Abstract
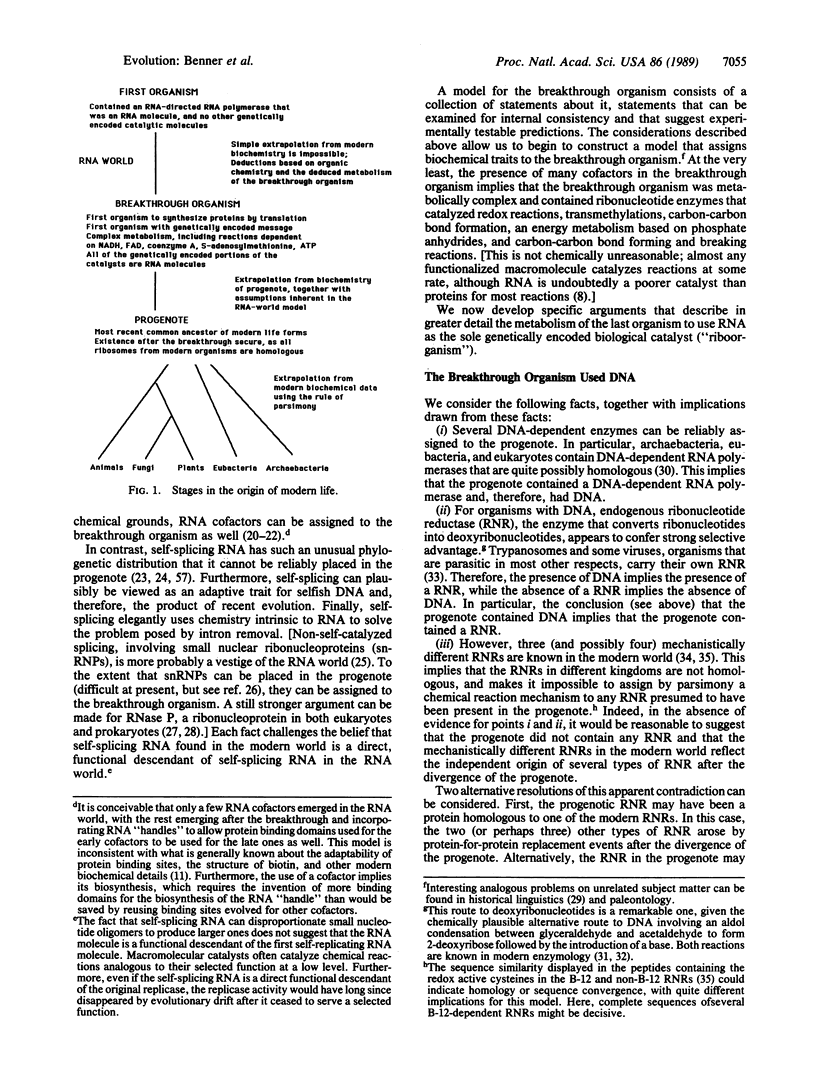
An approach is developed for constructing models of ancient organisms using data from metabolic pathways, genetic organization, chemical structure, and enzymatic reaction mechanisms found in contemporary organisms. This approach is illustrated by a partial reconstruction of a model for the "breakthrough organism," the last organism to use RNA as the sole genetically encoded biological catalyst. As reconstructed here, this organism had a complex metabolism that included dehydrogenations, transmethylations, carbon-carbon bond-forming reactions, and an energy metabolism based on phosphate esters. Furthermore, the breakthrough organism probably used DNA to store genetic information, biosynthesized porphyrins, and used terpenes as its major lipid component. This model differs significantly from prevailing models based primarily on genetic data.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ator M. A., Stubbe J., Spector T. Mechanism of ribonucleotide reductase from herpes simplex virus type 1. Evidence for 3' carbon-hydrogen bond cleavage and inactivation by nucleotide analogs. J Biol Chem. 1986 Mar 15;261(8):3595–3599. [PubMed] [Google Scholar]
- Benner S. A., Allemann R. K., Ellington A. D., Ge L., Glasfeld A., Leanz G. F., Krauch T., MacPherson L. J., Moroney S., Piccirilli J. A. Natural selection, protein engineering, and the last riboorganism: rational model building in biochemistry. Cold Spring Harb Symp Quant Biol. 1987;52:53–63. doi: 10.1101/sqb.1987.052.01.009. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Ellington A. D. Return of the 'last ribo-organism'. Nature. 1988 Apr 21;332(6166):688–689. doi: 10.1038/332688b0. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Ellington A. D. The last ribo-organism. Nature. 1987 Sep 24;329(6137):295–296. doi: 10.1038/329295a0. [DOI] [PubMed] [Google Scholar]
- Benner S., Ellington A. D. Interpreting the behavior of enzymes: purpose or pedigree? CRC Crit Rev Biochem. 1988;23(4):369–426. doi: 10.3109/10409238809082549. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Crick F. H. The origin of the genetic code. J Mol Biol. 1968 Dec;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Doolittle W. F. Speculations on the early course of evolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1271–1275. doi: 10.1073/pnas.83.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., van Belkum A., Pleij C. W., Altman S. Novel reactions of RNAase P with a tRNA-like structure in turnip yellow mosaic virus RNA. Cell. 1988 Apr 22;53(2):267–272. doi: 10.1016/0092-8674(88)90388-1. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ROSEN O. M., HORECKER B. L. THE MECHANISM OF ACTION OF ALDOLASES. VI. CRYSTALLIZATION OF DEOXYRIBOSE 5-PHOSPHATE ALDOLASE AND THE NUMBER OF ACTIVE SITES. J Biol Chem. 1965 Apr;240:1512–1516. [PubMed] [Google Scholar]
- Hauska G. Elucidation of methanogenesis seems well on its way. Trends Biochem Sci. 1988 Jan;13(1):2–4. doi: 10.1016/0968-0004(88)90003-5. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Iwanow A., Sayer B. G., Wysocka W., Spenser I. D. The regiochemistry and stereochemistry of the biosynthesis of vitamin B6 from triose units. J Biol Chem. 1987 Jun 5;262(16):7463–7471. [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y., Gough S. P., Kannangara C. G. delta-Aminolevulinic acid-synthesizing enzymes need an RNA moiety for activity. Science. 1984 Sep 28;225(4669):1482–1484. doi: 10.1126/science.6206568. [DOI] [PubMed] [Google Scholar]
- Inouye M., Delihas N. Small RNAs in the prokaryotes: a growing list of diverse roles. Cell. 1988 Apr 8;53(1):5–7. doi: 10.1016/0092-8674(88)90480-1. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Gough S. P., Bruyant P., Hoober J. K., Kahn A., von Wettstein D. tRNA(Glu) as a cofactor in delta-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988 Apr;13(4):139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Lang B. F. The mitochondrial genome of the fission yeast Schizosaccharomyces pombe: highly homologous introns are inserted at the same position of the otherwise less conserved cox1 genes in Schizosaccharomyces pombe and Aspergillus nidulans. EMBO J. 1984 Sep;3(9):2129–2136. doi: 10.1002/j.1460-2075.1984.tb02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. Fish to bacterium gene transfer. Science. 1985 Mar 1;227(4690):1020–1020. doi: 10.1126/science.227.4690.1020. [DOI] [PubMed] [Google Scholar]
- Lewin R. RNA Catalysis Gives Fresh Perspective on the Origin of Life: The old chicken-and-egg problem of the origin of life is illuminated in unexpected ways by recent results on the splicing of RNA precursors. Science. 1986 Feb 7;231(4738):545–546. doi: 10.1126/science.231.4738.545. [DOI] [PubMed] [Google Scholar]
- Lin A. N., Ashley G. W., Stubbe J. Location of the redox-active thiols of ribonucleotide reductase: sequence similarity between the Escherichia coli and Lactobacillus leichmannii enzymes. Biochemistry. 1987 Nov 3;26(22):6905–6909. doi: 10.1021/bi00396a006. [DOI] [PubMed] [Google Scholar]
- Maizels N., Weiner A. M. The 'last ribo-organism' was no breakthrough. Nature. 1987 Dec 17;330(6149):616–616. doi: 10.1038/330616a0. [DOI] [PubMed] [Google Scholar]
- McClung C. R., Somerville J. E., Guerinot M. L., Chelm B. K. Structure of the Bradyrhizobium japonicum gene hemA encoding 5-aminolevulinic acid synthase. Gene. 1987;54(1):133–139. doi: 10.1016/0378-1119(87)90355-6. [DOI] [PubMed] [Google Scholar]
- McElwain M. C., Pollack J. D. Synthesis of deoxyribomononucleotides in Mollicutes: dependence on deoxyribose-1-phosphate and PPi. J Bacteriol. 1987 Aug;169(8):3647–3653. doi: 10.1128/jb.169.8.3647-3653.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Dujon B. Genetic exchanges between bacteriophage T4 and filamentous fungi? Cell. 1986 Aug 1;46(3):323–323. doi: 10.1016/0092-8674(86)90651-3. [DOI] [PubMed] [Google Scholar]
- Miller G. Animal model for Epstein-Barr lymphoma. Nature. 1986 Feb 20;319(6055):626–626. doi: 10.1038/319626c0. [DOI] [PubMed] [Google Scholar]
- Nandi D. L. Delta-aminolevulinic acid synthase of rhodopseudomonas spheroides. Binding of pyridoxal phosphate to the enzyme. Arch Biochem Biophys. 1978 Jun;188(2):266–271. doi: 10.1016/s0003-9861(78)80008-3. [DOI] [PubMed] [Google Scholar]
- Neunlist S., Rohmer M. A novel hopanoid, 30-(5'-adenosyl)hopane, from the purple non-sulphur bacterium Rhodopseudomonas acidophila, with possible DNA interactions. Biochem J. 1985 Jun 15;228(3):769–771. doi: 10.1042/bj2280769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M., Söll D., Willis I. Yeast RNase P: catalytic activity and substrate binding are separate functions. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1379–1383. doi: 10.1073/pnas.85.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E. Evolution of the genetic apparatus. J Mol Biol. 1968 Dec;38(3):381–393. doi: 10.1016/0022-2836(68)90393-8. [DOI] [PubMed] [Google Scholar]
- Orgel L. E. RNA catalysis and the origins of life. J Theor Biol. 1986 Nov 21;123(2):127–149. doi: 10.1016/s0022-5193(86)80149-7. [DOI] [PubMed] [Google Scholar]
- Pezacka E., Walerych W. Biosynthesis of vitamin B-12. Part I. Role of the ribosomal proteins in vitamin B-12 biosynthesis. Biochim Biophys Acta. 1981 Dec 18;678(3):300–315. doi: 10.1016/0304-4165(81)90107-0. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Wassef M. K., Kates M. Inhibition of fatty acid synthetase in Halobacterium cutirubrum and Escherichia coli by high salt concentrations. Can J Biochem. 1971 Aug;49(8):953–958. doi: 10.1139/o71-138. [DOI] [PubMed] [Google Scholar]
- SIU P. M., WOOD H. G. Phosphoenolpyruvic carboxytransphosphorylase, a CO2 fixation enzyme from propionic acid bacteria. J Biol Chem. 1962 Oct;237:3044–3051. [PubMed] [Google Scholar]
- Sogin M. L., Ingold A., Karlok M., Nielsen H., Engberg J. Phylogenetic evidence for the acquisition of ribosomal RNA introns subsequent to the divergence of some of the major Tetrahymena groups. EMBO J. 1986 Dec 20;5(13):3625–3630. doi: 10.1002/j.1460-2075.1986.tb04691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvanen M. Cross-species gene transfer; implications for a new theory of evolution. J Theor Biol. 1985 Jan 21;112(2):333–343. doi: 10.1016/s0022-5193(85)80291-5. [DOI] [PubMed] [Google Scholar]
- Visser C. M. Evolution of biocatalysis 1. Possible pre-genetic-code RNA catalysts which are their own replicase. Orig Life. 1984;14(1-4):291–300. doi: 10.1007/BF00933670. [DOI] [PubMed] [Google Scholar]
- Visser C. M., Kellogg R. M. Biotin. Its place in evolution. J Mol Evol. 1978 Jun 20;11(2):171–187. doi: 10.1007/BF01733892. [DOI] [PubMed] [Google Scholar]
- Weiner A. M. Eukaryotic nuclear telomeres: molecular fossils of the RNP world? Cell. 1988 Jan 29;52(2):155–158. doi: 10.1016/0092-8674(88)90501-6. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Maizels N. tRNA-like structures tag the 3' ends of genomic RNA molecules for replication: implications for the origin of protein synthesis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7383–7387. doi: 10.1073/pnas.84.21.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. B., 3rd Coenzymes as fossils of an earlier metabolic state. J Mol Evol. 1976 Mar 29;7(2):101–104. doi: 10.1007/BF01732468. [DOI] [PubMed] [Google Scholar]


