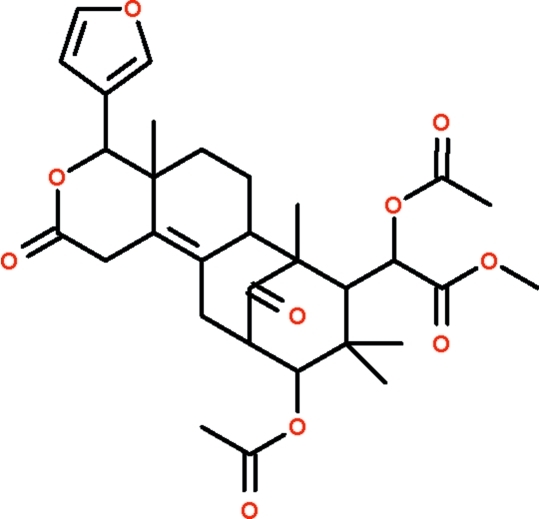
Experimental
Crystal data
C31H38O10
M r = 570.61
Orthorhombic,
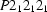
a = 12.5889 (11) Å
b = 13.7109 (12) Å
c = 17.0045 (14) Å
V = 2935.1 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 293 K
0.35 × 0.15 × 0.10 mm
Data collection
Bruker SMART APEX diffractometer
28065 measured reflections
3771 independent reflections
2491 reflections with I > 2σ(I)
R int = 0.077
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.127
S = 1.02
3771 reflections
377 parameters
H-atom parameters constrained
Δρmax = 0.16 e Å−3
Δρmin = −0.13 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810017733/bt5263sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810017733/bt5263Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
Sweietenia macrophylla is a large mahogany tree growing in the rainforests of Malaysia. The extracts of the seeds contain flavonoids, saponins and alkaloids that are commecialized in local herbal products. The isolation of the title compound (Scheme I, Fig. 1) has been reported a long time ago. The present crystal structure analysis confirms the spectroscopic structure determination.
Experimental
Swietenolide diacetate was isloated from the seeds of Swietenia macrophylla by using a reported procedure (Chan et al., 1976).
The finely ground seeds (600 g) were soaked in ethanol at room temperature for three days. The mixture was filtered and the sovlent evaporated to give a dark yellow crude material (70 g). A portion (40 g) was successively extracted with n-hexane, ethyl acetate and water to give an n-hexane-insoluble residue. The residue was partitioned between ethyl acetate-water (1:1) to give an ethyl acetate-soluble fraction (30 g, 80%).
This fraction (3 g) was subjected to column chromatography on silica gel (70-230 mesh, 300 g), with initial elution by n-hexane, followed by increasing proportions of chloroform. Eleven fractions were obtained. The fourth fraction (2 g) was further subjected to column chromatography (70-230 mesh,200 g), initially eluting with n-hexane and later with acetone to give twelve fractions.
The eighth fraction (600 mg) was dissolved in methanol and kept in a refrigerator. A white solid was obtained after two days, and a second crop was obtained after another two days. Recrystallization of the first crop from chloroform yielded colorless crystals of the title compound (yield 15 mg).
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.93 to 0.98 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2 to 1.5Ueq(C). The Flack parameter was fixed to be zero. 2976 Friedel pairs were merged.
Figures
Fig. 1.
Anisotropic displacement ellipsoid plot (Barbour, 2001) of C31H38O10 at the 50% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C31H38O10 | F(000) = 1216 |
| Mr = 570.61 | Dx = 1.291 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 4600 reflections |
| a = 12.5889 (11) Å | θ = 2.2–21.7° |
| b = 13.7109 (12) Å | µ = 0.10 mm−1 |
| c = 17.0045 (14) Å | T = 293 K |
| V = 2935.1 (4) Å3 | Prism, colorless |
| Z = 4 | 0.35 × 0.15 × 0.10 mm |
Data collection
| Bruker SMART APEX diffractometer | 2491 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.077 |
| graphite | θmax = 27.5°, θmin = 1.9° |
| ω scans | h = −14→16 |
| 28065 measured reflections | k = −17→17 |
| 3771 independent reflections | l = −21→22 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.043 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.127 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0642P)2 + 0.2454P] where P = (Fo2 + 2Fc2)/3 |
| 3771 reflections | (Δ/σ)max = 0.001 |
| 377 parameters | Δρmax = 0.16 e Å−3 |
| 0 restraints | Δρmin = −0.13 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.6572 (2) | 0.3236 (2) | −0.20580 (14) | 0.0747 (7) | |
| O2 | 0.7345 (2) | 0.35757 (17) | 0.04692 (14) | 0.0651 (6) | |
| O3 | 0.7606 (3) | 0.4265 (2) | 0.16075 (17) | 0.0924 (9) | |
| O4 | 0.9749 (2) | −0.1523 (2) | 0.17405 (16) | 0.0828 (8) | |
| O5 | 0.55375 (19) | −0.11379 (15) | −0.08732 (13) | 0.0571 (6) | |
| O6 | 0.61162 (19) | 0.03581 (15) | −0.06055 (15) | 0.0622 (6) | |
| O7 | 0.72148 (19) | −0.20206 (14) | −0.03800 (12) | 0.0521 (5) | |
| O8 | 0.7631 (3) | −0.19051 (19) | −0.16536 (15) | 0.0807 (8) | |
| O9 | 0.6687 (2) | 0.0434 (2) | 0.19007 (13) | 0.0720 (7) | |
| O10 | 0.5727 (4) | 0.0137 (4) | 0.2958 (3) | 0.169 (2) | |
| C1 | 0.6620 (3) | 0.2682 (3) | −0.13924 (19) | 0.0620 (9) | |
| H1 | 0.6269 | 0.2091 | −0.1325 | 0.074* | |
| C2 | 0.7183 (4) | 0.4023 (3) | −0.1918 (2) | 0.0801 (12) | |
| H2 | 0.7296 | 0.4527 | −0.2274 | 0.096* | |
| C3 | 0.7605 (4) | 0.3985 (3) | −0.1201 (2) | 0.0729 (11) | |
| H3 | 0.8053 | 0.4445 | −0.0975 | 0.087* | |
| C4 | 0.7242 (3) | 0.3105 (2) | −0.08472 (19) | 0.0520 (8) | |
| C5 | 0.7473 (3) | 0.2725 (2) | −0.00418 (18) | 0.0491 (7) | |
| H5 | 0.6923 | 0.2248 | 0.0095 | 0.059* | |
| C6 | 0.7706 (3) | 0.3546 (3) | 0.1211 (2) | 0.0622 (9) | |
| C7 | 0.8189 (4) | 0.2635 (3) | 0.1517 (2) | 0.0704 (11) | |
| H7A | 0.7672 | 0.2321 | 0.1856 | 0.084* | |
| H7B | 0.8790 | 0.2814 | 0.1844 | 0.084* | |
| C8 | 0.8569 (3) | 0.1887 (2) | 0.09253 (17) | 0.0486 (7) | |
| C9 | 0.8563 (2) | 0.2247 (2) | 0.00792 (16) | 0.0431 (7) | |
| C10 | 0.9466 (3) | 0.2977 (2) | −0.0049 (2) | 0.0582 (8) | |
| H10A | 1.0136 | 0.2651 | 0.0011 | 0.087* | |
| H10B | 0.9412 | 0.3493 | 0.0330 | 0.087* | |
| H10C | 0.9416 | 0.3244 | −0.0570 | 0.087* | |
| C11 | 0.8891 (3) | 0.0997 (2) | 0.11439 (17) | 0.0481 (7) | |
| C12 | 0.9384 (2) | 0.0273 (2) | 0.05864 (17) | 0.0485 (7) | |
| H12 | 1.0107 | 0.0171 | 0.0784 | 0.058* | |
| C13 | 0.9526 (3) | 0.0679 (2) | −0.02434 (17) | 0.0510 (7) | |
| H13A | 1.0207 | 0.1009 | −0.0273 | 0.061* | |
| H13B | 0.9543 | 0.0140 | −0.0613 | 0.061* | |
| C14 | 0.8658 (2) | 0.1386 (2) | −0.04903 (16) | 0.0455 (7) | |
| H14A | 0.7985 | 0.1043 | −0.0512 | 0.055* | |
| H14B | 0.8812 | 0.1630 | −0.1013 | 0.055* | |
| C15 | 0.8823 (2) | −0.0758 (2) | 0.06588 (17) | 0.0490 (7) | |
| C16 | 0.9398 (3) | −0.1515 (3) | 0.0156 (2) | 0.0609 (9) | |
| H16A | 0.9102 | −0.2149 | 0.0254 | 0.091* | |
| H16B | 1.0140 | −0.1518 | 0.0287 | 0.091* | |
| H16C | 0.9315 | −0.1354 | −0.0390 | 0.091* | |
| C17 | 0.7588 (2) | −0.0670 (2) | 0.05172 (17) | 0.0438 (7) | |
| H17 | 0.7446 | 0.0033 | 0.0537 | 0.053* | |
| C18 | 0.7268 (3) | −0.09720 (19) | −0.03206 (16) | 0.0432 (7) | |
| H18 | 0.7836 | −0.0755 | −0.0673 | 0.052* | |
| C19 | 0.6247 (3) | −0.0503 (2) | −0.06065 (16) | 0.0443 (7) | |
| C20 | 0.4543 (3) | −0.0743 (3) | −0.1149 (2) | 0.0680 (10) | |
| H20A | 0.4184 | −0.1222 | −0.1464 | 0.102* | |
| H20B | 0.4677 | −0.0172 | −0.1460 | 0.102* | |
| H20C | 0.4107 | −0.0572 | −0.0707 | 0.102* | |
| C21 | 0.7431 (3) | −0.2402 (2) | −0.1093 (2) | 0.0565 (8) | |
| C22 | 0.7397 (4) | −0.3485 (2) | −0.1083 (3) | 0.0834 (13) | |
| H22A | 0.8030 | −0.3738 | −0.1322 | 0.125* | |
| H22B | 0.6787 | −0.3706 | −0.1371 | 0.125* | |
| H22C | 0.7351 | −0.3709 | −0.0549 | 0.125* | |
| C23 | 0.6916 (3) | −0.1102 (3) | 0.12000 (18) | 0.0590 (9) | |
| C24 | 0.5717 (3) | −0.1013 (3) | 0.1041 (2) | 0.0760 (11) | |
| H24A | 0.5331 | −0.1158 | 0.1513 | 0.114* | |
| H24B | 0.5517 | −0.1465 | 0.0636 | 0.114* | |
| H24C | 0.5556 | −0.0361 | 0.0874 | 0.114* | |
| C25 | 0.7149 (4) | −0.2193 (3) | 0.1370 (2) | 0.0815 (13) | |
| H25A | 0.7901 | −0.2286 | 0.1426 | 0.122* | |
| H25B | 0.6893 | −0.2584 | 0.0941 | 0.122* | |
| H25C | 0.6797 | −0.2385 | 0.1846 | 0.122* | |
| C26 | 0.7198 (3) | −0.0507 (3) | 0.19481 (19) | 0.0641 (9) | |
| H26 | 0.6901 | −0.0852 | 0.2402 | 0.077* | |
| C27 | 0.8403 (3) | −0.0385 (3) | 0.20878 (18) | 0.0599 (9) | |
| H27 | 0.8563 | −0.0606 | 0.2623 | 0.072* | |
| C28 | 0.8853 (3) | 0.0659 (3) | 0.19881 (18) | 0.0620 (9) | |
| H28A | 0.8418 | 0.1109 | 0.2288 | 0.074* | |
| H28B | 0.9566 | 0.0679 | 0.2205 | 0.074* | |
| C29 | 0.9034 (3) | −0.0992 (3) | 0.1525 (2) | 0.0599 (9) | |
| C30 | 0.5932 (4) | 0.0658 (5) | 0.2431 (3) | 0.0993 (16) | |
| C31 | 0.5422 (5) | 0.1596 (5) | 0.2259 (3) | 0.135 (3) | |
| H31A | 0.4720 | 0.1484 | 0.2058 | 0.203* | |
| H31B | 0.5833 | 0.1943 | 0.1875 | 0.203* | |
| H31C | 0.5379 | 0.1976 | 0.2733 | 0.203* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0871 (19) | 0.0801 (18) | 0.0570 (15) | 0.0094 (16) | −0.0090 (14) | −0.0018 (13) |
| O2 | 0.0688 (15) | 0.0690 (14) | 0.0576 (14) | 0.0173 (13) | −0.0014 (12) | −0.0152 (12) |
| O3 | 0.123 (2) | 0.0782 (17) | 0.0757 (18) | 0.0140 (19) | 0.0052 (17) | −0.0303 (15) |
| O4 | 0.0820 (18) | 0.0923 (19) | 0.0742 (17) | 0.0214 (17) | −0.0234 (15) | 0.0242 (16) |
| O5 | 0.0546 (13) | 0.0524 (12) | 0.0643 (14) | −0.0015 (11) | −0.0160 (12) | −0.0047 (10) |
| O6 | 0.0572 (14) | 0.0449 (12) | 0.0844 (16) | 0.0046 (10) | −0.0142 (13) | 0.0017 (11) |
| O7 | 0.0649 (14) | 0.0394 (10) | 0.0521 (12) | 0.0058 (10) | −0.0030 (11) | 0.0013 (9) |
| O8 | 0.119 (2) | 0.0714 (15) | 0.0516 (15) | 0.0022 (17) | 0.0051 (15) | −0.0070 (13) |
| O9 | 0.0713 (16) | 0.103 (2) | 0.0417 (12) | 0.0149 (15) | 0.0016 (12) | −0.0055 (12) |
| O10 | 0.176 (5) | 0.226 (5) | 0.103 (3) | 0.026 (4) | 0.082 (3) | 0.017 (3) |
| C1 | 0.070 (2) | 0.064 (2) | 0.0519 (19) | −0.0011 (19) | −0.0007 (18) | −0.0028 (17) |
| C2 | 0.098 (3) | 0.065 (2) | 0.077 (3) | 0.011 (2) | −0.005 (3) | 0.014 (2) |
| C3 | 0.093 (3) | 0.0507 (19) | 0.075 (3) | 0.004 (2) | −0.019 (2) | 0.0056 (17) |
| C4 | 0.0514 (19) | 0.0491 (17) | 0.0555 (19) | 0.0085 (15) | −0.0043 (16) | −0.0057 (14) |
| C5 | 0.0475 (19) | 0.0506 (17) | 0.0493 (17) | −0.0008 (15) | 0.0021 (14) | −0.0059 (14) |
| C6 | 0.067 (2) | 0.066 (2) | 0.054 (2) | −0.0016 (19) | 0.0086 (18) | −0.0122 (17) |
| C7 | 0.099 (3) | 0.066 (2) | 0.0456 (18) | 0.001 (2) | 0.0063 (19) | −0.0097 (16) |
| C8 | 0.0516 (18) | 0.0564 (18) | 0.0377 (15) | −0.0057 (15) | 0.0005 (14) | −0.0047 (14) |
| C9 | 0.0406 (16) | 0.0487 (16) | 0.0400 (15) | −0.0035 (13) | 0.0003 (13) | −0.0020 (13) |
| C10 | 0.0514 (19) | 0.063 (2) | 0.060 (2) | −0.0124 (17) | −0.0019 (17) | 0.0026 (17) |
| C11 | 0.0483 (18) | 0.0587 (18) | 0.0374 (15) | −0.0050 (16) | −0.0054 (14) | 0.0009 (13) |
| C12 | 0.0408 (16) | 0.0629 (18) | 0.0419 (15) | 0.0054 (15) | −0.0063 (15) | 0.0020 (15) |
| C13 | 0.0502 (18) | 0.0547 (17) | 0.0481 (17) | 0.0027 (16) | 0.0063 (15) | 0.0004 (14) |
| C14 | 0.0504 (17) | 0.0508 (16) | 0.0353 (13) | −0.0019 (14) | 0.0025 (14) | 0.0018 (13) |
| C15 | 0.0468 (17) | 0.0568 (17) | 0.0435 (16) | 0.0072 (15) | −0.0059 (14) | 0.0059 (14) |
| C16 | 0.058 (2) | 0.0603 (19) | 0.064 (2) | 0.0185 (18) | −0.0057 (18) | 0.0014 (17) |
| C17 | 0.0451 (17) | 0.0465 (15) | 0.0397 (14) | 0.0014 (14) | −0.0031 (13) | 0.0044 (13) |
| C18 | 0.0523 (17) | 0.0366 (14) | 0.0407 (15) | 0.0025 (14) | −0.0043 (14) | 0.0012 (12) |
| C19 | 0.0543 (18) | 0.0414 (16) | 0.0372 (14) | −0.0011 (14) | −0.0023 (14) | 0.0008 (12) |
| C20 | 0.055 (2) | 0.080 (2) | 0.069 (2) | 0.002 (2) | −0.0195 (19) | −0.0008 (19) |
| C21 | 0.056 (2) | 0.0523 (18) | 0.061 (2) | 0.0036 (17) | −0.0063 (17) | −0.0084 (16) |
| C22 | 0.092 (3) | 0.052 (2) | 0.106 (3) | 0.009 (2) | 0.005 (3) | −0.022 (2) |
| C23 | 0.064 (2) | 0.070 (2) | 0.0426 (17) | −0.0084 (18) | −0.0010 (16) | 0.0080 (15) |
| C24 | 0.059 (2) | 0.116 (3) | 0.053 (2) | −0.016 (2) | 0.0049 (18) | 0.010 (2) |
| C25 | 0.096 (3) | 0.081 (3) | 0.067 (2) | −0.022 (2) | −0.007 (2) | 0.029 (2) |
| C26 | 0.067 (2) | 0.086 (2) | 0.0386 (17) | −0.005 (2) | 0.0029 (17) | 0.0158 (16) |
| C27 | 0.070 (2) | 0.076 (2) | 0.0341 (15) | 0.0009 (19) | −0.0117 (16) | 0.0160 (15) |
| C28 | 0.072 (2) | 0.075 (2) | 0.0385 (16) | 0.000 (2) | −0.0092 (16) | 0.0015 (16) |
| C29 | 0.061 (2) | 0.063 (2) | 0.056 (2) | 0.0001 (18) | −0.0147 (17) | 0.0156 (17) |
| C30 | 0.087 (3) | 0.162 (5) | 0.049 (2) | 0.010 (4) | 0.008 (2) | −0.016 (3) |
| C31 | 0.100 (4) | 0.208 (7) | 0.099 (4) | 0.070 (5) | −0.027 (3) | −0.056 (4) |
Geometric parameters (Å, °)
| O1—C2 | 1.347 (5) | C13—H13B | 0.9700 |
| O1—C1 | 1.364 (4) | C14—H14A | 0.9700 |
| O2—C6 | 1.342 (4) | C14—H14B | 0.9700 |
| O2—C5 | 1.463 (4) | C15—C16 | 1.528 (5) |
| O3—C6 | 1.200 (4) | C15—C29 | 1.531 (5) |
| O4—C29 | 1.214 (4) | C15—C17 | 1.578 (4) |
| O5—C19 | 1.327 (3) | C16—H16A | 0.9600 |
| O5—C20 | 1.442 (4) | C16—H16B | 0.9600 |
| O6—C19 | 1.193 (3) | C16—H16C | 0.9600 |
| O7—C21 | 1.348 (4) | C17—C18 | 1.538 (4) |
| O7—C18 | 1.443 (3) | C17—C23 | 1.554 (4) |
| O8—C21 | 1.198 (4) | C17—H17 | 0.9800 |
| O9—C30 | 1.345 (5) | C18—C19 | 1.517 (4) |
| O9—C26 | 1.445 (5) | C18—H18 | 0.9800 |
| O10—C30 | 1.175 (7) | C20—H20A | 0.9600 |
| C1—C4 | 1.345 (5) | C20—H20B | 0.9600 |
| C1—H1 | 0.9300 | C20—H20C | 0.9600 |
| C2—C3 | 1.330 (5) | C21—C22 | 1.486 (5) |
| C2—H2 | 0.9300 | C22—H22A | 0.9600 |
| C3—C4 | 1.423 (5) | C22—H22B | 0.9600 |
| C3—H3 | 0.9300 | C22—H22C | 0.9600 |
| C4—C5 | 1.494 (5) | C23—C24 | 1.538 (5) |
| C5—C9 | 1.535 (4) | C23—C26 | 1.552 (5) |
| C5—H5 | 0.9800 | C23—C25 | 1.552 (5) |
| C6—C7 | 1.484 (5) | C24—H24A | 0.9600 |
| C7—C8 | 1.514 (4) | C24—H24B | 0.9600 |
| C7—H7A | 0.9700 | C24—H24C | 0.9600 |
| C7—H7B | 0.9700 | C25—H25A | 0.9600 |
| C8—C11 | 1.339 (4) | C25—H25B | 0.9600 |
| C8—C9 | 1.521 (4) | C25—H25C | 0.9600 |
| C9—C10 | 1.530 (4) | C26—C27 | 1.544 (5) |
| C9—C14 | 1.532 (4) | C26—H26 | 0.9800 |
| C10—H10A | 0.9600 | C27—C29 | 1.497 (5) |
| C10—H10B | 0.9600 | C27—C28 | 1.549 (5) |
| C10—H10C | 0.9600 | C27—H27 | 0.9800 |
| C11—C12 | 1.506 (4) | C28—H28A | 0.9700 |
| C11—C28 | 1.509 (4) | C28—H28B | 0.9700 |
| C12—C13 | 1.528 (4) | C30—C31 | 1.467 (8) |
| C12—C15 | 1.585 (4) | C31—H31A | 0.9600 |
| C12—H12 | 0.9800 | C31—H31B | 0.9600 |
| C13—C14 | 1.520 (4) | C31—H31C | 0.9600 |
| C13—H13A | 0.9700 | ||
| C2—O1—C1 | 105.9 (3) | H16B—C16—H16C | 109.5 |
| C6—O2—C5 | 119.8 (3) | C18—C17—C23 | 116.6 (3) |
| C19—O5—C20 | 116.7 (2) | C18—C17—C15 | 112.3 (2) |
| C21—O7—C18 | 116.1 (2) | C23—C17—C15 | 113.1 (2) |
| C30—O9—C26 | 118.8 (4) | C18—C17—H17 | 104.5 |
| C4—C1—O1 | 111.0 (3) | C23—C17—H17 | 104.5 |
| C4—C1—H1 | 124.5 | C15—C17—H17 | 104.5 |
| O1—C1—H1 | 124.5 | O7—C18—C19 | 111.1 (2) |
| C3—C2—O1 | 111.0 (4) | O7—C18—C17 | 110.2 (2) |
| C3—C2—H2 | 124.5 | C19—C18—C17 | 113.9 (2) |
| O1—C2—H2 | 124.5 | O7—C18—H18 | 107.1 |
| C2—C3—C4 | 107.0 (4) | C19—C18—H18 | 107.1 |
| C2—C3—H3 | 126.5 | C17—C18—H18 | 107.1 |
| C4—C3—H3 | 126.5 | O6—C19—O5 | 123.8 (3) |
| C1—C4—C3 | 105.1 (3) | O6—C19—C18 | 122.4 (3) |
| C1—C4—C5 | 126.5 (3) | O5—C19—C18 | 113.7 (2) |
| C3—C4—C5 | 128.4 (3) | O5—C20—H20A | 109.5 |
| O2—C5—C4 | 104.2 (2) | O5—C20—H20B | 109.5 |
| O2—C5—C9 | 111.0 (2) | H20A—C20—H20B | 109.5 |
| C4—C5—C9 | 116.5 (3) | O5—C20—H20C | 109.5 |
| O2—C5—H5 | 108.3 | H20A—C20—H20C | 109.5 |
| C4—C5—H5 | 108.3 | H20B—C20—H20C | 109.5 |
| C9—C5—H5 | 108.3 | O8—C21—O7 | 122.5 (3) |
| O3—C6—O2 | 117.9 (3) | O8—C21—C22 | 125.7 (3) |
| O3—C6—C7 | 122.5 (3) | O7—C21—C22 | 111.8 (3) |
| O2—C6—C7 | 119.6 (3) | C21—C22—H22A | 109.5 |
| C6—C7—C8 | 117.8 (3) | C21—C22—H22B | 109.5 |
| C6—C7—H7A | 107.8 | H22A—C22—H22B | 109.5 |
| C8—C7—H7A | 107.8 | C21—C22—H22C | 109.5 |
| C6—C7—H7B | 107.8 | H22A—C22—H22C | 109.5 |
| C8—C7—H7B | 107.8 | H22B—C22—H22C | 109.5 |
| H7A—C7—H7B | 107.2 | C24—C23—C26 | 109.1 (3) |
| C11—C8—C7 | 121.9 (3) | C24—C23—C25 | 107.1 (3) |
| C11—C8—C9 | 124.0 (3) | C26—C23—C25 | 108.1 (3) |
| C7—C8—C9 | 114.0 (3) | C24—C23—C17 | 111.9 (3) |
| C8—C9—C10 | 110.1 (2) | C26—C23—C17 | 106.7 (3) |
| C8—C9—C14 | 110.3 (2) | C25—C23—C17 | 113.8 (3) |
| C10—C9—C14 | 110.8 (2) | C23—C24—H24A | 109.5 |
| C8—C9—C5 | 105.6 (2) | C23—C24—H24B | 109.5 |
| C10—C9—C5 | 111.5 (2) | H24A—C24—H24B | 109.5 |
| C14—C9—C5 | 108.3 (2) | C23—C24—H24C | 109.5 |
| C9—C10—H10A | 109.5 | H24A—C24—H24C | 109.5 |
| C9—C10—H10B | 109.5 | H24B—C24—H24C | 109.5 |
| H10A—C10—H10B | 109.5 | C23—C25—H25A | 109.5 |
| C9—C10—H10C | 109.5 | C23—C25—H25B | 109.5 |
| H10A—C10—H10C | 109.5 | H25A—C25—H25B | 109.5 |
| H10B—C10—H10C | 109.5 | C23—C25—H25C | 109.5 |
| C8—C11—C12 | 123.4 (3) | H25A—C25—H25C | 109.5 |
| C8—C11—C28 | 122.3 (3) | H25B—C25—H25C | 109.5 |
| C12—C11—C28 | 114.2 (3) | O9—C26—C27 | 110.4 (3) |
| C11—C12—C13 | 112.9 (3) | O9—C26—C23 | 108.8 (3) |
| C11—C12—C15 | 110.8 (2) | C27—C26—C23 | 114.1 (3) |
| C13—C12—C15 | 116.7 (3) | O9—C26—H26 | 107.8 |
| C11—C12—H12 | 105.1 | C27—C26—H26 | 107.8 |
| C13—C12—H12 | 105.1 | C23—C26—H26 | 107.8 |
| C15—C12—H12 | 105.1 | C29—C27—C26 | 111.3 (3) |
| C14—C13—C12 | 113.8 (3) | C29—C27—C28 | 104.4 (3) |
| C14—C13—H13A | 108.8 | C26—C27—C28 | 116.3 (3) |
| C12—C13—H13A | 108.8 | C29—C27—H27 | 108.2 |
| C14—C13—H13B | 108.8 | C26—C27—H27 | 108.2 |
| C12—C13—H13B | 108.8 | C28—C27—H27 | 108.2 |
| H13A—C13—H13B | 107.7 | C11—C28—C27 | 113.5 (3) |
| C13—C14—C9 | 111.9 (2) | C11—C28—H28A | 108.9 |
| C13—C14—H14A | 109.2 | C27—C28—H28A | 108.9 |
| C9—C14—H14A | 109.2 | C11—C28—H28B | 108.9 |
| C13—C14—H14B | 109.2 | C27—C28—H28B | 108.9 |
| C9—C14—H14B | 109.2 | H28A—C28—H28B | 107.7 |
| H14A—C14—H14B | 107.9 | O4—C29—C27 | 122.3 (3) |
| C16—C15—C29 | 108.3 (3) | O4—C29—C15 | 123.0 (3) |
| C16—C15—C17 | 115.7 (3) | C27—C29—C15 | 114.0 (3) |
| C29—C15—C17 | 109.6 (3) | O10—C30—O9 | 121.8 (6) |
| C16—C15—C12 | 110.5 (3) | O10—C30—C31 | 126.1 (5) |
| C29—C15—C12 | 100.6 (3) | O9—C30—C31 | 112.1 (5) |
| C17—C15—C12 | 111.0 (2) | C30—C31—H31A | 109.5 |
| C15—C16—H16A | 109.5 | C30—C31—H31B | 109.5 |
| C15—C16—H16B | 109.5 | H31A—C31—H31B | 109.5 |
| H16A—C16—H16B | 109.5 | C30—C31—H31C | 109.5 |
| C15—C16—H16C | 109.5 | H31A—C31—H31C | 109.5 |
| H16A—C16—H16C | 109.5 | H31B—C31—H31C | 109.5 |
| C2—O1—C1—C4 | 0.0 (4) | C12—C15—C17—C18 | 100.0 (3) |
| C1—O1—C2—C3 | 0.0 (5) | C16—C15—C17—C23 | 107.4 (3) |
| O1—C2—C3—C4 | 0.0 (5) | C29—C15—C17—C23 | −15.4 (4) |
| O1—C1—C4—C3 | 0.0 (4) | C12—C15—C17—C23 | −125.6 (3) |
| O1—C1—C4—C5 | 179.0 (3) | C21—O7—C18—C19 | 82.4 (3) |
| C2—C3—C4—C1 | 0.0 (4) | C21—O7—C18—C17 | −150.4 (3) |
| C2—C3—C4—C5 | −179.0 (3) | C23—C17—C18—O7 | −52.8 (3) |
| C6—O2—C5—C4 | −166.2 (3) | C15—C17—C18—O7 | 80.0 (3) |
| C6—O2—C5—C9 | −40.0 (4) | C23—C17—C18—C19 | 72.9 (3) |
| C1—C4—C5—O2 | −134.2 (3) | C15—C17—C18—C19 | −154.4 (2) |
| C3—C4—C5—O2 | 44.6 (4) | C20—O5—C19—O6 | −2.2 (4) |
| C1—C4—C5—C9 | 103.2 (4) | C20—O5—C19—C18 | 179.5 (3) |
| C3—C4—C5—C9 | −78.0 (4) | O7—C18—C19—O6 | 179.6 (3) |
| C5—O2—C6—O3 | 178.5 (3) | C17—C18—C19—O6 | 54.4 (4) |
| C5—O2—C6—C7 | −2.7 (5) | O7—C18—C19—O5 | −2.1 (3) |
| O3—C6—C7—C8 | −162.5 (4) | C17—C18—C19—O5 | −127.3 (3) |
| O2—C6—C7—C8 | 18.8 (5) | C18—O7—C21—O8 | −1.8 (5) |
| C6—C7—C8—C11 | −171.5 (3) | C18—O7—C21—C22 | 177.7 (3) |
| C6—C7—C8—C9 | 9.4 (5) | C18—C17—C23—C24 | −47.1 (4) |
| C11—C8—C9—C10 | −106.3 (3) | C15—C17—C23—C24 | −179.4 (3) |
| C7—C8—C9—C10 | 72.8 (4) | C18—C17—C23—C26 | −166.3 (3) |
| C11—C8—C9—C14 | 16.4 (4) | C15—C17—C23—C26 | 61.3 (4) |
| C7—C8—C9—C14 | −164.5 (3) | C18—C17—C23—C25 | 74.5 (4) |
| C11—C8—C9—C5 | 133.3 (3) | C15—C17—C23—C25 | −57.9 (4) |
| C7—C8—C9—C5 | −47.7 (3) | C30—O9—C26—C27 | −119.0 (4) |
| O2—C5—C9—C8 | 63.6 (3) | C30—O9—C26—C23 | 115.2 (4) |
| C4—C5—C9—C8 | −177.4 (3) | C24—C23—C26—O9 | −45.9 (4) |
| O2—C5—C9—C10 | −55.9 (3) | C25—C23—C26—O9 | −162.1 (3) |
| C4—C5—C9—C10 | 63.0 (3) | C17—C23—C26—O9 | 75.1 (3) |
| O2—C5—C9—C14 | −178.1 (2) | C24—C23—C26—C27 | −169.6 (3) |
| C4—C5—C9—C14 | −59.2 (3) | C25—C23—C26—C27 | 74.2 (4) |
| C7—C8—C11—C12 | −173.2 (3) | C17—C23—C26—C27 | −48.6 (4) |
| C9—C8—C11—C12 | 5.9 (5) | O9—C26—C27—C29 | −130.9 (3) |
| C7—C8—C11—C28 | 2.8 (5) | C23—C26—C27—C29 | −8.0 (4) |
| C9—C8—C11—C28 | −178.1 (3) | O9—C26—C27—C28 | −11.4 (4) |
| C8—C11—C12—C13 | 2.8 (4) | C23—C26—C27—C28 | 111.4 (4) |
| C28—C11—C12—C13 | −173.5 (3) | C8—C11—C28—C27 | 135.0 (3) |
| C8—C11—C12—C15 | −130.2 (3) | C12—C11—C28—C27 | −48.6 (4) |
| C28—C11—C12—C15 | 53.5 (3) | C29—C27—C28—C11 | 51.4 (4) |
| C11—C12—C13—C14 | −33.9 (4) | C26—C27—C28—C11 | −71.7 (4) |
| C15—C12—C13—C14 | 96.2 (3) | C26—C27—C29—O4 | −130.9 (4) |
| C12—C13—C14—C9 | 57.0 (3) | C28—C27—C29—O4 | 102.9 (4) |
| C8—C9—C14—C13 | −46.3 (3) | C26—C27—C29—C15 | 58.6 (4) |
| C10—C9—C14—C13 | 76.0 (3) | C28—C27—C29—C15 | −67.7 (4) |
| C5—C9—C14—C13 | −161.5 (2) | C16—C15—C29—O4 | 17.0 (5) |
| C11—C12—C15—C16 | −174.1 (2) | C17—C15—C29—O4 | 144.1 (3) |
| C13—C12—C15—C16 | 54.8 (3) | C12—C15—C29—O4 | −98.9 (4) |
| C11—C12—C15—C29 | −59.9 (3) | C16—C15—C29—C27 | −172.5 (3) |
| C13—C12—C15—C29 | 169.0 (3) | C17—C15—C29—C27 | −45.4 (4) |
| C11—C12—C15—C17 | 56.0 (3) | C12—C15—C29—C27 | 71.6 (3) |
| C13—C12—C15—C17 | −75.1 (3) | C26—O9—C30—O10 | 4.9 (7) |
| C16—C15—C17—C18 | −27.1 (4) | C26—O9—C30—C31 | −174.6 (4) |
| C29—C15—C17—C18 | −149.8 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5263).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chan, K. C., Tang, T. S. & Toh, H. T. (1976). Phytochemistry, 15, 429–430.
- Connolly, J. D. & Labbe, C. (1980). J. Chem. Soc. Perkin Trans. 1, pp. 529–530.
- Connolly, J. D., McCrindle, R., Overton, K. H. & Warnock, W. D. C. (1965). Tetrahedron Lett. pp. 2937–2940.
- Govindachari, T. R., Suresh, G., Banumathy, B., Masilamani, S., Gopalakrishnan, G. & Kumari, G. N. K. (1999). J. Chem. Ecol.25, 923–933.
- Kadota, S., Marpaung, L., Kikuchi, T. & Ekimoto, H. (1990). Chem. Pharm. Bull.38, 639–651. [DOI] [PubMed]
- Kadota, S., Yanagawa, K., Kikuchi, T. & Tanaka, K. (1990). Tetrahedron Lett.31, 5943–5946.
- Mootoo, B. S., Ali, A., Motilal, R., Pingal, R., Ramlal, A., Khan, A., Reynolds, W. F. & McLean, S. (1999). J. Nat. Prod.62, 1514–1517. [DOI] [PubMed]
- Narender,T., Khaliq, T. Shweta (2008). Nat. Prod. Res. A22, 763–800. [DOI] [PubMed]
- Schefer, A. B., Braumann, U., Tseng, L.-H., Spraul, M., Soares, M. G., Fernandes, J. B., da Silva, M. F. G. F., Vieira, P. C. & Ferreira, A. G. (2006). J. Chromatograph. A, 1128, 152–163. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Taylor, A. R. H. & Taylor, D. A. H. (1983). S. Afr. Phytochem.22, 2870–2871.
- Westrip, S. P. (2010). J. Appl. Cryst.43 Submitted.
- Yuan, T., Zhang, C.-R., Yang, S.-P. & Yue, J.-M. (2010). J. Nat. Prod.73, 669–674. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810017733/bt5263sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810017733/bt5263Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report