Abstract
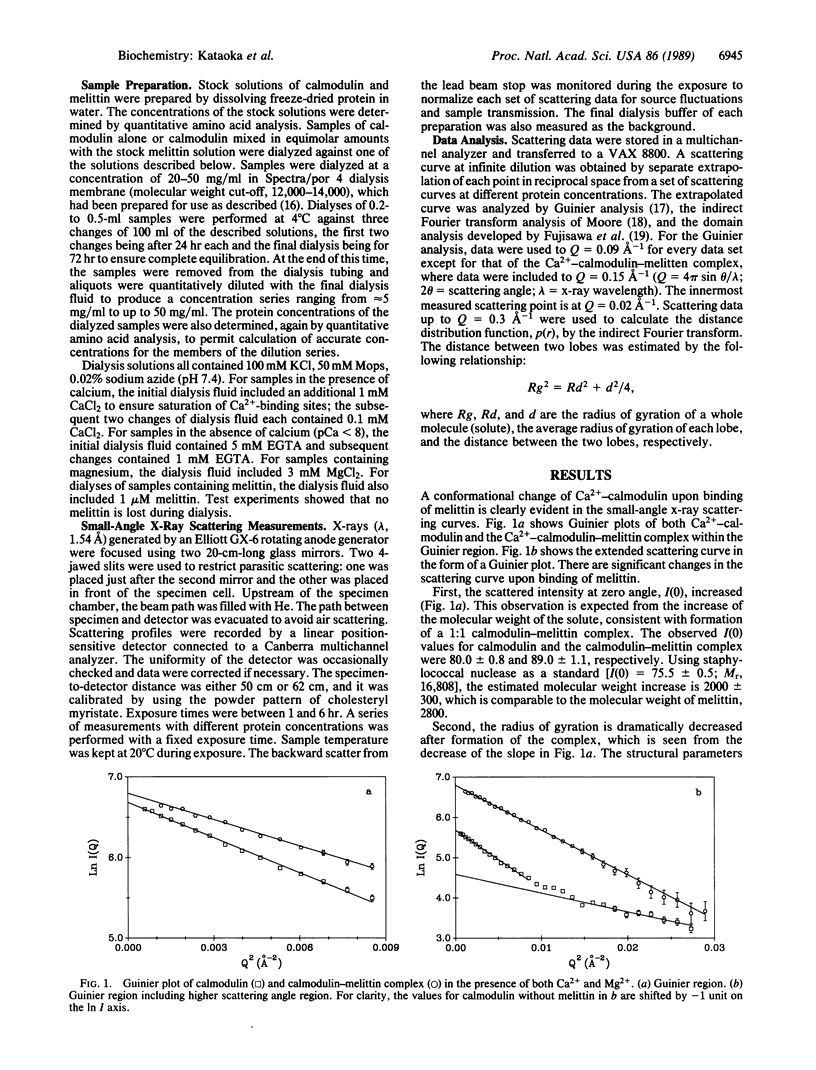
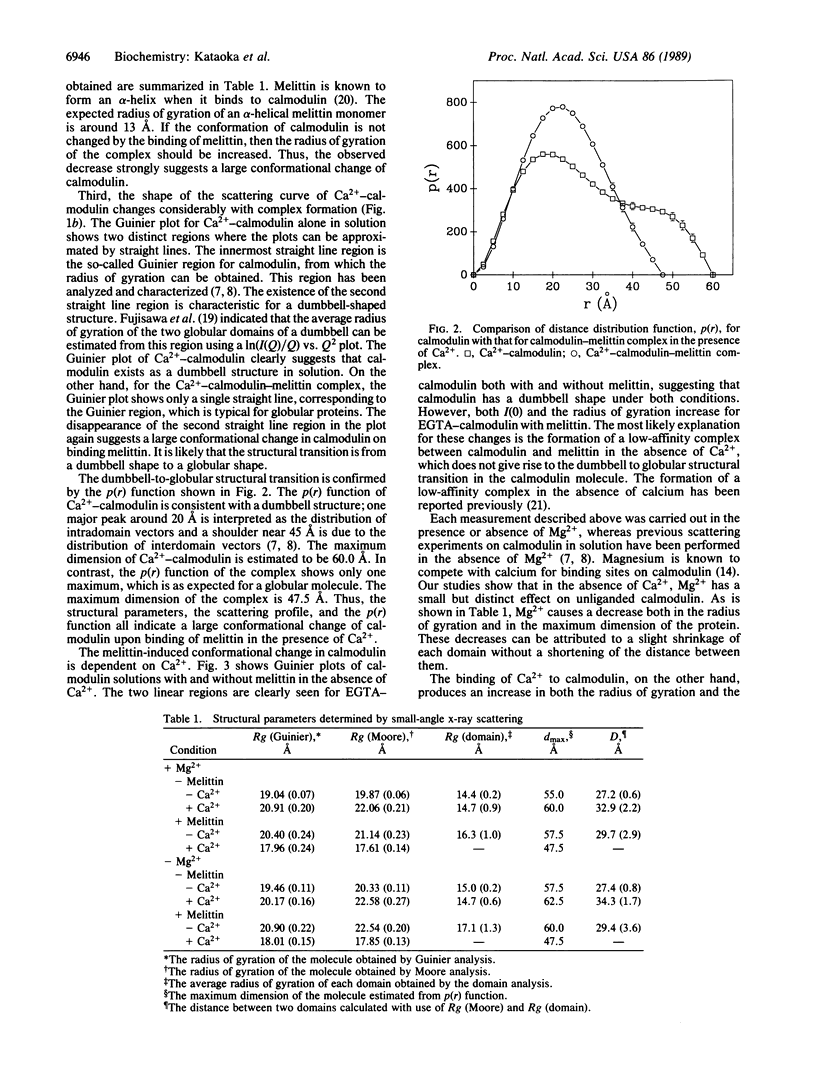
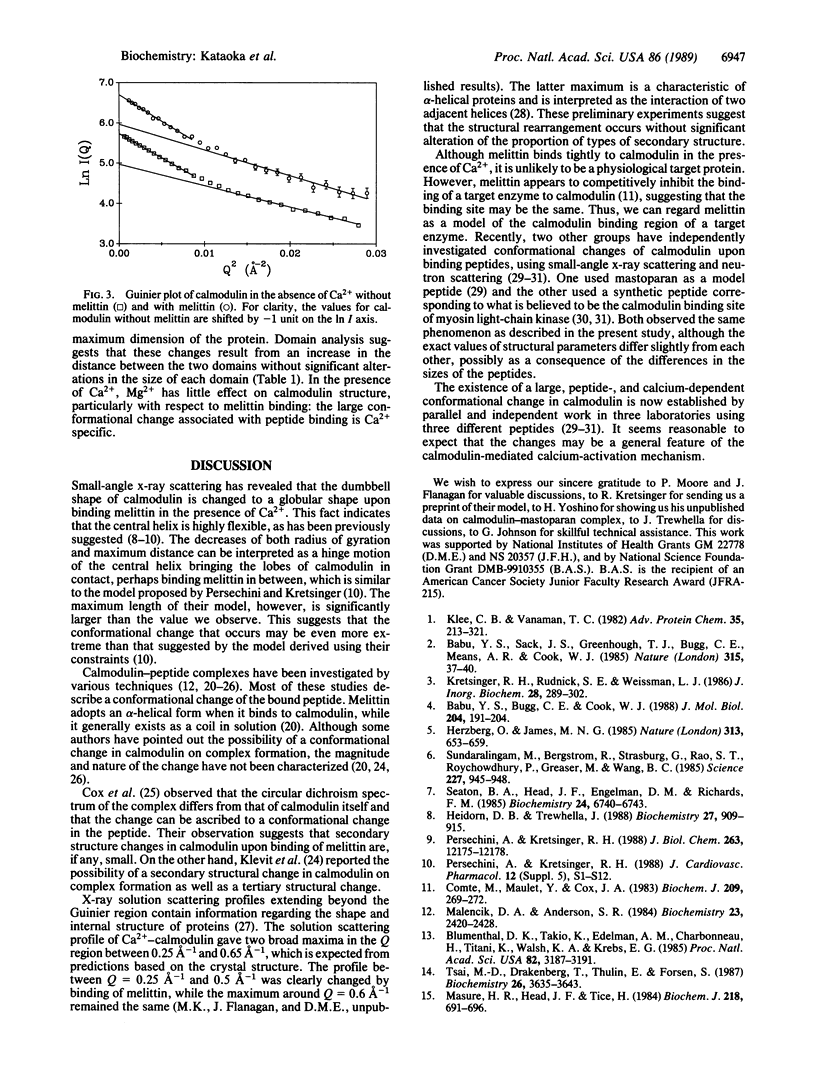
The interaction between calmodulin and its target protein is a key step in many calcium-regulated cellular functions. Melittin binds tightly to calmodulin in the presence of calcium and is a competitive inhibitor of calmodulin function. Using melittin as a model for the target peptide of calmodulin, we have found a large Ca2+-dependent conformational change of calmodulin in solution induced by peptide binding. Mg2+ does not substitute for Ca2+ in producing the conformation change. Small-angle x-ray scattering has shown that calmodulin exists as a dumbbell in solution, similar to that observed in the crystalline state. Our present measurements reveal that the overall structure of the Ca2+-calmodulin-melittin complex is not a dumbbell but a globular shape. Upon binding melittin, the radius of gyration decreases from 20.9 to 18.0 A and the largest dimension decreases from 60 to 47.5 A. In the absence of calcium, however, melittin has little effect on the solution structure of calmodulin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu Y. S., Bugg C. E., Cook W. J. Structure of calmodulin refined at 2.2 A resolution. J Mol Biol. 1988 Nov 5;204(1):191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Babu Y. S., Sack J. S., Greenhough T. J., Bugg C. E., Means A. R., Cook W. J. Three-dimensional structure of calmodulin. Nature. 1985 May 2;315(6014):37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. K., Takio K., Edelman A. M., Charbonneau H., Titani K., Walsh K. A., Krebs E. G. Identification of the calmodulin-binding domain of skeletal muscle myosin light chain kinase. Proc Natl Acad Sci U S A. 1985 May;82(10):3187–3191. doi: 10.1073/pnas.82.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comte M., Maulet Y., Cox J. A. Ca2+-dependent high-affinity complex formation between calmodulin and melittin. Biochem J. 1983 Jan 1;209(1):269–272. doi: 10.1042/bj2090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Fitton J. E., DeGrado W. F. The interaction of calmodulin with amphiphilic peptides. J Biol Chem. 1985 Feb 25;260(4):2527–2534. [PubMed] [Google Scholar]
- Heidorn D. B., Trewhella J. Comparison of the crystal and solution structures of calmodulin and troponin C. Biochemistry. 1988 Feb 9;27(3):909–915. doi: 10.1021/bi00403a011. [DOI] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature. 1985 Feb 21;313(6004):653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Klevit R. E., Blumenthal D. K., Wemmer D. E., Krebs E. G. Interaction of calmodulin and a calmodulin-binding peptide from myosin light chain kinase: major spectral changes in both occur as the result of complex formation. Biochemistry. 1985 Dec 31;24(27):8152–8157. doi: 10.1021/bi00348a047. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Rudnick S. E., Weissman L. J. Crystal structure of calmodulin. J Inorg Biochem. 1986 Oct-Nov;28(2-3):289–302. doi: 10.1016/0162-0134(86)80093-9. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of simple peptides, hormones, and neurotransmitters by calmodulin. Biochemistry. 1982 Jul 6;21(14):3480–3486. doi: 10.1021/bi00257a035. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Peptide binding by calmodulin and its proteolytic fragments and by troponin C. Biochemistry. 1984 May 22;23(11):2420–2428. doi: 10.1021/bi00306a016. [DOI] [PubMed] [Google Scholar]
- Masure H. R., Head J. F., Tice H. M. Studies on the alpha-subunit of bovine brain S-100 protein. Biochem J. 1984 Mar 15;218(3):691–696. doi: 10.1042/bj2180691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima N., Izumi Y., Matsuo T., Yoshino H., Ueki T., Miyake Y. Binding of both Ca2+ and mastoparan to calmodulin induces a large change in the tertiary structure. J Biochem. 1989 Jun;105(6):883–887. doi: 10.1093/oxfordjournals.jbchem.a122773. [DOI] [PubMed] [Google Scholar]
- Maulet Y., Cox J. A. Structural changes in melittin and calmodulin upon complex formation and their modulation by calcium. Biochemistry. 1983 Nov 22;22(24):5680–5686. doi: 10.1021/bi00293a035. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., Wolfe H. R., Jr, Erickson-Viitanen S., DeGrado W. F. Fluorescence properties of calmodulin-binding peptides reflect alpha-helical periodicity. Science. 1987 Jun 12;236(4807):1454–1456. doi: 10.1126/science.3589665. [DOI] [PubMed] [Google Scholar]
- Persechini A., Kretsinger R. H. The central helix of calmodulin functions as a flexible tether. J Biol Chem. 1988 Sep 5;263(25):12175–12178. [PubMed] [Google Scholar]
- Persechini A., Kretsinger R. H. Toward a model of the calmodulin-myosin light-chain kinase complex: implications for calmodulin function. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S1–12. [PubMed] [Google Scholar]
- Seaton B. A., Head J. F., Engelman D. M., Richards F. M. Calcium-induced increase in the radius of gyration and maximum dimension of calmodulin measured by small-angle X-ray scattering. Biochemistry. 1985 Nov 19;24(24):6740–6743. doi: 10.1021/bi00345a002. [DOI] [PubMed] [Google Scholar]
- Seeholzer S. H., Cohn M., Putkey J. A., Means A. R., Crespi H. L. NMR studies of a complex of deuterated calmodulin with melittin. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3634–3638. doi: 10.1073/pnas.83.11.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon A., Head J. F. Calcium-binding properties of two high affinity calcium-binding proteins from squid optic lobe. J Biol Chem. 1988 Oct 5;263(28):14384–14389. [PubMed] [Google Scholar]
- Sundaralingam M., Bergstrom R., Strasburg G., Rao S. T., Roychowdhury P., Greaser M., Wang B. C. Molecular structure of troponin C from chicken skeletal muscle at 3-angstrom resolution. Science. 1985 Feb 22;227(4689):945–948. doi: 10.1126/science.3969570. [DOI] [PubMed] [Google Scholar]
- Tsai M. D., Drakenberg T., Thulin E., Forsén S. Is the binding of magnesium (II) to calmodulin significant? An investigation by magnesium-25 nuclear magnetic resonance. Biochemistry. 1987 Jun 16;26(12):3635–3643. doi: 10.1021/bi00386a057. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Ikura M., Hikichi K., Ying L., Yagi K. Communication between two globular domains of calmodulin in the presence of mastoparan or caldesmon fragment. Ca2+ binding and 1H NMR. J Biol Chem. 1987 Aug 15;262(23):10951–10954. [PubMed] [Google Scholar]


