Abstract
In the title compound, C14H18N2O3S, the 1H-pyrazole ring is approximately planar, with a maximum deviation of 0.005 (1) Å. The dihedral angle formed between the 1H-pyrazole and phenyl rings is 79.09 (5)°. Pairs of intermolecular N—H⋯O and O⋯H⋯N hydrogen bonds form dimers between neighboring molecules, generating R 2 2(10) ring motifs. These dimers are further linked by intermolecular N—H⋯O and O—H⋯N hydrogen bonds into two-dimensional arrays parallel to the ac plane. The crystal structure is also stabilized by C—H⋯π interactions.
Related literature
For background to the biological activity of 3-ethyl-4-methyl-1H-pyrazol-5-ol, see: Brogden (1986 ▶); Gursoy et al. (2000 ▶); Ragavan et al. (2009 ▶, 2010 ▶); Watanabe et al. (1984 ▶); Kawai et al. (1997 ▶); Wu et al. (2002 ▶). For related structures, see: Shahani et al. (2009 ▶, 2010a ▶,b
▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For reference bond-length data, see: Allen et al. (1987 ▶). For the stability of the temperature controller used for the data collection, see: Cosier & Glazer (1986 ▶).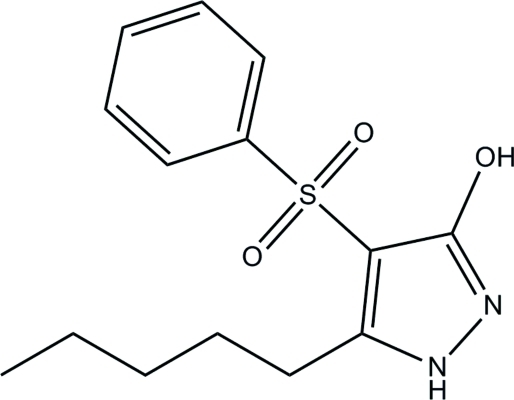
Experimental
Crystal data
C14H18N2O3S
M r = 294.36
Monoclinic,
a = 10.3425 (3) Å
b = 11.2963 (3) Å
c = 12.8911 (3) Å
β = 107.419 (1)°
V = 1437.02 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.23 mm−1
T = 100 K
0.48 × 0.33 × 0.11 mm
Data collection
Bruker APEXII DUO CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.896, T max = 0.976
28969 measured reflections
7810 independent reflections
6336 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.122
S = 1.14
7810 reflections
253 parameters
All H-atom parameters refined
Δρmax = 0.73 e Å−3
Δρmin = −0.41 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810019458/wn2386sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810019458/wn2386Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the 1H-pyrazole ring (C7–C9/N1/N2).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O3—H1O3⋯N1i | 0.945 (19) | 1.79 (2) | 2.7287 (10) | 171.5 (17) |
| N2—H1N2⋯O2ii | 0.880 (19) | 1.959 (19) | 2.7162 (10) | 143.4 (17) |
| C12—H12A⋯Cg1iii | 1.005 (16) | 2.952 (16) | 3.5692 (10) | 120.5 (11) |
Symmetry codes: (i) 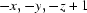 ; (ii)
; (ii) 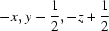 ; (iii)
; (iii) 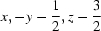 .
.
Acknowledgments
TSH and HKF thank Universiti Sains Malaysia (USM) for the Research University Golden Goose Grant (1001/PFIZIK/811012). VV is grateful to the DST-India for funding through the Young Scientist Scheme (Fast Track Proposal).
supplementary crystallographic information
Comment
Pyrazolone derivatives have a broad spectrum of biological activities, being used as analgesic, antipyretic and anti-inflammatory therapeutical drugs (Brogden, 1986; Gursoy et al., 2000). A class of new compounds with the pyrazolone unit has been synthesized and reported to possess antibacterial and antifungal activities (Ragavan et al., 2009, 2010). A new pyrazolone derivative, edaravone (3-methyl-1-phenyl-2-pyrazoline-5-one), is being used as a drug in clinical practice for brain ischemia (Watanabe et al., 1984; Kawai et al., 1997) and the same has been found to be effective against myocardial ischemia (Wu et al., 2002).
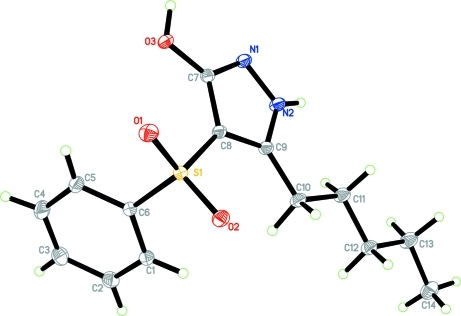
In the crystal structure (Fig. 1), the 1H-pyrazole ring (C1–C2/N1/N2) is approximately planar, with a maximum deviation of 0.005 (1) Å at atoms C9 and N2. The dihedral angle formed between the 1H-pyrazole and phenyl rings (C1—C6) is 79.09 (5)°. The bond lengths (Allen et al.,1987) and angles are within normal ranges and comparable to those in closely related crystal structures (Shahani et al., 2009, 2010a,b).
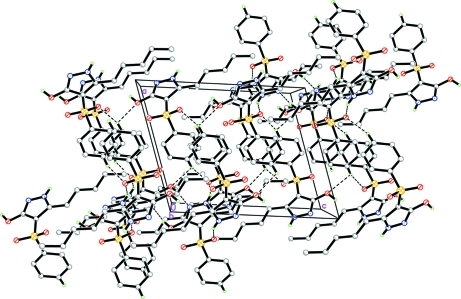
In the crystal packing (Fig. 2), pairs of intermolecular O3—H1O3···N1, N2—H1N2···O2 hydrogen bonds form dimers between neighbouring molecules, generating R22(8) ring motifs (Bernstein et al., 1995). These dimers are linked into two-dimensional arrays parallel to the ac plane by intermolecular O3—H1O3···N1 and N2—H1N2···O2 hydrogen bonds (Table 1). The crystal structure is also stabilized by C12—H12A···Cg1 interactions (Table 1) involving the C7–C9/N1/N2 pyrazole ring.
Experimental
The compound 5-pentyl-4-(phenylsulfonyl)-1H-pyrazol-3-ol was synthesized according to the procedure available in the literature (Ragavan et al., 2009, 2010), which in turn was dissolved using a THF/water (1:1) mixture. Oxone (4 mmol) was then added and the solution was stirred at room temperature for 3 h. The reaction mixture was diluted with water (20 ml), and then was extracted with ethyl acetate (2 x 50 ml). The combined extract was washed with water (20 ml) and brine solution (10 ml). Crystallization was carried out using absolute ethanol.
Refinement
All hydrogen atoms were located in a difference map and were refined freely [C—H = 0.910 (19) – 1.035 (18); N—H = 0.880 (18); O—H = 0.944 (19) Å].
Figures
Fig. 1.
The molecular structure of the title compound, showing 30% probability displacement ellipsoids and the atom numbering scheme. Hydrogen atoms are shown as spheres of arbitrary radius.
Fig. 2.
The crystal packing of the title compound, viewed along the b axis, showing 2D arrays parallel to the ac plane. Dashed lines indicate hydrogen bonds. H atoms not involved in the hydrogen bond interactions have been omitted for clarity.
Crystal data
| C14H18N2O3S | F(000) = 624 |
| Mr = 294.36 | Dx = 1.361 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 9972 reflections |
| a = 10.3425 (3) Å | θ = 2.5–37.9° |
| b = 11.2963 (3) Å | µ = 0.23 mm−1 |
| c = 12.8911 (3) Å | T = 100 K |
| β = 107.419 (1)° | Plate, colourless |
| V = 1437.02 (7) Å3 | 0.48 × 0.33 × 0.11 mm |
| Z = 4 |
Data collection
| Bruker APEXII DUO CCD area-detector diffractometer | 7810 independent reflections |
| Radiation source: fine-focus sealed tube | 6336 reflections with I > 2σ(I) |
| graphite | Rint = 0.035 |
| φ and ω scans | θmax = 38.1°, θmin = 2.1° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −17→17 |
| Tmin = 0.896, Tmax = 0.976 | k = −19→19 |
| 28969 measured reflections | l = −22→22 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.122 | All H-atom parameters refined |
| S = 1.14 | w = 1/[σ2(Fo2) + (0.0676P)2 + 0.1076P] where P = (Fo2 + 2Fc2)/3 |
| 7810 reflections | (Δ/σ)max < 0.001 |
| 253 parameters | Δρmax = 0.73 e Å−3 |
| 0 restraints | Δρmin = −0.41 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cyrosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.252485 (19) | 0.286811 (16) | 0.369236 (14) | 0.01071 (5) | |
| O1 | 0.28657 (7) | 0.32531 (6) | 0.48049 (5) | 0.01659 (12) | |
| O2 | 0.21016 (7) | 0.37440 (5) | 0.28352 (5) | 0.01507 (11) | |
| O3 | 0.16163 (6) | 0.10556 (6) | 0.53216 (5) | 0.01448 (11) | |
| N1 | −0.00782 (8) | 0.03707 (6) | 0.38100 (6) | 0.01404 (12) | |
| N2 | −0.04649 (8) | 0.06672 (6) | 0.27303 (6) | 0.01496 (12) | |
| C1 | 0.40090 (9) | 0.19978 (8) | 0.24384 (7) | 0.01491 (13) | |
| C2 | 0.50570 (9) | 0.13165 (8) | 0.22799 (7) | 0.01884 (15) | |
| C3 | 0.59982 (10) | 0.07844 (9) | 0.31610 (8) | 0.02123 (16) | |
| C4 | 0.59168 (10) | 0.09408 (9) | 0.42112 (8) | 0.02094 (16) | |
| C5 | 0.48687 (9) | 0.16047 (8) | 0.43838 (7) | 0.01639 (14) | |
| C6 | 0.39226 (8) | 0.21172 (6) | 0.34913 (6) | 0.01197 (12) | |
| C7 | 0.09692 (8) | 0.10767 (7) | 0.42646 (6) | 0.01194 (12) | |
| C8 | 0.12549 (8) | 0.18213 (7) | 0.34698 (6) | 0.01188 (12) | |
| C9 | 0.02975 (8) | 0.15129 (7) | 0.24810 (6) | 0.01305 (13) | |
| C10 | 0.00436 (9) | 0.19276 (8) | 0.13344 (7) | 0.01604 (14) | |
| C11 | −0.11449 (9) | 0.12961 (8) | 0.05249 (7) | 0.01717 (15) | |
| C12 | −0.13109 (9) | 0.16318 (8) | −0.06542 (7) | 0.01677 (14) | |
| C13 | −0.24983 (11) | 0.09921 (9) | −0.14480 (7) | 0.02131 (17) | |
| C14 | −0.25766 (12) | 0.11849 (10) | −0.26364 (8) | 0.02367 (18) | |
| H1A | 0.3342 (16) | 0.2363 (14) | 0.1852 (12) | 0.022 (3)* | |
| H2A | 0.5111 (16) | 0.1238 (14) | 0.1558 (13) | 0.025 (4)* | |
| H3A | 0.6683 (17) | 0.0309 (15) | 0.3017 (13) | 0.029 (4)* | |
| H4A | 0.6624 (17) | 0.0595 (14) | 0.4792 (13) | 0.031 (4)* | |
| H5A | 0.4772 (17) | 0.1722 (14) | 0.5069 (13) | 0.026 (4)* | |
| H10A | 0.0893 (16) | 0.1812 (13) | 0.1116 (12) | 0.021 (3)* | |
| H10B | −0.0097 (16) | 0.2808 (13) | 0.1271 (13) | 0.022 (4)* | |
| H11A | −0.1995 (16) | 0.1492 (13) | 0.0666 (12) | 0.021 (3)* | |
| H11B | −0.1003 (15) | 0.0412 (13) | 0.0581 (12) | 0.020 (3)* | |
| H12A | −0.1437 (15) | 0.2511 (14) | −0.0758 (12) | 0.023 (3)* | |
| H12B | −0.0479 (18) | 0.1422 (15) | −0.0867 (14) | 0.031 (4)* | |
| H13A | −0.2409 (17) | 0.0115 (17) | −0.1291 (13) | 0.036 (5)* | |
| H13B | −0.3394 (18) | 0.1256 (16) | −0.1315 (14) | 0.034 (4)* | |
| H14A | −0.2628 (16) | 0.2016 (13) | −0.2746 (13) | 0.023 (4)* | |
| H14B | −0.3452 (19) | 0.0787 (16) | −0.3118 (15) | 0.037 (4)* | |
| H14C | −0.1814 (19) | 0.0873 (16) | −0.2740 (15) | 0.037 (4)* | |
| H1O3 | 0.1158 (19) | 0.0555 (17) | 0.5682 (14) | 0.042 (5)* | |
| H1N2 | −0.1111 (19) | 0.0241 (16) | 0.2289 (15) | 0.039 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.01150 (9) | 0.01041 (8) | 0.01036 (8) | −0.00003 (5) | 0.00348 (6) | 0.00008 (5) |
| O1 | 0.0196 (3) | 0.0179 (3) | 0.0126 (2) | −0.0023 (2) | 0.0053 (2) | −0.0042 (2) |
| O2 | 0.0168 (3) | 0.0120 (2) | 0.0166 (2) | 0.00187 (19) | 0.0053 (2) | 0.00367 (19) |
| O3 | 0.0152 (3) | 0.0173 (2) | 0.0106 (2) | −0.0011 (2) | 0.0033 (2) | 0.00243 (18) |
| N1 | 0.0157 (3) | 0.0151 (3) | 0.0112 (2) | −0.0019 (2) | 0.0038 (2) | 0.0015 (2) |
| N2 | 0.0165 (3) | 0.0162 (3) | 0.0115 (2) | −0.0045 (2) | 0.0031 (2) | 0.0013 (2) |
| C1 | 0.0139 (3) | 0.0187 (3) | 0.0123 (3) | 0.0007 (2) | 0.0043 (3) | 0.0005 (2) |
| C2 | 0.0170 (4) | 0.0240 (4) | 0.0173 (3) | 0.0017 (3) | 0.0078 (3) | −0.0014 (3) |
| C3 | 0.0164 (4) | 0.0244 (4) | 0.0240 (4) | 0.0054 (3) | 0.0078 (3) | 0.0012 (3) |
| C4 | 0.0168 (4) | 0.0251 (4) | 0.0200 (4) | 0.0075 (3) | 0.0043 (3) | 0.0050 (3) |
| C5 | 0.0152 (3) | 0.0198 (3) | 0.0134 (3) | 0.0034 (3) | 0.0032 (3) | 0.0031 (3) |
| C6 | 0.0108 (3) | 0.0131 (3) | 0.0119 (3) | 0.0000 (2) | 0.0032 (2) | 0.0010 (2) |
| C7 | 0.0128 (3) | 0.0122 (3) | 0.0114 (3) | 0.0008 (2) | 0.0043 (2) | 0.0006 (2) |
| C8 | 0.0123 (3) | 0.0123 (3) | 0.0112 (3) | −0.0010 (2) | 0.0038 (2) | 0.0008 (2) |
| C9 | 0.0138 (3) | 0.0136 (3) | 0.0119 (3) | −0.0021 (2) | 0.0040 (2) | 0.0005 (2) |
| C10 | 0.0171 (3) | 0.0186 (3) | 0.0112 (3) | −0.0048 (3) | 0.0025 (3) | 0.0019 (2) |
| C11 | 0.0181 (4) | 0.0196 (3) | 0.0126 (3) | −0.0052 (3) | 0.0026 (3) | 0.0008 (3) |
| C12 | 0.0183 (4) | 0.0184 (3) | 0.0127 (3) | −0.0032 (3) | 0.0033 (3) | 0.0012 (2) |
| C13 | 0.0239 (4) | 0.0243 (4) | 0.0132 (3) | −0.0059 (3) | 0.0016 (3) | 0.0004 (3) |
| C14 | 0.0290 (5) | 0.0268 (4) | 0.0134 (3) | 0.0016 (4) | 0.0037 (3) | 0.0000 (3) |
Geometric parameters (Å, °)
| S1—O1 | 1.4379 (6) | C5—H5A | 0.928 (16) |
| S1—O2 | 1.4498 (6) | C7—C8 | 1.4233 (11) |
| S1—C8 | 1.7263 (8) | C8—C9 | 1.4039 (11) |
| S1—C6 | 1.7610 (8) | C9—C10 | 1.4969 (11) |
| O3—C7 | 1.3264 (10) | C10—C11 | 1.5300 (12) |
| O3—H1O3 | 0.944 (19) | C10—H10A | 1.009 (15) |
| N1—C7 | 1.3315 (11) | C10—H10B | 1.004 (15) |
| N1—N2 | 1.3697 (10) | C11—C12 | 1.5255 (12) |
| N2—C9 | 1.3377 (10) | C11—H11A | 0.976 (16) |
| N2—H1N2 | 0.880 (18) | C11—H11B | 1.009 (14) |
| C1—C6 | 1.3932 (11) | C12—C13 | 1.5248 (13) |
| C1—C2 | 1.3934 (12) | C12—H12A | 1.005 (16) |
| C1—H1A | 0.952 (15) | C12—H12B | 1.006 (17) |
| C2—C3 | 1.3917 (14) | C13—C14 | 1.5254 (13) |
| C2—H2A | 0.952 (16) | C13—H13A | 1.010 (19) |
| C3—C4 | 1.3926 (14) | C13—H13B | 1.035 (18) |
| C3—H3A | 0.950 (17) | C14—H14A | 0.949 (15) |
| C4—C5 | 1.3897 (13) | C14—H14B | 1.034 (18) |
| C4—H4A | 0.959 (16) | C14—H14C | 0.910 (19) |
| C5—C6 | 1.3937 (11) | ||
| O1—S1—O2 | 118.80 (4) | C7—C8—S1 | 126.64 (6) |
| O1—S1—C8 | 108.67 (4) | N2—C9—C8 | 105.38 (7) |
| O2—S1—C8 | 107.44 (4) | N2—C9—C10 | 121.26 (7) |
| O1—S1—C6 | 109.00 (4) | C8—C9—C10 | 133.35 (7) |
| O2—S1—C6 | 106.88 (4) | C9—C10—C11 | 113.28 (7) |
| C8—S1—C6 | 105.24 (4) | C9—C10—H10A | 109.0 (8) |
| C7—O3—H1O3 | 110.0 (11) | C11—C10—H10A | 109.8 (8) |
| C7—N1—N2 | 104.62 (6) | C9—C10—H10B | 111.6 (9) |
| C9—N2—N1 | 113.89 (7) | C11—C10—H10B | 109.8 (9) |
| C9—N2—H1N2 | 128.6 (12) | H10A—C10—H10B | 102.9 (12) |
| N1—N2—H1N2 | 117.2 (12) | C12—C11—C10 | 113.05 (7) |
| C6—C1—C2 | 118.42 (8) | C12—C11—H11A | 106.9 (9) |
| C6—C1—H1A | 119.3 (9) | C10—C11—H11A | 110.6 (9) |
| C2—C1—H1A | 122.2 (9) | C12—C11—H11B | 106.7 (8) |
| C3—C2—C1 | 120.24 (8) | C10—C11—H11B | 110.1 (8) |
| C3—C2—H2A | 121.7 (10) | H11A—C11—H11B | 109.4 (12) |
| C1—C2—H2A | 118.0 (10) | C13—C12—C11 | 112.25 (7) |
| C2—C3—C4 | 120.50 (9) | C13—C12—H12A | 109.4 (9) |
| C2—C3—H3A | 117.7 (10) | C11—C12—H12A | 110.5 (8) |
| C4—C3—H3A | 121.8 (10) | C13—C12—H12B | 106.7 (10) |
| C5—C4—C3 | 120.07 (8) | C11—C12—H12B | 111.3 (10) |
| C5—C4—H4A | 122.9 (10) | H12A—C12—H12B | 106.5 (13) |
| C3—C4—H4A | 117.0 (10) | C12—C13—C14 | 113.34 (8) |
| C4—C5—C6 | 118.72 (8) | C12—C13—H13A | 108.9 (10) |
| C4—C5—H5A | 122.7 (10) | C14—C13—H13A | 108.4 (9) |
| C6—C5—H5A | 118.5 (10) | C12—C13—H13B | 109.5 (10) |
| C1—C6—C5 | 122.01 (8) | C14—C13—H13B | 110.1 (10) |
| C1—C6—S1 | 119.01 (6) | H13A—C13—H13B | 106.4 (13) |
| C5—C6—S1 | 118.88 (6) | C13—C14—H14A | 106.0 (9) |
| O3—C7—N1 | 122.36 (7) | C13—C14—H14B | 108.3 (10) |
| O3—C7—C8 | 126.91 (7) | H14A—C14—H14B | 109.9 (14) |
| N1—C7—C8 | 110.73 (7) | C13—C14—H14C | 107.7 (11) |
| C9—C8—C7 | 105.37 (7) | H14A—C14—H14C | 112.0 (15) |
| C9—C8—S1 | 127.99 (6) | H14B—C14—H14C | 112.7 (15) |
| C7—N1—N2—C9 | 0.83 (10) | O3—C7—C8—S1 | 0.59 (12) |
| C6—C1—C2—C3 | −0.77 (14) | N1—C7—C8—S1 | −179.79 (6) |
| C1—C2—C3—C4 | −0.99 (15) | O1—S1—C8—C9 | 155.48 (7) |
| C2—C3—C4—C5 | 1.82 (16) | O2—S1—C8—C9 | 25.76 (9) |
| C3—C4—C5—C6 | −0.84 (14) | C6—S1—C8—C9 | −87.89 (8) |
| C2—C1—C6—C5 | 1.76 (13) | O1—S1—C8—C7 | −25.08 (8) |
| C2—C1—C6—S1 | −174.63 (7) | O2—S1—C8—C7 | −154.80 (7) |
| C4—C5—C6—C1 | −0.96 (13) | C6—S1—C8—C7 | 91.55 (8) |
| C4—C5—C6—S1 | 175.44 (7) | N1—N2—C9—C8 | −0.98 (10) |
| O1—S1—C6—C1 | −159.60 (6) | N1—N2—C9—C10 | 178.47 (7) |
| O2—S1—C6—C1 | −30.03 (7) | C7—C8—C9—N2 | 0.71 (9) |
| C8—S1—C6—C1 | 84.00 (7) | S1—C8—C9—N2 | −179.75 (6) |
| O1—S1—C6—C5 | 23.90 (8) | C7—C8—C9—C10 | −178.65 (9) |
| O2—S1—C6—C5 | 153.46 (7) | S1—C8—C9—C10 | 0.89 (14) |
| C8—S1—C6—C5 | −92.50 (7) | N2—C9—C10—C11 | 0.26 (12) |
| N2—N1—C7—O3 | 179.32 (7) | C8—C9—C10—C11 | 179.54 (9) |
| N2—N1—C7—C8 | −0.31 (9) | C9—C10—C11—C12 | −174.25 (8) |
| O3—C7—C8—C9 | −179.86 (8) | C10—C11—C12—C13 | 179.91 (8) |
| N1—C7—C8—C9 | −0.25 (9) | C11—C12—C13—C14 | −172.45 (8) |
Hydrogen-bond geometry (Å, °)
| Cg1 is the centroid of the 1H-pyrazole ring (C7–C9/N1/N2). |
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H1O3···N1i | 0.945 (19) | 1.79 (2) | 2.7287 (10) | 171.5 (17) |
| N2—H1N2···O2ii | 0.880 (19) | 1.959 (19) | 2.7162 (10) | 143.4 (17) |
| C12—H12A···Cg1iii | 1.005 (16) | 2.952 (16) | 3.5692 (10) | 120.5 (11) |
Symmetry codes: (i) −x, −y, −z+1; (ii) −x, y−1/2, −z+1/2; (iii) x, −y−1/2, z−3/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WN2386).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Brogden, R. N. (1986). Pyrazolone Derivatives Drugs, 32, 60–70. [DOI] [PubMed]
- Bruker (2009). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wiscosin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
- Gursoy, A., Demirayak, S., Capan, G., Erol, K. & Vural, K. (2000). Eur. J. Med. Chem.35, 359–364. [DOI] [PubMed]
- Kawai, H., Nakai, H., Suga, M., Yuki, S., Watanabe, T. & Saito, K. I. (1997). J. Pharmcol. Exp. Ther.281, 921–927. [PubMed]
- Ragavan, R. V., Vijayakumar, V. & Kumari, N. S. (2009). Eur. J. Med. Chem.44, 3852–3857.
- Ragavan, R. V., Vijayakumar, V. & Kumari, N. S. (2010). Eur. J. Med. Chem.45, 1173–1180. [DOI] [PubMed]
- Shahani, T., Fun, H.-K., Ragavan, R. V., Vijayakumar, V. & Sarveswari, S. (2009). Acta Cryst. E65, o3249–o3250. [DOI] [PMC free article] [PubMed]
- Shahani, T., Fun, H.-K., Ragavan, R. V., Vijayakumar, V. & Sarveswari, S. (2010a). Acta Cryst. E66, o142–o143. [DOI] [PMC free article] [PubMed]
- Shahani, T., Fun, H.-K., Ragavan, R. V., Vijayakumar, V. & Sarveswari, S. (2010b). Acta Cryst. E66, o1357–o1358. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Watanabe, T., Yuki, S., Egawa, M. & Nishi, H. (1984). J. Pharmacol. Exp. Ther.268, 1597–1604. [PubMed]
- Wu, T. W., Zeng, L. H., Wu, J. & Fung, K. P. (2002). Life Sci.71, 2249–2255. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810019458/wn2386sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810019458/wn2386Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report