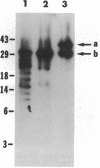
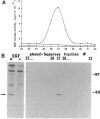
Abstract
pp42, a low-abundance 42-kDa protein, becomes transiently phosphorylated on tyrosine after stimulation of fibroblasts by a variety of mitogens, including epidermal growth factor, platelet-derived growth factor, phorbol 12-myristate 13-acetate, thrombin, and insulin-like growth factor II. The induction of pp42 phosphorylation on tyrosine by such diverse mitogenic agents suggests an important role for pp42 in the cascade of events necessary for cell transition from G0 into the cell cycle. However, as with most proteins identified on the basis of their tyrosine phosphorylation, the function of pp42 in cellular regulation is unknown. In this manuscript we report evidence that suggests that pp42 is a serine/threonine-specific protein kinase. Stimulation of 3T3-L1 cells with insulin has been shown to activate a cytosolic serine/threonine kinase capable of phosphorylating microtubule-associated protein 2 (MAP-2) and ribosomal protein S6 kinase II. This cytosolic serine/threonine protein kinase, which itself is phosphorylated on tyrosine, has been termed "MAP kinase". We now report that pp42 phosphorylation and MAP kinase activation occur in fibroblasts in response to similar mitogens, that the two proteins comigrate on one- and two-dimensional polyacrylamide gels, and that the two proteins copurify chromatographically. The major peptides generated from purified MAP kinase by V8 protease digestion are present as a subset of the peptides in digests of pp42 excised from two-dimensional gels. Thus, the results suggest that MAP kinase is tyrosine-phosphorylated pp42.
Full text
PDF

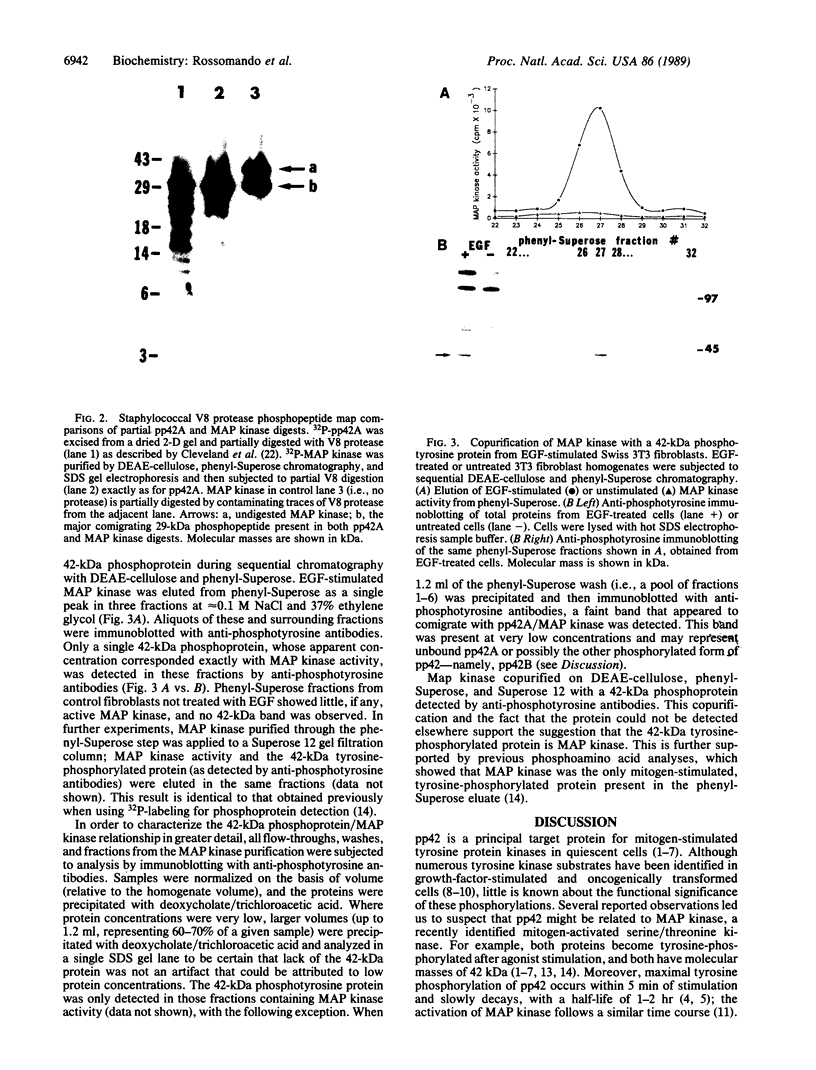

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Contor L., Lamy F., Lecocq R. E. Use of electroblotting to detect and analyze phosphotyrosine containing peptides separated by two-dimensional gel electrophoresis. Anal Biochem. 1987 Feb 1;160(2):414–420. doi: 10.1016/0003-2697(87)90069-8. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Major substrate for growth factor-activated protein-tyrosine kinases is a low-abundance protein. Mol Cell Biol. 1985 Nov;5(11):3304–3309. doi: 10.1128/mcb.5.11.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J., Nakamura K. D., Hunter T., Weber M. J. Phosphotyrosine-containing proteins and expression of transformation parameters in cells infected with partial transformation mutants of Rous sarcoma virus. J Virol. 1983 Apr;46(1):15–28. doi: 10.1128/jvi.46.1.15-28.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Protein kinase C mediates platelet-derived growth factor-induced tyrosine phosphorylation of p42. J Cell Biol. 1988 Apr;106(4):1395–1402. doi: 10.1083/jcb.106.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinez R., Nakamura K. D., Weber M. J. Identification of phosphotyrosine-containing proteins in untransformed and Rous sarcoma virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1982 Jun;2(6):653–665. doi: 10.1128/mcb.2.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. D., Martinez R., Weber M. J. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol Cell Biol. 1983 Mar;3(3):380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Talbot P. J., Knobler R. L., Buchmeier M. J. Western and dot immunoblotting analysis of viral antigens and antibodies: application to murine hepatitis virus. J Immunol Methods. 1984 Oct 12;73(1):177–188. doi: 10.1016/0022-1759(84)90043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila J., Weber M. J. Mitogen-stimulated tyrosine phosphorylation of a 42-kD cellular protein: evidence for a protein kinase-C requirement. J Cell Physiol. 1988 May;135(2):285–292. doi: 10.1002/jcp.1041350216. [DOI] [PubMed] [Google Scholar]