Abstract
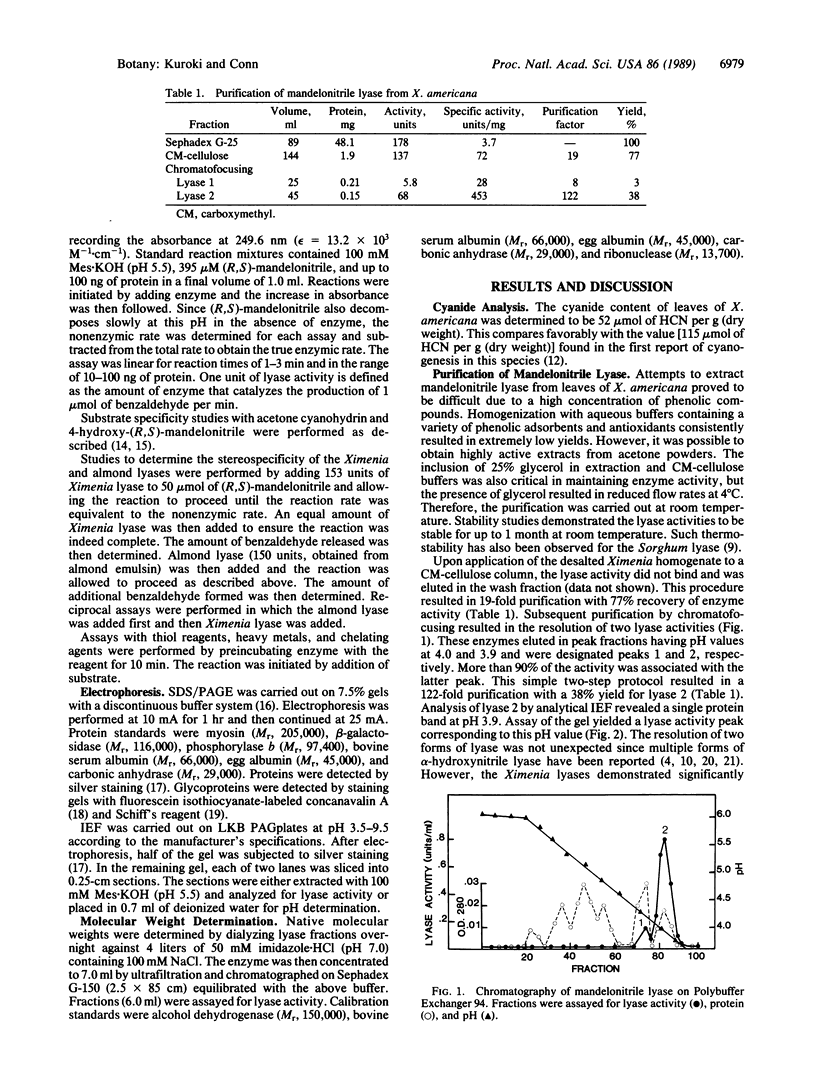
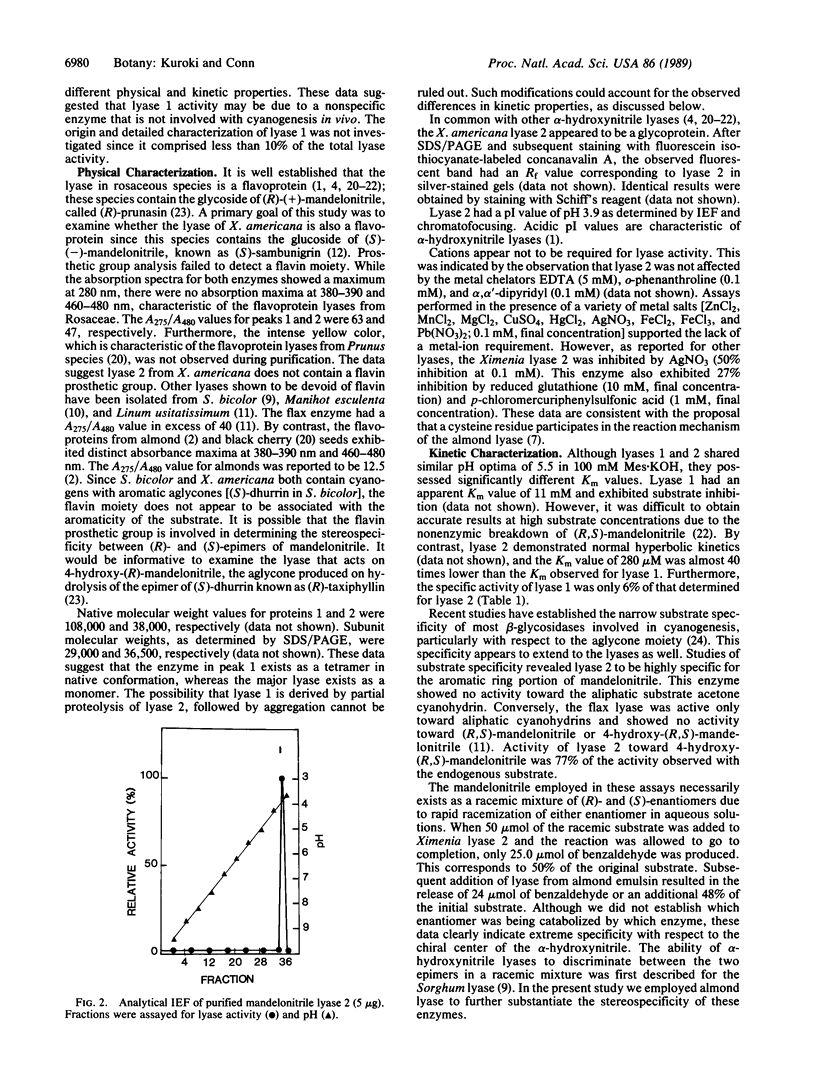
A mandelonitrile lyase (EC 4.1.2.10) that catalyzes the dissociation of (S)-(-)-mandelonitrile to benzaldehyde and hydrogen cyanide has been purified to apparent homogeneity from leaves of Ximenia americana L. (Olacaceae). The lyase was purified 122-fold with 38% yield by chromatography on carboxymethyl-cellulose and chromatofocusing. The enzyme had a pH optimum of 5.5, with a Km value of 280 microM. Activity toward 4-hydroxy-(R,S)-mandelonitrile was 77% of that observed with the endogenous substrate; no activity was observed toward the aliphatic substrate acetone cyanohydrin. The enzyme was stable at 4 degrees C and at room temperature for at least 1 month. Native and subunit molecular weights of 38,000 and 36,500, respectively, suggest the enzyme is a monomer. The isoelectric point was pH 3.9 as determined by isoelectric focusing. Staining with periodic acid-Schiff and fluorescein-labeled concanavalin A reagents indicate this enzyme is a glycoprotein. In contrast to (R)-mandelonitrile lyases isolated from Prunus species, the Ximenia lyase does not appear to be a flavoprotein. A second enzyme that eluted from the chromatofocusing column at pH 4.0 was also active toward mandelonitrile. However, this form accounted for less than 10% of the total activity, and its specific activity was only 6% of that of the major component. Additional physical and kinetic studies suggested this activity may be due to a nonspecific enzyme that is active toward mandelonitrile.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschhoff H. J., Pfeil E. Auftrennung und Charakterisierung der Isoenzyme von D-Hydroxynitril-Lyase (D-Oxynitrilase) aus Mandeln. Hoppe Seylers Z Physiol Chem. 1970 Jul;351(7):818–826. [PubMed] [Google Scholar]
- BECKER W., BENTHIN U., ESCHENHOF E., PFEIL E. [On the knowledge of cyanhydrin synthesis. II. Purification and properties of hydroxynitrilase from bitter almonds (Prunus communis Stokes)]. Biochem Z. 1963;337:156–166. [PubMed] [Google Scholar]
- Fargeaud D., Jeannin C. B., Kato F., Chappuis G. Biochemical study of the Feline Herpesvirus 1. Identification of glycoproteins by affinity. Arch Virol. 1984;80(2-3):69–82. doi: 10.1007/BF01310650. [DOI] [PubMed] [Google Scholar]
- Gerstner E., Kiel U. Eine neue Mandelsäurenitril-Lyase (D-Oxynitrilase) aus Prunus laurocerasus (Kirschlorbeer) Hoppe Seylers Z Physiol Chem. 1975 Dec;356(12):1853–1857. [PubMed] [Google Scholar]
- Gerstner E., Pfeil E. Zur Kenntnis des Flavinenzyms Hydroxynitril-Lyase (D-Oxynitrilase. Hoppe Seylers Z Physiol Chem. 1972 Mar;353(3):271–286. doi: 10.1515/bchm2.1972.353.1.271. [DOI] [PubMed] [Google Scholar]
- Jaenicke L., Preun J. Chemical modification of hydroxynitrile lyase by selective reaction of an essential cysteine-SH group with alpha, beta-unsaturated propiophenones as pseudo-substrates. Eur J Biochem. 1984 Jan 16;138(2):319–325. doi: 10.1111/j.1432-1033.1984.tb07917.x. [DOI] [PubMed] [Google Scholar]
- Jorns M. S. Comments on: 'Chemical modification of hydroxynitrile lyase by selective reaction of an essential cysteine-SH group with alpha, beta-unsaturated propriophenones as pseudo-substrates' by L. Jaenicke and J. Preun. Eur J Biochem. 1985 Jan 15;146(2):481–482. doi: 10.1111/j.1432-1033.1985.tb08676.x. [DOI] [PubMed] [Google Scholar]
- Jorns M. S. Mechanism of catalysis by the flavoenzyme oxynitrilase. J Biol Chem. 1979 Dec 10;254(23):12145–12152. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Seely M. K., Criddle R. S., Conn E. E. The metabolism of aromatic compounds in higher plants. 8. On the requirement of hydroxynitrile lyase for flavin. J Biol Chem. 1966 Oct 10;241(19):4457–4462. [PubMed] [Google Scholar]
- Selmar D., Carvalho F. J., Conn E. E. A colorimetric assay for alpha-hydroxynitrile lyase. Anal Biochem. 1987 Oct;166(1):208–211. doi: 10.1016/0003-2697(87)90565-3. [DOI] [PubMed] [Google Scholar]
- Xu L. L., Singh B. K., Conn E. E. Purification and characterization of acetone cyanohydrin lyase from Linum usitatissimum. Arch Biochem Biophys. 1988 Jun;263(2):256–263. doi: 10.1016/0003-9861(88)90634-0. [DOI] [PubMed] [Google Scholar]
- Xu L. L., Singh B. K., Conn E. E. Purification and characterization of mandelonitrile lyase from Prunus lyonii. Arch Biochem Biophys. 1986 Nov 1;250(2):322–328. doi: 10.1016/0003-9861(86)90733-2. [DOI] [PubMed] [Google Scholar]
- Yemm R. S., Poulton J. E. Isolation and characterization of multiple forms of mandelonitrile lyase from mature black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1986 Jun;247(2):440–445. doi: 10.1016/0003-9861(86)90604-1. [DOI] [PubMed] [Google Scholar]