Abstract
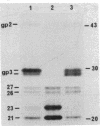
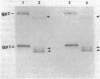
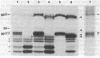
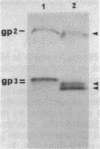
We previously showed that glycophorins are expressed in virus-transformed, murine erythroleukemia cells; we detected four glycophorin precursors (two more than in normal erythroblasts) and found that two of them are not translocated or are inefficiently translocated across the endoplasmic reticulum (ER) membrane. By using the drug brefeldin A to block intracellular transport of proteins from the ER to the Golgi complex, the translocated precursors were shown to accumulate in the ER, while the untranslocated forms were rapidly degraded with an intracellular half-life of approximately 20 min. Brefeldin A did not inhibit the synthesis of fatty acylation of the precursors but substantially delayed their acquisition of O-linked oligosaccharides, which indicates that murine glycophorins are fatty acylated in the ER and O-glycosylated in the Golgi complex. Even after 6 hr in brefeldin A, glycophorins were only partially glycosylated, resulting in the accumulation of glycoproteins apparently sialylated but lower in apparent molecular mass than mature glycophorins. Complete glycophorin processing resumed only after removal of the drug. In murine erythroleukemia cells, brefeldin A caused a rapid and extensive disorganization of the entire Golgi complex accompanied by the accumulation of membranes in a part of the ER closely associated with ER transitional elements. These findings extend recently published results [Lippincott-Schwartz, J., Yuan, L. C., Bonifacino, J. S. & Klausner, R. D. (1989) Cell 56, 801-813] and suggest that brefeldin A induces net membrane flow from the entire Golgi complex to the ER.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Subcellular site of synthesis of the N-acetylgalactosamine (alpha 1-0) serine (or threonine) linkage in rat liver. J Biol Chem. 1987 Mar 25;262(9):4153–4159. [PubMed] [Google Scholar]
- Andrews D. W., Perara E., Lesser C., Lingappa V. R. Sequences beyond the cleavage site influence signal peptide function. J Biol Chem. 1988 Oct 25;263(30):15791–15798. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dolci E. D., Palade G. E. The biosynthesis and fatty acid acylation of the murine erythrocyte sialoglycoproteins. J Biol Chem. 1985 Sep 5;260(19):10728–10735. [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Gilmore R., Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A. 1986 Feb;83(3):581–585. doi: 10.1073/pnas.83.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988 Dec 5;263(34):18545–18552. [PubMed] [Google Scholar]
- High S., Tanner M. J. Human erythrocyte membrane sialoglycoprotein beta. The cDNA sequence suggests the absence of a cleaved N-terminal signal sequence. Biochem J. 1987 Apr 1;243(1):277–280. doi: 10.1042/bj2430277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal and membrane anchor functions overlap in the type II membrane protein I gamma CAT. J Cell Biol. 1988 Jun;106(6):1813–1820. doi: 10.1083/jcb.106.6.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. P., Topping T. B., Cover W. H., Randall L. L. Retardation of folding as a possible means of suppression of a mutation in the leader sequence of an exported protein. J Biol Chem. 1988 Oct 15;263(29):14790–14793. [PubMed] [Google Scholar]
- Matsui Y., Natori S., Obinata M. Isolation of the cDNA clone for mouse glycophorin, erythroid-specific membrane protein. Gene. 1989 Apr 30;77(2):325–332. doi: 10.1016/0378-1119(89)90080-2. [DOI] [PubMed] [Google Scholar]
- Meyer D. I. Preprotein conformation: the year's major theme in translocation studies. Trends Biochem Sci. 1988 Dec;13(12):471–474. doi: 10.1016/0968-0004(88)90233-2. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Mize N. K., Andrews D. W., Lingappa V. R. A stop transfer sequence recognizes receptors for nascent chain translocation across the endoplasmic reticulum membrane. Cell. 1986 Dec 5;47(5):711–719. doi: 10.1016/0092-8674(86)90514-3. [DOI] [PubMed] [Google Scholar]
- Oda K., Hirose S., Takami N., Misumi Y., Takatsuki A., Ikehara Y. Brefeldin A arrests the intracellular transport of a precursor of complement C3 before its conversion site in rat hepatocytes. FEBS Lett. 1987 Apr 6;214(1):135–138. doi: 10.1016/0014-5793(87)80028-5. [DOI] [PubMed] [Google Scholar]
- Pathak R. K., Merkle R. K., Cummings R. D., Goldstein J. L., Brown M. S., Anderson R. G. Immunocytochemical localization of mutant low density lipoprotein receptors that fail to reach the Golgi complex. J Cell Biol. 1988 Jun;106(6):1831–1841. doi: 10.1083/jcb.106.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Weber B. Early O-glycosidic glycosylation of proglucagon in pancreatic islets: an unusual type of prohormonal modification. EMBO J. 1986 Sep;5(9):2103–2108. doi: 10.1002/j.1460-2075.1986.tb04472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel V. S., Liu A. Y., Miura Y., Magner J. A. The effects of brefeldin-A on the high mannose oligosaccharides of mouse thyrotropin, free alpha-subunits, and total glycoproteins. Endocrinology. 1988 Jul;123(1):310–318. doi: 10.1210/endo-123-1-310. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rizzolo L. J., Kornfeld R. Post-translational protein modification in the endoplasmic reticulum. Demonstration of fatty acylase and deoxymannojirimycin-sensitive alpha-mannosidase activities. J Biol Chem. 1988 Jul 5;263(19):9520–9525. [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sarris A. H., Palade G. E. Isolation and partial characterization of the sialoglycoprotein fraction of murine erythrocyte ghosts. J Cell Biol. 1982 Jun;93(3):583–590. doi: 10.1083/jcb.93.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
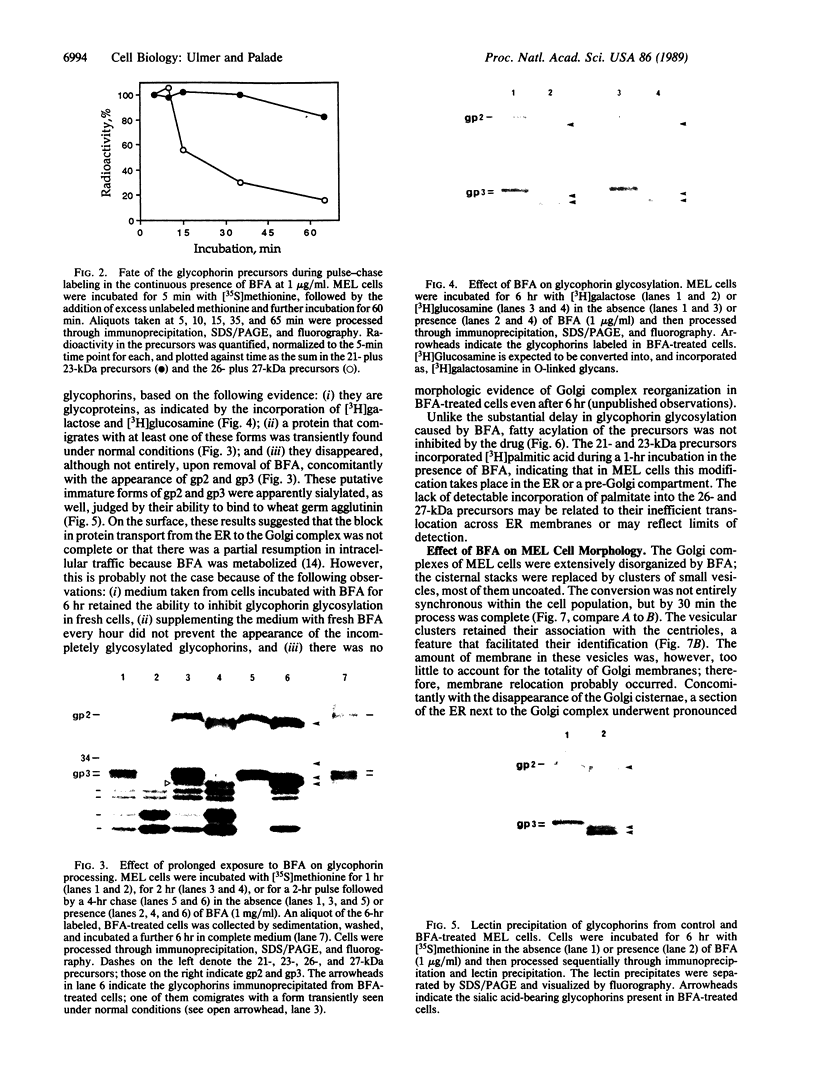
- Sarris A. H., Palade G. E. The sialoglycoproteins of murine erythrocyte ghosts. A modified periodic acid-Schiff stain procedure staining nonsubstituted and O-acetylated sialyl residues on glycopeptides. J Biol Chem. 1979 Jul 25;254(14):6724–6731. [PubMed] [Google Scholar]
- Schnitzer J. E., Carley W. W., Palade G. E. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6773–6777. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. The affinity of signal recognition particle for presecretory proteins is dependent on nascent chain length. EMBO J. 1988 Jun;7(6):1769–1775. doi: 10.1002/j.1460-2075.1988.tb03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany A., Tsukada H., Zalesna G., Slomiany B. L. Cotranslational fatty acylation of mucus glycoprotein. Addition of palmitic acid to peptidyl-tRNA occurs prior to peptide chain completion and its release. Int J Biochem. 1988;20(12):1381–1390. doi: 10.1016/s0020-711x(98)90006-4. [DOI] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. An internal signal sequence: the asialoglycoprotein receptor membrane anchor. Cell. 1986 Jan 17;44(1):177–185. doi: 10.1016/0092-8674(86)90496-4. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Tooze J., Warren G. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J Cell Biol. 1988 May;106(5):1475–1487. doi: 10.1083/jcb.106.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Dolci E. D., Palade G. E. Glycophorin expression in murine erythroleukaemia cells. J Cell Sci. 1989 Feb;92(Pt 2):163–171. doi: 10.1242/jcs.92.2.163. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Anomalies in the translocation and processing of glycophorin precursors in murine erythroleukemia cells. J Biol Chem. 1989 Jan 15;264(2):1084–1091. [PubMed] [Google Scholar]
- Walter R. J., Tandler B. Viruses and annulate lamellae in Friend erythroleukemia cells. J Submicrosc Cytol Pathol. 1989 Jan;21(1):93–101. [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Bielka H., Rapoport T. A. Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific cross-linking. J Cell Biol. 1987 Feb;104(2):201–208. doi: 10.1083/jcb.104.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]