Abstract
The title compound, [Zn(C10H11N4O2)2]·2H2O, was prepared by the reaction between Zn(CH3COO)2·2H2O and 2-hydroxyimino-N′-[1-(2-pyridyl)ethylidene]propanohydrazide (Hpop). The central ZnII atom has a distorted tetragonal-bipyramidal coordination geometry formed by two amide O atoms and four N atoms of two azomethine and two pyridine groups. In the crystal, complex molecules form layers parallel to the crystallographic b direction. The layers are connected by O—H⋯N and O—H⋯O hydrogen bonds involving the solvent water molecules.
Related literature
For zinc(II)-containing complexes with similiar ligands, see: Petrusenko et al. (1997 ▶); Comba et al. (2002 ▶); Kasuga et al. (2003 ▶). For the structural parameters of amide derivatives of 2-hydroxyiminopropanoic acid, see: Onindo et al. (1995 ▶); Sliva et al. (1997a
▶,b
▶); Mokhir et al. (2002 ▶); Moroz et al. (2009a
▶,b
▶). For the preparation and characterization of 3d-metal complexes with 2-hydroxyimino-N′-[1-(2-pyridyl)ethylidene]propanohydrazone, see: Moroz et al. (2008a
▶,b
▶).
Experimental
Crystal data
[Zn(C10H11N4O2)2]·2H2O
M r = 539.86
Triclinic,
a = 8.3241 (3) Å
b = 10.6299 (4) Å
c = 13.9006 (5) Å
α = 94.184 (2)°
β = 101.389 (2)°
γ = 108.052 (2)°
V = 1134.48 (7) Å3
Z = 2
Mo Kα radiation
μ = 1.14 mm−1
T = 100 K
0.28 × 0.07 × 0.02 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2008 ▶) T min = 0.743, T max = 0.977
21551 measured reflections
5171 independent reflections
4253 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.092
S = 1.05
5171 reflections
320 parameters
H-atom parameters constrained
Δρmax = 0.95 e Å−3
Δρmin = −0.39 e Å−3
Data collection: COLLECT (Bruker, 2004 ▶); cell refinement: DENZO/SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO/SCALEPACK; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810003351/jh2129sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810003351/jh2129Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Zn1—N2 | 2.061 (2) |
| Zn1—N6 | 2.085 (2) |
| Zn1—O1 | 2.0880 (15) |
| Zn1—O3 | 2.1470 (15) |
| Zn1—N5 | 2.1955 (19) |
| Zn1—N1 | 2.2877 (19) |
| N2—Zn1—O1 | 76.10 (7) |
| N6—Zn1—O3 | 74.17 (6) |
| N6—Zn1—N5 | 75.07 (7) |
| N2—Zn1—N1 | 73.97 (7) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2O⋯N7i | 0.92 | 1.89 | 2.801 (3) | 170 |
| O4—H4O⋯O5ii | 0.93 | 1.77 | 2.675 (3) | 164 |
| O5—H5P⋯O3 | 0.91 | 1.93 | 2.811 (3) | 161 |
| O5—H5O⋯O6 | 0.86 | 2.08 | 2.889 (3) | 157 |
| O6—H6O⋯N4iii | 0.92 | 2.12 | 2.934 (3) | 148 |
| O6—H6P⋯N8ii | 0.93 | 2.10 | 2.971 (3) | 154 |
Symmetry codes: (i) 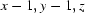 ; (ii)
; (ii) 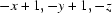 ; (iii)
; (iii) 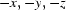 .
.
Acknowledgments
The authors thank the Ministry of Education and Science of Ukraine for financial support (grant No. F28/241–2009).
supplementary crystallographic information
Comment
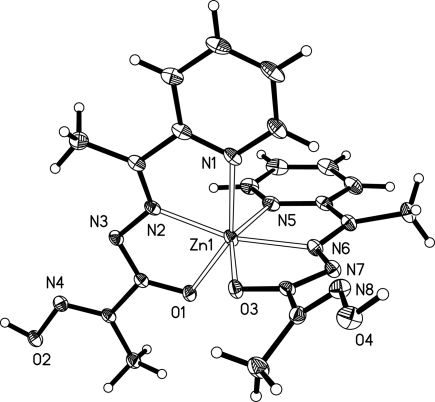
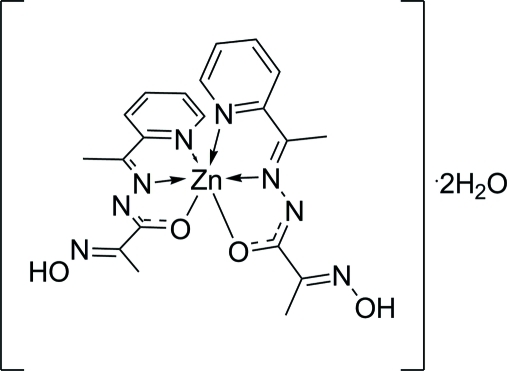
As a part of our study of coordination compounds based on oxime-containing Schiff bases we would like to present the structure of the title compound 1, Fig. 1, which is based on polynucleative strand-type ligand 2-hydroxyimino-N'-[1-(2-pyridyl)ethylidene]propanohydrazone (Hpop) (Fig. 1). It has been shown previously that Hpop is able to form mono- and tetranuclear [2 x 2] grid-like assemblies with 3d-metal ions (Moroz et al., 2008a,b).
The title compound consists of neutral complex molecules and solvating water molecules. Zinc ion has a distorted tetragonal bipyramidal geometry. The coordination polyhedron is formed by two oxygen atoms from the amide groups and four nitrogen atoms belonging to two azomethine and two pyridine groups. The Zn—N and Zn—O bond lengths are comparable to previously reported zinc complexes with thiosemicarbasone and semicarbasone derivatives (Kasuga et al., (2003)), ligands with pyridine groups complexed to the metal ion (Petrusenko et al. (1997), Comba et al., (2002)) and the zinc-containing complex based on Hpop (Moroz et al., 2008b) (Table 1). The bite angles around the central atom deviate from an ideal square-planar configuration, that is a consequence of the formation of four almost flat five-membered chelate rings (Table 1). The ligands exist in complex molecule in singly charged form due to deprotonation of the amide group, C–N, C–O and N–N' bond distances are typical for deprotonated functions. In Hpop the oxime group is situated in anti- position to the amide group which was early shown in the structures of the free ligand and similiar compounds - amide derivatives of 2-hydroxyiminopropanoic acid (Onindo et al. (1995); Sliva et al. (1997a,b); Mokhir et al. (2002); Moroz et al., 2009a,b).
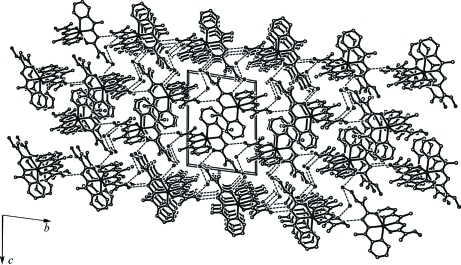
In the crystal packing the molecules of 1 form columns along a crystallographic direction due to hydrogen bonds and π-stacking interaction (Fig. 2). The columns are connected in 3D structure by a variety of hydrogen bonds where solvated water molecules act as donors and O and N atoms of the oxime group and O atom of the amide group of the ligand act as acceptors (Table 2).
Experimental
Zinc(II) acetate (0.011 g, 0.05 mmol) in 5 ml H2O was added to 10 ml of hot methanol solution of Hpop (0.022 g, 0.1 mmol) and followed by 1 ml of alkali solution (0.1 M KOH). The mixture was left for slow evaporation at room temperature. After 5 days cubic yellowish crystals of 1 suitable for X-ray analysis were obtained.
Refinement
The H2O hydrogen atoms were located from the difference Fourier map but constrained to ride on their parent atom, with Uiso = 1.5 Ueq(parent atom). Other hydrogen atoms were positioned geometrically and were also constrained to ride on their parent atoms, with C—H = 0.95-0.98 Å, and Uiso = 1.2-1.5 Ueq(parent atom). The highest peak is located 1.15 Å from atom H5O and the deepest hole is located 0.82 Å from atom Zn1.
Figures
Fig. 1.
1 A view of compound 1, with displacement ellipsoids shown at the 40% probability level.
Fig. 2.
A packing diagram for 1 viewed in projection down the a axis. Hydrogen bonds are indicated by dashed lines; H atoms are omitted for clarity.
Crystal data
| [Zn(C10H11N4O2)2]·2H2O | Z = 2 |
| Mr = 539.86 | F(000) = 560 |
| Triclinic, P1 | Dx = 1.580 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.3241 (3) Å | Cell parameters from 4952 reflections |
| b = 10.6299 (4) Å | θ = 1.0–27.5° |
| c = 13.9006 (5) Å | µ = 1.14 mm−1 |
| α = 94.184 (2)° | T = 100 K |
| β = 101.389 (2)° | Needle, yellow |
| γ = 108.052 (2)° | 0.28 × 0.07 × 0.02 mm |
| V = 1134.48 (7) Å3 |
Data collection
| Nonius KappaCCD diffractometer | 5171 independent reflections |
| Radiation source: fine-focus sealed tube | 4253 reflections with I > 2σ(I) |
| horizontally mounted graphite crystal | Rint = 0.048 |
| Detector resolution: 9 pixels mm-1 | θmax = 27.5°, θmin = 2.4° |
| φ scans and ω scans with κ offset | h = −10→10 |
| Absorption correction: multi-scan (SADABS; Version 2008/1; Sheldrick, 2008) | k = −13→13 |
| Tmin = 0.743, Tmax = 0.977 | l = −18→18 |
| 21551 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.092 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0417P)2 + 0.8841P] where P = (Fo2 + 2Fc2)/3 |
| 5171 reflections | (Δ/σ)max < 0.001 |
| 320 parameters | Δρmax = 0.95 e Å−3 |
| 0 restraints | Δρmin = −0.39 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Zn1 | 0.39746 (3) | 0.27581 (3) | 0.304853 (19) | 0.01820 (9) | |
| O1 | 0.16169 (19) | 0.20226 (15) | 0.34643 (12) | 0.0202 (3) | |
| O2 | −0.32670 (19) | −0.16202 (17) | 0.32111 (13) | 0.0239 (4) | |
| H2O | −0.3576 | −0.2526 | 0.3012 | 0.036* | |
| O3 | 0.30726 (19) | 0.37780 (16) | 0.18911 (12) | 0.0204 (3) | |
| O4 | 0.4981 (2) | 0.70910 (18) | 0.00903 (13) | 0.0290 (4) | |
| H4O | 0.6065 | 0.7601 | 0.0013 | 0.044* | |
| O5 | 0.1803 (2) | 0.1858 (2) | 0.01793 (14) | 0.0367 (4) | |
| H5P | 0.2119 | 0.2596 | 0.0646 | 0.055* | |
| H5O | 0.1264 | 0.1984 | −0.0383 | 0.055* | |
| O6 | 0.0879 (2) | 0.29498 (19) | −0.16109 (14) | 0.0351 (4) | |
| H6O | 0.0697 | 0.2177 | −0.2017 | 0.053* | |
| H6P | 0.2077 | 0.3384 | −0.1471 | 0.053* | |
| N1 | 0.5988 (2) | 0.2424 (2) | 0.22445 (14) | 0.0207 (4) | |
| N2 | 0.3039 (2) | 0.07661 (19) | 0.24489 (14) | 0.0175 (4) | |
| N3 | 0.1450 (2) | 0.00054 (19) | 0.25994 (14) | 0.0181 (4) | |
| N4 | −0.1642 (2) | −0.11100 (19) | 0.29900 (15) | 0.0205 (4) | |
| N5 | 0.5549 (2) | 0.2876 (2) | 0.45404 (14) | 0.0212 (4) | |
| N6 | 0.5524 (2) | 0.4762 (2) | 0.34417 (14) | 0.0190 (4) | |
| N7 | 0.5460 (2) | 0.55914 (19) | 0.27198 (14) | 0.0188 (4) | |
| N8 | 0.5314 (3) | 0.6441 (2) | 0.09127 (15) | 0.0230 (4) | |
| C1 | 0.7541 (3) | 0.3275 (3) | 0.22043 (18) | 0.0258 (5) | |
| H1 | 0.7857 | 0.4185 | 0.2482 | 0.031* | |
| C2 | 0.8707 (3) | 0.2868 (3) | 0.17679 (19) | 0.0306 (6) | |
| H2 | 0.9811 | 0.3486 | 0.1767 | 0.037* | |
| C3 | 0.8235 (3) | 0.1560 (3) | 0.13393 (18) | 0.0288 (6) | |
| H3 | 0.9005 | 0.1263 | 0.1035 | 0.035* | |
| C4 | 0.6620 (3) | 0.0680 (3) | 0.13569 (18) | 0.0255 (5) | |
| H4 | 0.6257 | −0.0224 | 0.1055 | 0.031* | |
| C5 | 0.5538 (3) | 0.1148 (2) | 0.18269 (16) | 0.0203 (5) | |
| C6 | 0.3825 (3) | 0.0232 (2) | 0.19170 (16) | 0.0190 (5) | |
| C7 | 0.3161 (3) | −0.1184 (3) | 0.14258 (19) | 0.0264 (5) | |
| H7A | 0.3898 | −0.1670 | 0.1744 | 0.040* | |
| H7B | 0.3181 | −0.1209 | 0.0722 | 0.040* | |
| H7C | 0.1966 | −0.1606 | 0.1488 | 0.040* | |
| C8 | 0.0873 (3) | 0.0788 (2) | 0.31405 (16) | 0.0165 (4) | |
| C9 | −0.0868 (3) | 0.0123 (2) | 0.33642 (16) | 0.0174 (4) | |
| C10 | −0.1580 (3) | 0.0927 (2) | 0.39855 (18) | 0.0223 (5) | |
| H10A | −0.2852 | 0.0583 | 0.3795 | 0.034* | |
| H10B | −0.1168 | 0.1865 | 0.3883 | 0.034* | |
| H10C | −0.1187 | 0.0860 | 0.4686 | 0.034* | |
| C11 | 0.5453 (3) | 0.1915 (3) | 0.51063 (18) | 0.0249 (5) | |
| H11 | 0.4600 | 0.1061 | 0.4865 | 0.030* | |
| C12 | 0.6559 (3) | 0.2109 (3) | 0.60431 (19) | 0.0302 (6) | |
| H12 | 0.6500 | 0.1389 | 0.6417 | 0.036* | |
| C13 | 0.7726 (3) | 0.3356 (3) | 0.64108 (19) | 0.0305 (6) | |
| H13 | 0.8473 | 0.3514 | 0.7051 | 0.037* | |
| C14 | 0.7817 (3) | 0.4389 (3) | 0.58458 (18) | 0.0270 (5) | |
| H14 | 0.8602 | 0.5265 | 0.6099 | 0.032* | |
| C15 | 0.6724 (3) | 0.4112 (2) | 0.48927 (17) | 0.0212 (5) | |
| C16 | 0.6797 (3) | 0.5119 (2) | 0.42118 (16) | 0.0194 (5) | |
| C17 | 0.8289 (3) | 0.6397 (2) | 0.44150 (18) | 0.0255 (5) | |
| H17A | 0.8476 | 0.6708 | 0.3786 | 0.038* | |
| H17B | 0.9338 | 0.6250 | 0.4774 | 0.038* | |
| H17C | 0.8032 | 0.7074 | 0.4818 | 0.038* | |
| C18 | 0.4150 (3) | 0.4947 (2) | 0.19584 (17) | 0.0183 (4) | |
| C19 | 0.3910 (3) | 0.5686 (2) | 0.10840 (17) | 0.0199 (5) | |
| C20 | 0.2114 (3) | 0.5440 (3) | 0.04913 (19) | 0.0290 (6) | |
| H20A | 0.2124 | 0.6132 | 0.0061 | 0.043* | |
| H20B | 0.1349 | 0.5468 | 0.0940 | 0.043* | |
| H20C | 0.1688 | 0.4560 | 0.0083 | 0.043* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Zn1 | 0.01308 (13) | 0.01868 (15) | 0.01989 (15) | 0.00111 (10) | 0.00272 (10) | 0.00585 (10) |
| O1 | 0.0168 (8) | 0.0165 (8) | 0.0252 (9) | 0.0013 (6) | 0.0066 (6) | 0.0030 (7) |
| O2 | 0.0147 (8) | 0.0208 (9) | 0.0335 (10) | −0.0003 (7) | 0.0097 (7) | 0.0043 (7) |
| O3 | 0.0172 (8) | 0.0173 (8) | 0.0217 (8) | 0.0009 (6) | 0.0003 (6) | 0.0054 (7) |
| O4 | 0.0324 (9) | 0.0346 (10) | 0.0252 (9) | 0.0134 (8) | 0.0100 (7) | 0.0171 (8) |
| O5 | 0.0357 (10) | 0.0396 (12) | 0.0305 (10) | 0.0057 (9) | 0.0089 (8) | 0.0057 (9) |
| O6 | 0.0248 (9) | 0.0351 (11) | 0.0401 (11) | 0.0065 (8) | 0.0062 (8) | −0.0079 (9) |
| N1 | 0.0138 (9) | 0.0276 (11) | 0.0197 (10) | 0.0043 (8) | 0.0034 (7) | 0.0100 (8) |
| N2 | 0.0132 (8) | 0.0207 (10) | 0.0195 (10) | 0.0047 (7) | 0.0051 (7) | 0.0083 (8) |
| N3 | 0.0134 (9) | 0.0175 (10) | 0.0224 (10) | 0.0016 (7) | 0.0067 (7) | 0.0050 (8) |
| N4 | 0.0154 (9) | 0.0199 (10) | 0.0255 (10) | 0.0022 (8) | 0.0084 (8) | 0.0050 (8) |
| N5 | 0.0182 (9) | 0.0266 (11) | 0.0214 (10) | 0.0080 (8) | 0.0076 (8) | 0.0088 (8) |
| N6 | 0.0158 (9) | 0.0230 (10) | 0.0161 (9) | 0.0039 (8) | 0.0025 (7) | 0.0038 (8) |
| N7 | 0.0169 (9) | 0.0198 (10) | 0.0183 (9) | 0.0038 (8) | 0.0035 (7) | 0.0065 (8) |
| N8 | 0.0282 (11) | 0.0239 (11) | 0.0213 (10) | 0.0116 (9) | 0.0080 (8) | 0.0113 (8) |
| C1 | 0.0178 (11) | 0.0333 (14) | 0.0240 (12) | 0.0037 (10) | 0.0042 (9) | 0.0119 (11) |
| C2 | 0.0159 (11) | 0.0499 (18) | 0.0265 (13) | 0.0069 (11) | 0.0083 (10) | 0.0175 (12) |
| C3 | 0.0204 (12) | 0.0486 (17) | 0.0238 (13) | 0.0153 (12) | 0.0098 (10) | 0.0134 (12) |
| C4 | 0.0224 (12) | 0.0377 (15) | 0.0207 (12) | 0.0136 (11) | 0.0072 (10) | 0.0092 (11) |
| C5 | 0.0171 (11) | 0.0318 (14) | 0.0146 (11) | 0.0095 (10) | 0.0044 (9) | 0.0112 (10) |
| C6 | 0.0156 (10) | 0.0267 (13) | 0.0153 (11) | 0.0068 (9) | 0.0037 (8) | 0.0071 (9) |
| C7 | 0.0252 (12) | 0.0289 (14) | 0.0269 (13) | 0.0087 (10) | 0.0110 (10) | 0.0032 (11) |
| C8 | 0.0141 (10) | 0.0185 (11) | 0.0163 (11) | 0.0044 (9) | 0.0023 (8) | 0.0070 (9) |
| C9 | 0.0132 (10) | 0.0196 (12) | 0.0189 (11) | 0.0039 (9) | 0.0040 (8) | 0.0060 (9) |
| C10 | 0.0161 (11) | 0.0220 (12) | 0.0270 (12) | 0.0026 (9) | 0.0072 (9) | 0.0019 (10) |
| C11 | 0.0239 (12) | 0.0290 (14) | 0.0259 (13) | 0.0099 (10) | 0.0104 (10) | 0.0115 (10) |
| C12 | 0.0324 (14) | 0.0428 (16) | 0.0264 (13) | 0.0204 (12) | 0.0137 (11) | 0.0198 (12) |
| C13 | 0.0254 (13) | 0.0471 (17) | 0.0229 (13) | 0.0151 (12) | 0.0068 (10) | 0.0128 (12) |
| C14 | 0.0197 (11) | 0.0385 (15) | 0.0204 (12) | 0.0073 (10) | 0.0022 (9) | 0.0057 (11) |
| C15 | 0.0149 (10) | 0.0300 (13) | 0.0189 (11) | 0.0074 (9) | 0.0052 (9) | 0.0035 (10) |
| C16 | 0.0154 (10) | 0.0240 (12) | 0.0164 (11) | 0.0041 (9) | 0.0028 (8) | 0.0015 (9) |
| C17 | 0.0200 (11) | 0.0252 (13) | 0.0238 (12) | 0.0005 (10) | −0.0001 (9) | 0.0016 (10) |
| C18 | 0.0136 (10) | 0.0202 (12) | 0.0218 (11) | 0.0053 (9) | 0.0054 (9) | 0.0054 (9) |
| C19 | 0.0207 (11) | 0.0176 (11) | 0.0205 (11) | 0.0058 (9) | 0.0034 (9) | 0.0029 (9) |
| C20 | 0.0241 (12) | 0.0321 (15) | 0.0277 (13) | 0.0083 (11) | −0.0011 (10) | 0.0096 (11) |
Geometric parameters (Å, °)
| Zn1—N2 | 2.061 (2) | C3—C4 | 1.385 (3) |
| Zn1—N6 | 2.085 (2) | C3—H3 | 0.9500 |
| Zn1—O1 | 2.0880 (15) | C4—C5 | 1.396 (3) |
| Zn1—O3 | 2.1470 (15) | C4—H4 | 0.9500 |
| Zn1—N5 | 2.1955 (19) | C5—C6 | 1.492 (3) |
| Zn1—N1 | 2.2877 (19) | C6—C7 | 1.491 (3) |
| O1—C8 | 1.268 (3) | C7—H7A | 0.9800 |
| O2—N4 | 1.397 (2) | C7—H7B | 0.9800 |
| O2—H2O | 0.9213 | C7—H7C | 0.9800 |
| O3—C18 | 1.272 (3) | C8—C9 | 1.508 (3) |
| O4—N8 | 1.404 (2) | C9—C10 | 1.495 (3) |
| O4—H4O | 0.9287 | C10—H10A | 0.9800 |
| O5—H5P | 0.9140 | C10—H10B | 0.9800 |
| O5—H5O | 0.8626 | C10—H10C | 0.9800 |
| O6—H6O | 0.9154 | C11—C12 | 1.398 (4) |
| O6—H6P | 0.9335 | C11—H11 | 0.9500 |
| N1—C5 | 1.342 (3) | C12—C13 | 1.367 (4) |
| N1—C1 | 1.343 (3) | C12—H12 | 0.9500 |
| N2—C6 | 1.287 (3) | C13—C14 | 1.388 (4) |
| N2—N3 | 1.385 (2) | C13—H13 | 0.9500 |
| N3—C8 | 1.337 (3) | C14—C15 | 1.404 (3) |
| N4—C9 | 1.284 (3) | C14—H14 | 0.9500 |
| N5—C11 | 1.327 (3) | C15—C16 | 1.475 (3) |
| N5—C15 | 1.357 (3) | C16—C17 | 1.492 (3) |
| N6—C16 | 1.287 (3) | C17—H17A | 0.9800 |
| N6—N7 | 1.387 (3) | C17—H17B | 0.9800 |
| N7—C18 | 1.325 (3) | C17—H17C | 0.9800 |
| N8—C19 | 1.274 (3) | C18—C19 | 1.508 (3) |
| C1—C2 | 1.398 (4) | C19—C20 | 1.491 (3) |
| C1—H1 | 0.9500 | C20—H20A | 0.9800 |
| C2—C3 | 1.375 (4) | C20—H20B | 0.9800 |
| C2—H2 | 0.9500 | C20—H20C | 0.9800 |
| N2—Zn1—N6 | 162.52 (7) | C6—C7—H7A | 109.5 |
| N2—Zn1—O1 | 76.10 (7) | C6—C7—H7B | 109.5 |
| N6—Zn1—O1 | 121.37 (7) | H7A—C7—H7B | 109.5 |
| N2—Zn1—O3 | 105.12 (7) | C6—C7—H7C | 109.5 |
| N6—Zn1—O3 | 74.17 (6) | H7A—C7—H7C | 109.5 |
| O1—Zn1—O3 | 96.43 (6) | H7B—C7—H7C | 109.5 |
| N2—Zn1—N5 | 106.20 (7) | O1—C8—N3 | 127.01 (19) |
| N6—Zn1—N5 | 75.07 (7) | O1—C8—C9 | 117.25 (19) |
| O1—Zn1—N5 | 93.88 (7) | N3—C8—C9 | 115.72 (19) |
| O3—Zn1—N5 | 148.54 (7) | N4—C9—C10 | 124.75 (19) |
| N2—Zn1—N1 | 73.97 (7) | N4—C9—C8 | 116.43 (19) |
| N6—Zn1—N1 | 88.55 (7) | C10—C9—C8 | 118.82 (19) |
| O1—Zn1—N1 | 150.07 (7) | C9—C10—H10A | 109.5 |
| O3—Zn1—N1 | 90.90 (6) | C9—C10—H10B | 109.5 |
| N5—Zn1—N1 | 94.81 (7) | H10A—C10—H10B | 109.5 |
| C8—O1—Zn1 | 111.22 (13) | C9—C10—H10C | 109.5 |
| N4—O2—H2O | 103.0 | H10A—C10—H10C | 109.5 |
| C18—O3—Zn1 | 110.35 (13) | H10B—C10—H10C | 109.5 |
| N8—O4—H4O | 105.6 | N5—C11—C12 | 122.6 (2) |
| H5P—O5—H5O | 111.3 | N5—C11—H11 | 118.7 |
| H6O—O6—H6P | 104.7 | C12—C11—H11 | 118.7 |
| C5—N1—C1 | 118.2 (2) | C13—C12—C11 | 118.7 (2) |
| C5—N1—Zn1 | 112.02 (14) | C13—C12—H12 | 120.7 |
| C1—N1—Zn1 | 129.54 (18) | C11—C12—H12 | 120.7 |
| C6—N2—N3 | 119.76 (19) | C12—C13—C14 | 119.8 (2) |
| C6—N2—Zn1 | 123.02 (15) | C12—C13—H13 | 120.1 |
| N3—N2—Zn1 | 117.15 (14) | C14—C13—H13 | 120.1 |
| C8—N3—N2 | 108.51 (18) | C13—C14—C15 | 118.6 (2) |
| C9—N4—O2 | 112.04 (18) | C13—C14—H14 | 120.7 |
| C11—N5—C15 | 119.1 (2) | C15—C14—H14 | 120.7 |
| C11—N5—Zn1 | 127.97 (17) | N5—C15—C14 | 121.1 (2) |
| C15—N5—Zn1 | 112.92 (14) | N5—C15—C16 | 116.1 (2) |
| C16—N6—N7 | 120.20 (19) | C14—C15—C16 | 122.7 (2) |
| C16—N6—Zn1 | 119.99 (16) | N6—C16—C15 | 114.0 (2) |
| N7—N6—Zn1 | 117.29 (14) | N6—C16—C17 | 125.2 (2) |
| C18—N7—N6 | 108.84 (18) | C15—C16—C17 | 120.8 (2) |
| C19—N8—O4 | 111.39 (19) | C16—C17—H17A | 109.5 |
| N1—C1—C2 | 122.3 (3) | C16—C17—H17B | 109.5 |
| N1—C1—H1 | 118.9 | H17A—C17—H17B | 109.5 |
| C2—C1—H1 | 118.9 | C16—C17—H17C | 109.5 |
| C3—C2—C1 | 119.1 (2) | H17A—C17—H17C | 109.5 |
| C3—C2—H2 | 120.5 | H17B—C17—H17C | 109.5 |
| C1—C2—H2 | 120.5 | O3—C18—N7 | 126.8 (2) |
| C2—C3—C4 | 119.1 (2) | O3—C18—C19 | 116.84 (19) |
| C2—C3—H3 | 120.4 | N7—C18—C19 | 116.34 (19) |
| C4—C3—H3 | 120.4 | N8—C19—C20 | 126.5 (2) |
| C3—C4—C5 | 118.7 (3) | N8—C19—C18 | 114.9 (2) |
| C3—C4—H4 | 120.6 | C20—C19—C18 | 118.58 (19) |
| C5—C4—H4 | 120.6 | C19—C20—H20A | 109.5 |
| N1—C5—C4 | 122.5 (2) | C19—C20—H20B | 109.5 |
| N1—C5—C6 | 116.25 (19) | H20A—C20—H20B | 109.5 |
| C4—C5—C6 | 121.2 (2) | C19—C20—H20C | 109.5 |
| N2—C6—C7 | 125.1 (2) | H20A—C20—H20C | 109.5 |
| N2—C6—C5 | 114.6 (2) | H20B—C20—H20C | 109.5 |
| C7—C6—C5 | 120.3 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2O···N7i | 0.92 | 1.89 | 2.801 (3) | 170 |
| O4—H4O···O5ii | 0.93 | 1.77 | 2.675 (3) | 164 |
| O5—H5P···O3 | 0.91 | 1.93 | 2.811 (3) | 161 |
| O5—H5O···O6 | 0.86 | 2.08 | 2.889 (3) | 157 |
| O6—H6O···N4iii | 0.92 | 2.12 | 2.934 (3) | 148 |
| O6—H6P···N8ii | 0.93 | 2.10 | 2.971 (3) | 154 |
Symmetry codes: (i) x−1, y−1, z; (ii) −x+1, −y+1, −z; (iii) −x, −y, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JH2129).
References
- Bruker (2004). COLLECT Bruker AXS Inc., Madison, Wisconsin, USA.
- Comba, P., Kerscher, M., Merz, M., Muller, V., Pritzkow, H., Remenyi, R., Schiek, W. & Xiong, Y. (2002). Chem. Eur. J.8, 5750–5760. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Kasuga, N. C., Sekino, K., Ishikawa, M., Honda, A., Yokoyama, M., Nakano, S., Shimada, N., Koumo, S. & Nomiya, K. (2003). J. Inorg. Biochem.96, 298–310. [DOI] [PubMed]
- Mokhir, A. A., Gumienna-Kontecka, E. S., Wiatek-Kozłowska, J., Petkova, E. G., Fritsky, I. O., Jerzykiewicz, L., Kapshuk, A. A. & Sliva, T. Yu. (2002). Inorg. Chim. Acta, 329, 113–121.
- Moroz, Yu. S., Kulon, K., Haukka, M., Gumienna-Kontecka, E., Kozłowski, H., Meyer, F. & Fritsky, I. O. (2008a). Inorg. Chem.47, 5656–5665. [DOI] [PubMed]
- Moroz, Y. S., Sliva, T. Yu., Kulon, K., Kozłowski, H. & Fritsky, I. O. (2008b). Acta Cryst. E64, m353–m354. [DOI] [PMC free article] [PubMed]
- Moroz, Y. S., Kalibabchuk, V. A., Gumienna-Kontecka, E., Skopenko, V. V. & Pavlova, S. V. (2009a). Acta Cryst. E65, o2413. [DOI] [PMC free article] [PubMed]
- Moroz, Y. S., Konovalova, I. S., Iskenderov, T. S., Pavlova, S. V. & Shishkin, O. V. (2009b). Acta Cryst. E65, o2242. [DOI] [PMC free article] [PubMed]
- Onindo, C. O., Sliva, T. Yu., Kowalik-Jankowska, T., Fritsky, I. O., Buglyo, P., Pettit, L. D., Kozłowski, H. & Kiss, T. (1995). J. Chem. Soc. Dalton Trans. pp. 3911–3915.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Petrusenko, S. R., Kokozay, V. N. & Fritsky, I. O. (1997). Polyhedron, 16, 267–274.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sliva, T. Yu., Duda, A. M., Głowiak, T., Fritsky, I. O., Amirkhanov, V. M., Mokhir, A. A. & Kozłowski, H. (1997a). J. Chem. Soc. Dalton Trans. pp. 273–276.
- Sliva, T. Yu., Kowalik-Jankowska, T., Amirkhanov, V. M., Głowiak, T., Onindo, C. O., Fritsky, I. O. & Kozłowski, H. (1997b). J. Inorg. Biochem.65, 287–294.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810003351/jh2129sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810003351/jh2129Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report