Abstract
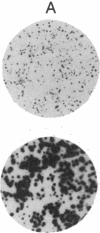
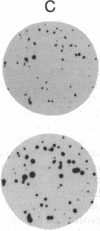
Hybrids constructed by fusion of wild-type Chinese hamster ovary cells (CHO-K1) to peroxisome-deficient CHO mutants (ZR-78.1) contain normal peroxisomes, demonstrating that the mutation(s) are recessive. "Nuclear hybrids" prepared by fusion of CHO-K1 karyoplasts to mutant ZR-78.1 occasionally fail to regain intact peroxisomes (approximately 1/300 cells). These peroxisome-deficient nuclear hybrids closely resemble the original mutant cells by biochemical criteria, but their modal chromosome number is 36-38, the same as that of CHO hybrids generated from intact cells. When the peroxisome-deficient nuclear hybrids are fused to wild-type cytoplasts, a fraction of the fusion products (at least 70%) continue to propagate normal peroxisomes indefinitely. Peroxisome biogenesis cannot be reinitiated in cells of mutant ZR-78.1 by fusion to wild-type cytoplasts. Our results suggest that a wild-type nucleus by itself is necessary but not sufficient for restoration of normal peroxisome biogenesis and that a cytoplasmic component of wild-type cells, possibly a normal peroxisome, is also required.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Borst P. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim Biophys Acta. 1986 May 5;866(4):179–203. doi: 10.1016/0167-4781(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Brasaemle D. L., Attie A. D. Microelisa reader quantitation of fixed, stained, solubilized cells in microtitre dishes. Biotechniques. 1988 May;6(5):418–419. [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- HIGASHI T., PETERS T., Jr STUDIES ON RAT LIVER CATALASE. II. INCORPORATION OF 14-C-LEUCINE INTO CATALASE OF LIVER CELL FRACTIONS IN VIVO. J Biol Chem. 1963 Dec;238:3952–3954. [PubMed] [Google Scholar]
- Kamiryo T., Abe M., Okazaki K., Kato S., Shimamoto N. Absence of DNA in peroxisomes of Candida tropicalis. J Bacteriol. 1982 Oct;152(1):269–274. doi: 10.1128/jb.152.1.269-274.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Johnson R. T., Puck T. T. Complementation analysis on virus-fused Chinese hamster cells with nutritional markers. Science. 1969 Apr 18;164(3877):312–314. doi: 10.1126/science.164.3877.312. [DOI] [PubMed] [Google Scholar]
- Krieger M. The use of amphotericin B to detect inhibitors of cellular cholesterol biosynthesis. Anal Biochem. 1983 Dec;135(2):383–391. doi: 10.1016/0003-2697(83)90700-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Robbi M., Fujiki Y., Wong L. Biogenesis of peroxisomal proteins in vivo and in vitro. Ann N Y Acad Sci. 1982;386:285–300. doi: 10.1111/j.1749-6632.1982.tb21423.x. [DOI] [PubMed] [Google Scholar]
- Linder S., Brzeski H., Ringertz N. R. Phenotypic expression in cybrids derived from teratocarcinoma cells fused with myoblast cytoplasms. Exp Cell Res. 1979 Apr;120(1):1–14. doi: 10.1016/0014-4827(79)90529-9. [DOI] [PubMed] [Google Scholar]
- Morand O. H., Zoeller R. A., Raetz C. R. Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J Biol Chem. 1988 Aug 15;263(23):11597–11606. [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Wermuth M. M., McIntyre T. M., Esko J. D., Wing D. C. Somatic cell cloning in polyester stacks. Proc Natl Acad Sci U S A. 1982 May;79(10):3223–3227. doi: 10.1073/pnas.79.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Small G. M., Lazarow P. B. Peroxisomal membrane ghosts in Zellweger syndrome--aberrant organelle assembly. Science. 1988 Mar 25;239(4847):1536–1538. doi: 10.1126/science.3281254. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Microsomal sn-glycerol 3-phosphate and dihydroxyacetone phosphate acyltransferase activities from liver and other tissues. Evidence for a single enzyme catalizing both reactions. Arch Biochem Biophys. 1977 Aug;182(2):732–742. doi: 10.1016/0003-9861(77)90555-0. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Evidence that the sn-glycerol-3-phosphate and dihydroxyacetone phosphate acyltransferase activities are dual catalytic functions of a single microsomal enzyme. J Biol Chem. 1976 Sep 25;251(18):5738–5744. [PubMed] [Google Scholar]
- Shay J. W., Porter K. R., Prescott D. M. The surface morphology and fine structure of CHO (Chinese hamster ovary) cells following enucleation. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3059–3063. doi: 10.1073/pnas.71.8.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Duff C. Karyotyping. Methods Enzymol. 1979;58:322–344. doi: 10.1016/s0076-6879(79)58148-8. [DOI] [PubMed] [Google Scholar]
- Zoeller R. A., Morand O. H., Raetz C. R. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988 Aug 15;263(23):11590–11596. [PubMed] [Google Scholar]
- Zoeller R. A., Raetz C. R. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5170–5174. doi: 10.1073/pnas.83.14.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]