Abstract
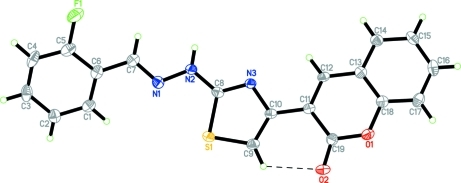
In the title compound, C19H12FN3O2S, the chromene ring system and the thiazole ring are approximately planar [maximum deviations of 0.023 (3) Å and 0.004 (2) Å, respectively]. The chromene ring system is inclined at angles of 4.78 (10) and 26.51 (10)° with respect to the thiazole and benzene rings, respectively, while the thiazole ring makes a dihedral angle of 23.07 (12)° with the benzene ring. The molecular structure is stabilized by an intramolecular C—H⋯O hydrogen bond, which generates an S(6) ring motif. The crystal packing is consolidated by intermolecular N—H⋯O hydrogen bonds, which link the molecules into chains parallel to [100], and by C—H⋯π and π–π [centroid–centroid distance = 3.4954 (15) Å] stacking interactions.
Related literature
For the synthesis of the title compound, see: Lv et al. (2010 ▶); Siddiqui et al. (2009 ▶). For general background to and the biological activity of coumarin derivatives, see: Anderson et al. (2002 ▶); Tassies et al. (2002 ▶); Mitscher (2002 ▶); Lafitte et al. (2002 ▶); Moffett (1964 ▶); Weber et al. (1998 ▶). For the biological activity of aminothiazoles derivatives, see: Hiremath et al. (1992 ▶); Habib & Khalil (1984 ▶); Karah et al. (1998 ▶); Gursoy & Karah (2000 ▶); Lednicer et al. (1990 ▶); Kim et al. (2002 ▶); Wattenberg et al. (1979 ▶). For the stability of the temperature controller used for the data collection, see: Cosier & Glazer (1986 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶).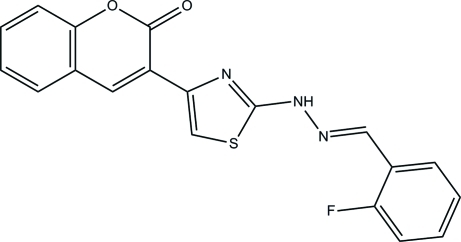
Experimental
Crystal data
C19H12FN3O2S
M r = 365.38
Orthorhombic,
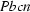
a = 12.303 (2) Å
b = 10.4477 (17) Å
c = 25.247 (4) Å
V = 3245.2 (9) Å3
Z = 8
Mo Kα radiation
μ = 0.23 mm−1
T = 100 K
0.37 × 0.08 × 0.04 mm
Data collection
Bruker SMART APEXII DUO CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.919, T max = 0.991
13589 measured reflections
2855 independent reflections
1971 reflections with I > 2σ(I)
R int = 0.082
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.102
S = 1.04
2855 reflections
239 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.22 e Å−3
Δρmin = −0.33 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL ; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810018647/tk2674sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810018647/tk2674Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the C13–C18 benzene ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C9—H9⋯O2 | 0.93 | 2.27 | 2.823 (3) | 118 |
| N2—H12N⋯O2i | 0.85 (3) | 2.04 (3) | 2.852 (3) | 161 (3) |
| C4—H4⋯Cg1ii | 0.93 | 2.96 | 3.701 (3) | 138 |
Symmetry codes: (i) 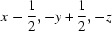 ; (ii)
; (ii) 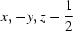 .
.
Acknowledgments
We thank the Malaysian Government and Universiti Sains Malaysia (USM) for a short-term grant (304/PKIMIA/639004) to conduct this work. HKF and CKQ thank USM for the Research University Golden Goose Grant (1001/PFIZIK/811012). CKQ also thanks USM for the award of USM Fellowship. AA thanks the Pakistan Government and PCSIR for financial support through a scholarship.
supplementary crystallographic information
Comment
Coumarin derivatives constitute an important class of heterocyclic compounds having pronounced biological activities. For example, warfarin and cenocoumarol are used as anti-coagulants (Anderson et al., 2002; Tassies et al., 2002). These compounds also possess very good anti-bacterial (Mitscher, 2002; Lafitte et al., 2002), anti-fungal (Moffett, 1964) and cytotoxic activities (Weber et al., 1998). On the other hand, aminothiazole derivatives have been reported to exhibit significant anti-fungal (Hiremath et al., 1992), anti-bacterial (Habib & Khalil, 1984), and anti-tuberculosis activities (Karah et al., 1998; Gursoy & Karah, 2000). These compounds also have very important pharmaceutical value because of their anti-inflammatory (Lednicer et al., 1990), enzyme inhibition (Kim et al., 2002) and anti-tumour activities (Wattenberg et al., 1979). Our approach is the synthesis of biologically active compounds based on the combination of different substructures to enhance the biological activity of known compounds. The title compound is a new coumarin derivative having aminothiazole moiety. We present here its crystal structure, Fig. 1.
The chromene (O1/C11–C19) ring system and thiazole (S1/N3/C8–C10) ring are approximately planar, with the maximum deviation of 0.023 (3) Å for atom C19 and 0.004 (2) Å for atom N3, respectively. The chromene ring system is inclined at angles of 4.78 (10) and 26.51 (10) ° with respect to the thiazole and benzene (C1–C6) rings, respectively. The thiazole ring makes a dihedral angle of 23.07 (12) ° with benzene ring. The molecular structure is stabilized by an intramolecular C9—H9···O2 hydrogen bond which generates an S(6) ring motif (Bernstein et al., 1995).
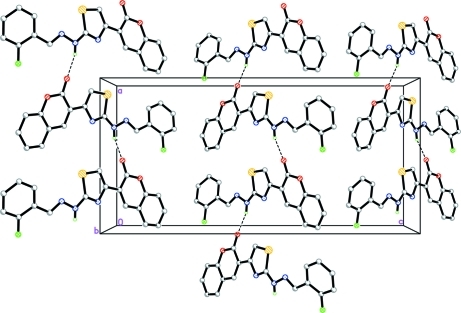
The crystal packing is consolidated by intermolecular N2—H12N···O2 hydrogen bonds (Fig. 2) which link the independent molecules into chains parallel to [1 0 0]. The crystal packing is consolidated by C—H···π (Table 1) and π–π stacking interactions between symmetry related S1/N3/C8—C10 (centroid Cg2) and O1/C11—C13/C18/C19 (centroid Cg3) rings, with Cg2···Cg3 distance of 3.4954 (15) Å [symmetry code: 3/2-x, -1/2+y, z].
Experimental
Thiosemicarbazide (5.00 mmol) was slowly added to a solution of 2-fluorobenzaldehyde in hot absolute ethanol (10 ml) while stirring. The resulting solution was refluxed for 2 h and then cooled on an ice bath for 45 minutes to get white precipitates. These precipitates were filtered and recrystallized from ethanol-water to obtain 2-fluorobenzaldehyde thiosemicarbazone (Lv et al., 2010). 3-[ω-Bromoacetyl coumarin] was prepared as reported in the literature (Siddiqui et al., 2009). A solution of 3-[ω-bromoacetyl coumarin] (2.50 mmol) and 2-fluorobenzaldehyde thiosemicarbazone (2.50 mmol) in chloroform-ethanol (2:1) was refluxed for 30 min. Precipitates formed were filtered and boiled in water containing sodium acetate. The product was purified by recrystallization with ethanol-chloroform (8:2).
Refinement
Atom H12N was located in a difference Fourier map and allowed to refined freely. The remaining hydrogen atoms were positioned geometrically and refined using a riding model with C—H = 0.93 Å and Uiso(H) = 1.2 Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids for non-H atoms and the atom-numbering scheme.The intramolecular C–H···O interaction is shown as a dashed line.
Fig. 2.
The crystal structure of the title compound viewed along the b axis. H atoms not involved in intermolecular interactions (dashed lines) have been omitted for clarity.
Crystal data
| C19H12FN3O2S | F(000) = 1504 |
| Mr = 365.38 | Dx = 1.496 Mg m−3 |
| Orthorhombic, Pbcn | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2n 2ab | Cell parameters from 1466 reflections |
| a = 12.303 (2) Å | θ = 3.2–27.1° |
| b = 10.4477 (17) Å | µ = 0.23 mm−1 |
| c = 25.247 (4) Å | T = 100 K |
| V = 3245.2 (9) Å3 | Needle, yellow |
| Z = 8 | 0.37 × 0.08 × 0.04 mm |
Data collection
| Bruker SMART APEXII DUO CCD area-detector diffractometer | 2855 independent reflections |
| Radiation source: fine-focus sealed tube | 1971 reflections with I > 2σ(I) |
| graphite | Rint = 0.082 |
| φ and ω scans | θmax = 25.0°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −12→14 |
| Tmin = 0.919, Tmax = 0.991 | k = −12→12 |
| 13589 measured reflections | l = −25→30 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.102 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0409P)2 + 0.4898P] where P = (Fo2 + 2Fc2)/3 |
| 2855 reflections | (Δ/σ)max < 0.001 |
| 239 parameters | Δρmax = 0.22 e Å−3 |
| 0 restraints | Δρmin = −0.33 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cyrosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.88953 (5) | 0.06999 (6) | 0.02651 (3) | 0.02149 (19) | |
| F1 | 0.54027 (12) | −0.24735 (15) | 0.18957 (7) | 0.0376 (5) | |
| O1 | 0.85525 (13) | 0.52077 (17) | −0.12371 (7) | 0.0230 (5) | |
| O2 | 0.97044 (14) | 0.41533 (18) | −0.07423 (8) | 0.0309 (5) | |
| N1 | 0.72862 (17) | −0.0844 (2) | 0.07751 (9) | 0.0205 (5) | |
| N2 | 0.68221 (19) | 0.0019 (2) | 0.04366 (9) | 0.0211 (5) | |
| N3 | 0.71706 (16) | 0.17627 (19) | −0.01285 (9) | 0.0179 (5) | |
| C1 | 0.8140 (2) | −0.2887 (2) | 0.14056 (11) | 0.0225 (6) | |
| H1 | 0.8600 | −0.2571 | 0.1144 | 0.027* | |
| C2 | 0.8520 (2) | −0.3806 (2) | 0.17528 (11) | 0.0244 (7) | |
| H2 | 0.9232 | −0.4097 | 0.1725 | 0.029* | |
| C3 | 0.7845 (2) | −0.4299 (3) | 0.21429 (11) | 0.0293 (7) | |
| H3 | 0.8099 | −0.4933 | 0.2370 | 0.035* | |
| C4 | 0.6792 (2) | −0.3843 (3) | 0.21930 (11) | 0.0296 (7) | |
| H4 | 0.6334 | −0.4155 | 0.2456 | 0.035* | |
| C5 | 0.6442 (2) | −0.2922 (3) | 0.18451 (12) | 0.0258 (7) | |
| C6 | 0.7077 (2) | −0.2424 (2) | 0.14401 (11) | 0.0214 (6) | |
| C7 | 0.6640 (2) | −0.1475 (2) | 0.10749 (11) | 0.0210 (6) | |
| H7 | 0.5895 | −0.1329 | 0.1060 | 0.025* | |
| C8 | 0.7499 (2) | 0.0851 (2) | 0.01854 (10) | 0.0181 (6) | |
| C9 | 0.9051 (2) | 0.1990 (2) | −0.01532 (11) | 0.0216 (6) | |
| H9 | 0.9717 | 0.2340 | −0.0250 | 0.026* | |
| C10 | 0.80743 (19) | 0.2424 (2) | −0.03219 (10) | 0.0169 (6) | |
| C11 | 0.78532 (19) | 0.3487 (2) | −0.06865 (11) | 0.0176 (6) | |
| C12 | 0.6839 (2) | 0.3765 (2) | −0.08582 (10) | 0.0181 (6) | |
| H12 | 0.6256 | 0.3283 | −0.0735 | 0.022* | |
| C13 | 0.6639 (2) | 0.4783 (2) | −0.12245 (11) | 0.0197 (6) | |
| C14 | 0.5599 (2) | 0.5110 (3) | −0.14162 (11) | 0.0225 (6) | |
| H14 | 0.4993 | 0.4657 | −0.1300 | 0.027* | |
| C15 | 0.5474 (2) | 0.6092 (2) | −0.17731 (11) | 0.0244 (7) | |
| H15 | 0.4785 | 0.6302 | −0.1897 | 0.029* | |
| C16 | 0.6378 (2) | 0.6774 (3) | −0.19495 (11) | 0.0264 (7) | |
| H16 | 0.6288 | 0.7433 | −0.2193 | 0.032* | |
| C17 | 0.7405 (2) | 0.6480 (2) | −0.17669 (11) | 0.0243 (7) | |
| H17 | 0.8008 | 0.6940 | −0.1881 | 0.029* | |
| C18 | 0.7515 (2) | 0.5489 (2) | −0.14105 (11) | 0.0197 (6) | |
| C19 | 0.8764 (2) | 0.4266 (2) | −0.08730 (11) | 0.0206 (6) | |
| H12N | 0.615 (2) | 0.019 (3) | 0.0458 (11) | 0.030 (8)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0159 (3) | 0.0227 (4) | 0.0259 (4) | 0.0030 (3) | −0.0001 (3) | 0.0013 (3) |
| F1 | 0.0327 (10) | 0.0392 (10) | 0.0409 (12) | −0.0040 (8) | 0.0141 (8) | 0.0027 (9) |
| O1 | 0.0179 (10) | 0.0250 (10) | 0.0261 (12) | −0.0044 (8) | 0.0005 (8) | 0.0017 (9) |
| O2 | 0.0147 (10) | 0.0368 (11) | 0.0413 (14) | −0.0049 (9) | −0.0028 (9) | 0.0075 (10) |
| N1 | 0.0232 (12) | 0.0150 (11) | 0.0233 (14) | 0.0024 (10) | 0.0011 (10) | −0.0016 (10) |
| N2 | 0.0160 (12) | 0.0197 (12) | 0.0277 (15) | 0.0023 (11) | 0.0025 (10) | 0.0032 (11) |
| N3 | 0.0142 (11) | 0.0176 (11) | 0.0219 (14) | 0.0009 (9) | 0.0013 (9) | 0.0004 (10) |
| C1 | 0.0245 (15) | 0.0192 (13) | 0.0237 (17) | −0.0059 (12) | 0.0010 (12) | −0.0045 (13) |
| C2 | 0.0299 (16) | 0.0183 (14) | 0.0251 (18) | −0.0026 (12) | −0.0030 (13) | −0.0016 (13) |
| C3 | 0.0457 (19) | 0.0181 (14) | 0.0240 (18) | −0.0078 (14) | −0.0051 (13) | 0.0032 (14) |
| C4 | 0.0436 (19) | 0.0239 (15) | 0.0212 (18) | −0.0147 (14) | 0.0058 (14) | 0.0001 (13) |
| C5 | 0.0262 (16) | 0.0240 (15) | 0.0273 (18) | −0.0067 (13) | 0.0053 (13) | −0.0072 (14) |
| C6 | 0.0290 (15) | 0.0144 (13) | 0.0207 (17) | −0.0052 (12) | 0.0006 (12) | −0.0050 (12) |
| C7 | 0.0242 (15) | 0.0159 (13) | 0.0228 (17) | −0.0007 (12) | 0.0038 (12) | −0.0051 (12) |
| C8 | 0.0171 (13) | 0.0158 (12) | 0.0213 (16) | 0.0024 (11) | −0.0012 (11) | −0.0051 (12) |
| C9 | 0.0170 (14) | 0.0227 (14) | 0.0252 (17) | 0.0010 (12) | 0.0042 (12) | −0.0024 (12) |
| C10 | 0.0148 (13) | 0.0177 (12) | 0.0183 (15) | −0.0012 (11) | −0.0001 (11) | −0.0049 (12) |
| C11 | 0.0165 (14) | 0.0175 (13) | 0.0189 (16) | −0.0013 (11) | 0.0017 (11) | −0.0044 (12) |
| C12 | 0.0153 (13) | 0.0189 (13) | 0.0202 (16) | −0.0009 (11) | 0.0040 (11) | −0.0034 (12) |
| C13 | 0.0218 (15) | 0.0182 (13) | 0.0191 (16) | 0.0009 (11) | 0.0026 (11) | −0.0040 (12) |
| C14 | 0.0219 (15) | 0.0242 (15) | 0.0215 (17) | 0.0026 (12) | 0.0023 (12) | −0.0015 (13) |
| C15 | 0.0263 (16) | 0.0250 (15) | 0.0220 (18) | 0.0087 (13) | −0.0023 (12) | −0.0016 (13) |
| C16 | 0.0399 (18) | 0.0188 (14) | 0.0206 (18) | 0.0042 (13) | −0.0005 (13) | 0.0003 (13) |
| C17 | 0.0310 (16) | 0.0208 (14) | 0.0210 (17) | −0.0051 (13) | 0.0027 (13) | −0.0020 (13) |
| C18 | 0.0211 (14) | 0.0175 (13) | 0.0206 (16) | 0.0002 (11) | 0.0007 (12) | −0.0051 (12) |
| C19 | 0.0214 (15) | 0.0183 (13) | 0.0222 (16) | −0.0009 (12) | −0.0004 (11) | −0.0010 (13) |
Geometric parameters (Å, °)
| S1—C9 | 1.723 (3) | C5—C6 | 1.388 (4) |
| S1—C8 | 1.736 (2) | C6—C7 | 1.456 (4) |
| F1—C5 | 1.367 (3) | C7—H7 | 0.9300 |
| O1—C19 | 1.372 (3) | C9—C10 | 1.354 (3) |
| O1—C18 | 1.381 (3) | C9—H9 | 0.9300 |
| O2—C19 | 1.209 (3) | C10—C11 | 1.469 (4) |
| N1—C7 | 1.281 (3) | C11—C12 | 1.353 (3) |
| N1—N2 | 1.368 (3) | C11—C19 | 1.462 (4) |
| N2—C8 | 1.361 (3) | C12—C13 | 1.431 (4) |
| N2—H12N | 0.85 (3) | C12—H12 | 0.9300 |
| N3—C8 | 1.303 (3) | C13—C18 | 1.387 (4) |
| N3—C10 | 1.397 (3) | C13—C14 | 1.410 (4) |
| C1—C2 | 1.382 (4) | C14—C15 | 1.374 (4) |
| C1—C6 | 1.397 (4) | C14—H14 | 0.9300 |
| C1—H1 | 0.9300 | C15—C16 | 1.394 (4) |
| C2—C3 | 1.387 (4) | C15—H15 | 0.9300 |
| C2—H2 | 0.9300 | C16—C17 | 1.379 (4) |
| C3—C4 | 1.386 (4) | C16—H16 | 0.9300 |
| C3—H3 | 0.9300 | C17—C18 | 1.379 (4) |
| C4—C5 | 1.372 (4) | C17—H17 | 0.9300 |
| C4—H4 | 0.9300 | ||
| C9—S1—C8 | 88.17 (12) | C10—C9—H9 | 124.6 |
| C19—O1—C18 | 122.7 (2) | S1—C9—H9 | 124.6 |
| C7—N1—N2 | 116.7 (2) | C9—C10—N3 | 115.5 (2) |
| C8—N2—N1 | 117.2 (2) | C9—C10—C11 | 128.0 (2) |
| C8—N2—H12N | 119.4 (19) | N3—C10—C11 | 116.5 (2) |
| N1—N2—H12N | 121 (2) | C12—C11—C19 | 119.0 (2) |
| C8—N3—C10 | 109.1 (2) | C12—C11—C10 | 122.3 (2) |
| C2—C1—C6 | 121.2 (3) | C19—C11—C10 | 118.7 (2) |
| C2—C1—H1 | 119.4 | C11—C12—C13 | 121.7 (2) |
| C6—C1—H1 | 119.4 | C11—C12—H12 | 119.2 |
| C1—C2—C3 | 120.4 (3) | C13—C12—H12 | 119.2 |
| C1—C2—H2 | 119.8 | C18—C13—C14 | 117.4 (2) |
| C3—C2—H2 | 119.8 | C18—C13—C12 | 118.7 (2) |
| C4—C3—C2 | 119.8 (3) | C14—C13—C12 | 123.9 (2) |
| C4—C3—H3 | 120.1 | C15—C14—C13 | 120.5 (2) |
| C2—C3—H3 | 120.1 | C15—C14—H14 | 119.8 |
| C5—C4—C3 | 118.4 (3) | C13—C14—H14 | 119.8 |
| C5—C4—H4 | 120.8 | C14—C15—C16 | 120.1 (2) |
| C3—C4—H4 | 120.8 | C14—C15—H15 | 120.0 |
| F1—C5—C4 | 118.3 (2) | C16—C15—H15 | 120.0 |
| F1—C5—C6 | 117.8 (3) | C17—C16—C15 | 120.7 (3) |
| C4—C5—C6 | 123.9 (3) | C17—C16—H16 | 119.6 |
| C5—C6—C1 | 116.3 (3) | C15—C16—H16 | 119.6 |
| C5—C6—C7 | 120.9 (2) | C16—C17—C18 | 118.4 (3) |
| C1—C6—C7 | 122.8 (3) | C16—C17—H17 | 120.8 |
| N1—C7—C6 | 119.7 (2) | C18—C17—H17 | 120.8 |
| N1—C7—H7 | 120.2 | C17—C18—O1 | 117.2 (2) |
| C6—C7—H7 | 120.2 | C17—C18—C13 | 122.9 (2) |
| N3—C8—N2 | 124.1 (2) | O1—C18—C13 | 119.8 (2) |
| N3—C8—S1 | 116.36 (19) | O2—C19—O1 | 115.7 (2) |
| N2—C8—S1 | 119.6 (2) | O2—C19—C11 | 126.3 (2) |
| C10—C9—S1 | 110.85 (19) | O1—C19—C11 | 118.0 (2) |
| C7—N1—N2—C8 | 168.7 (2) | N3—C10—C11—C12 | −4.5 (4) |
| C6—C1—C2—C3 | 0.5 (4) | C9—C10—C11—C19 | −4.9 (4) |
| C1—C2—C3—C4 | −1.6 (4) | N3—C10—C11—C19 | 176.0 (2) |
| C2—C3—C4—C5 | 1.0 (4) | C19—C11—C12—C13 | 1.2 (4) |
| C3—C4—C5—F1 | −179.9 (2) | C10—C11—C12—C13 | −178.3 (2) |
| C3—C4—C5—C6 | 0.7 (4) | C11—C12—C13—C18 | 0.7 (4) |
| F1—C5—C6—C1 | 178.9 (2) | C11—C12—C13—C14 | 179.9 (2) |
| C4—C5—C6—C1 | −1.7 (4) | C18—C13—C14—C15 | 0.0 (4) |
| F1—C5—C6—C7 | −1.3 (4) | C12—C13—C14—C15 | −179.2 (3) |
| C4—C5—C6—C7 | 178.1 (2) | C13—C14—C15—C16 | 0.1 (4) |
| C2—C1—C6—C5 | 1.0 (4) | C14—C15—C16—C17 | −0.5 (4) |
| C2—C1—C6—C7 | −178.7 (2) | C15—C16—C17—C18 | 0.7 (4) |
| N2—N1—C7—C6 | 179.2 (2) | C16—C17—C18—O1 | 179.5 (2) |
| C5—C6—C7—N1 | 166.1 (2) | C16—C17—C18—C13 | −0.6 (4) |
| C1—C6—C7—N1 | −14.2 (4) | C19—O1—C18—C17 | 178.3 (2) |
| C10—N3—C8—N2 | −179.4 (2) | C19—O1—C18—C13 | −1.6 (4) |
| C10—N3—C8—S1 | −0.9 (3) | C14—C13—C18—C17 | 0.3 (4) |
| N1—N2—C8—N3 | −176.7 (2) | C12—C13—C18—C17 | 179.5 (2) |
| N1—N2—C8—S1 | 4.9 (3) | C14—C13—C18—O1 | −179.8 (2) |
| C9—S1—C8—N3 | 0.9 (2) | C12—C13—C18—O1 | −0.6 (4) |
| C9—S1—C8—N2 | 179.4 (2) | C18—O1—C19—O2 | −177.4 (2) |
| C8—S1—C9—C10 | −0.5 (2) | C18—O1—C19—C11 | 3.5 (3) |
| S1—C9—C10—N3 | 0.1 (3) | C12—C11—C19—O2 | 177.7 (3) |
| S1—C9—C10—C11 | −179.0 (2) | C10—C11—C19—O2 | −2.7 (4) |
| C8—N3—C10—C9 | 0.5 (3) | C12—C11—C19—O1 | −3.3 (4) |
| C8—N3—C10—C11 | 179.7 (2) | C10—C11—C19—O1 | 176.3 (2) |
| C9—C10—C11—C12 | 174.6 (3) |
Hydrogen-bond geometry (Å, °)
| Cg1 is the centroid of the C13–C18 benzene ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C9—H9···O2 | 0.93 | 2.27 | 2.823 (3) | 118 |
| N2—H12N···O2i | 0.85 (3) | 2.04 (3) | 2.852 (3) | 161 (3) |
| C4—H4···Cg1ii | 0.93 | 2.96 | 3.701 (3) | 138 |
Symmetry codes: (i) x−1/2, −y+1/2, −z; (ii) x, −y, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2674).
References
- Anderson, D. M., Shelley, S., Crick, N. & Buraglio, L. (2002). J. Clin. Pharmacol.42, 1358–1365. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
- Gursoy, A. & Karah, N. (2000). Arzneim. Forsch. Drug Res.50, 167–172.
- Habib, N. S. & Khalil, M. A. (1984). J. Pharm. Sci.73, 982–985. [DOI] [PubMed]
- Hiremath, S. P., Swamy, K. M. K. & Mrnthyunjayaswamy, B. H. M. (1992). J. Indian Chem. Soc.69, 87–89.
- Karah, N., Terzioglu, N. & Gursoy, A. (1998). Arzneim. Forsch. Drug Res.48, 758–763. [PubMed]
- Kim, K. S., Kimball, S. D., Misra, R. N., Rawlins, D. B., Hunt, J. T., Xiao, S. L., Qian, L., Han, W. C., Shan, W., Mitt, T., Cai, Z. W., Poss, M. A., Zhu, H., Sack, J. S., Torarski, J. S., Chang, C. G., Pavletic, N., Kamath, A., Humphrey, W. G., Marathe, P., Bursuker, J., Kellar, K. A., Rongta, U., Batorsky, R., Mulheron, J. G., Bol, D., Fairchild, C. R., Lee, F. Y. & Webster, K. R. (2002). J. Med. Chem.45, 3905–3927. [DOI] [PubMed]
- Lafitte, D., Lamour, V., Tsvetkov, P. O., Makarov, A. A., Klich, M., Deprez, P., Moras, D., Braind, C. & Gilli, R. (2002). Biochemistry, 41, 7217–7223. [DOI] [PubMed]
- Lednicer, D., Mitscher, L. A. & Georg, G. I. (1990). The Organic Chemistry of Drug Synthesis, Vol. 4, New York: J. Wiley & Sons.
- Lv, P.-C., Zhou, C.-F., Chen, J., Liu, P.-G., Wang, K.-L., Mao, W.-J., Li, H.-Q., Yang, Y., Xiong, J. & Zhu, H.-L. (2010). Bioorg. Med. Chem.18, 314–319. [DOI] [PubMed]
- Mitscher, L. A. (2002). Principles of Medicinal Chemistry, 5th ed., pp. 819–864. Baltimore: Williams & Wilkinsons.
- Moffett, R. B. (1964). J. Med. Chem.7, 446–449. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siddiqui, N., Arshad, M. F. & Khan, S. A. (2009). Acta Pol. Pharm. Drug Res.66, 161–167. [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tassies, D., Freire, C., Puoan, J., Maragall, S., Moonteagudo, J., Ordinas, A. & Reverter, J. C. (2002). Haematologica, 87, 1185–1191. [PubMed]
- Wattenberg, L. W., Low, L. K. T. & Fladmoe, A. V. (1979). Cancer Res.39, 1651–1654. [PubMed]
- Weber, U. S., Steffen, B. & Siegers, C. (1998). Res. Commun. Mol. Pathol. Pharmacol.99, 193–206. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810018647/tk2674sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810018647/tk2674Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report