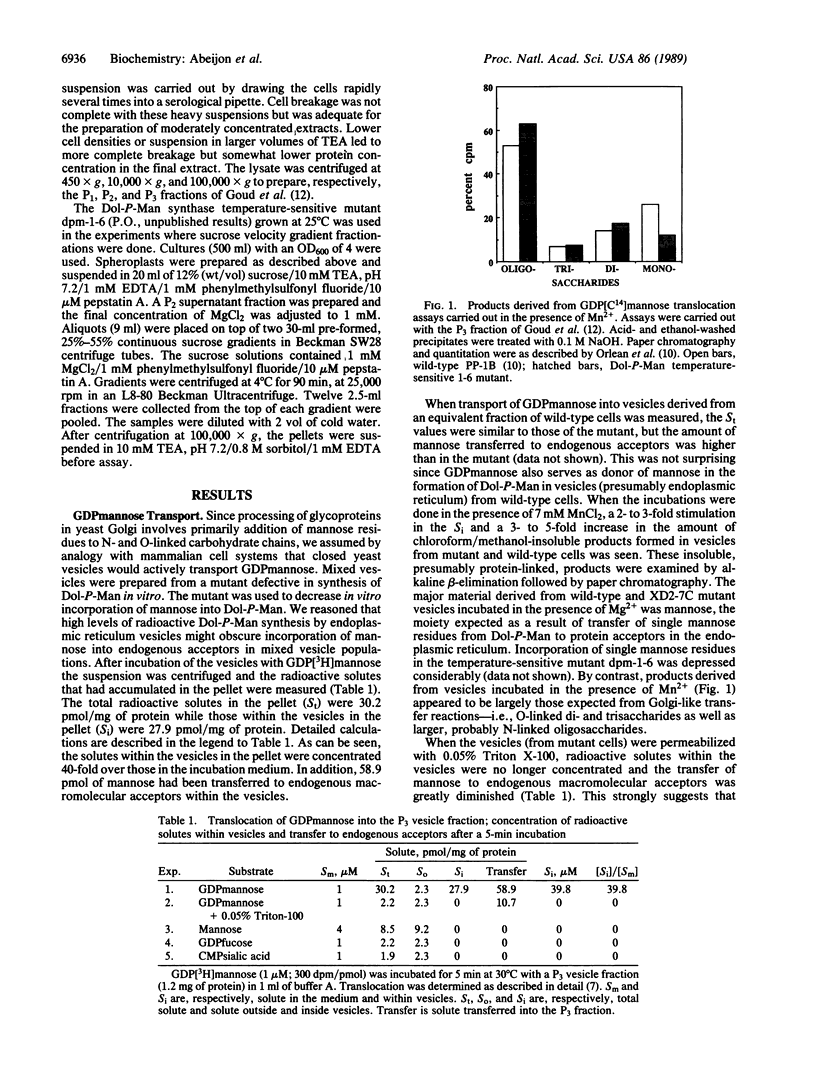
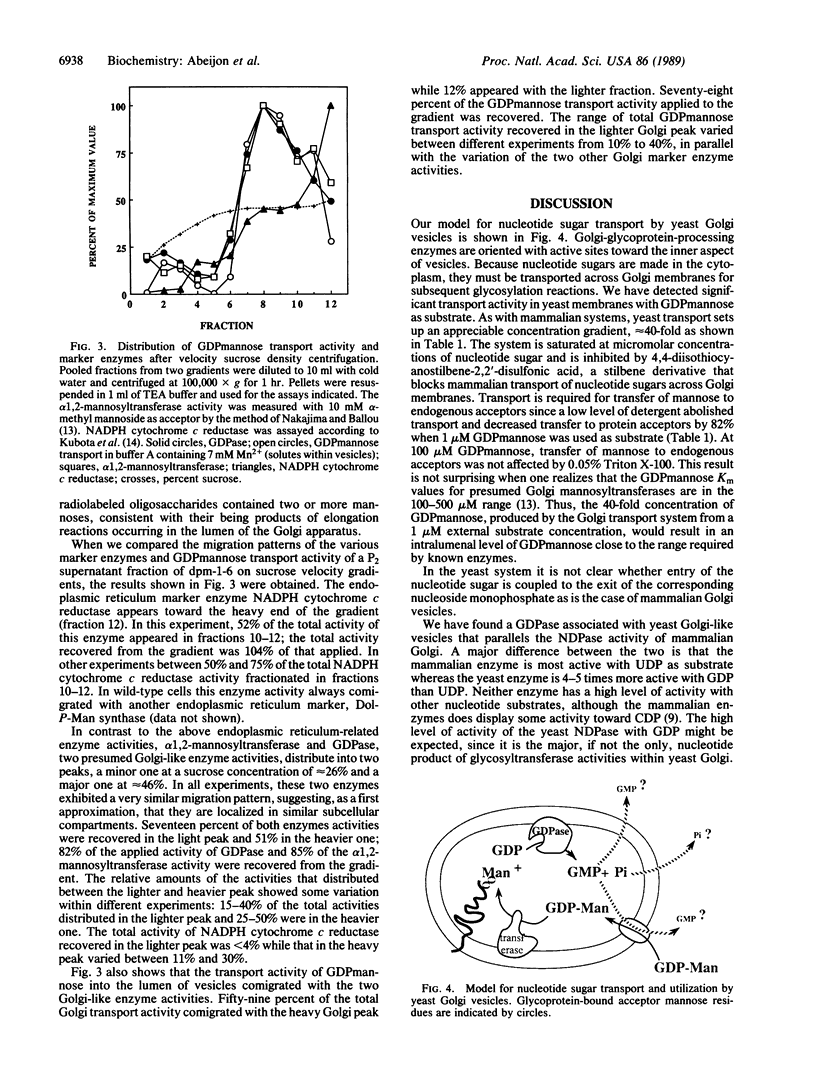
Abstract
"Outer-chain" addition of mannose residues to yeast glycoproteins occurs in the Golgi compartment of the cell. Essential steps in this process are thought to include transport of GDPmannose from the cytoplasm into the lumen of Golgi vesicles, transfer of mannose to glycoprotein acceptors, hydrolysis of the resulting GDP to GMP, and return of GMP and inorganic phosphate to the cytoplasm. We report detection and characterization of a GDPmannose transport activity and a GDPase by yeast vesicles. The active transport of GDPmannose as well as the GDPase and another presumed Golgi enzyme, alpha 1,2-mannosyltransferase, are concentrated in a subcellular fraction that can be partially separated, by velocity sucrose gradient centrifugation, from a fraction enriched in an endoplasmic reticulum marker enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou L., Cohen R. E., Ballou C. E. Saccharomyces cerevisiae mutants that make mannoproteins with a truncated carbohydrate outer chain. J Biol Chem. 1980 Jun 25;255(12):5986–5991. [PubMed] [Google Scholar]
- Brandan E., Fleischer B. Orientation and role of nucleosidediphosphatase and 5'-nucleotidase in Golgi vesicles from rat liver. Biochemistry. 1982 Sep 14;21(19):4640–4645. doi: 10.1021/bi00262a019. [DOI] [PubMed] [Google Scholar]
- Cunningham K. W., Wickner W. T. Yeast KEX2 protease and mannosyltransferase I are localized to distinct compartments of the secretory pathway. Yeast. 1989 Jan-Feb;5(1):25–33. doi: 10.1002/yea.320050105. [DOI] [PubMed] [Google Scholar]
- Gopal P. K., Ballou C. E. Regulation of the protein glycosylation pathway in yeast: structural control of N-linked oligosaccharide elongation. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8824–8828. doi: 10.1073/pnas.84.24.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Kubota S., Yoshida Y., Kumaoka H., Furumichi A. Studies on the microsomal electron-transport system of anaerobically grown yeast. V. Purification and characterization of NADPH-cytochrome c reductase. J Biochem. 1977 Jan;81(1):197–205. doi: 10.1093/oxfordjournals.jbchem.a131436. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Yeast manno-protein biosynthesis: solubilization and selective assay of four mannosyltransferases. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3912–3916. doi: 10.1073/pnas.72.10.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Orlean P., Albright C., Robbins P. W. Cloning and sequencing of the yeast gene for dolichol phosphate mannose synthase, an essential protein. J Biol Chem. 1988 Nov 25;263(33):17499–17507. [PubMed] [Google Scholar]
- Orlean P., Ammer H., Watzele M., Tanner W. Synthesis of an O-glycosylated cell surface protein induced in yeast by alpha factor. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6263–6266. doi: 10.1073/pnas.83.17.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J Biol Chem. 1986 May 25;261(15):6822–6830. [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Transport of sugar nucleotides into the lumen of vesicles derived from rat liver rough endoplasmic reticulum and Golgi apparatus. Methods Enzymol. 1987;138:709–715. doi: 10.1016/0076-6879(87)38061-9. [DOI] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Walworth N. C., Novick P. J. Purification and characterization of constitutive secretory vesicles from yeast. J Cell Biol. 1987 Jul;105(1):163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]



