Abstract
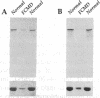
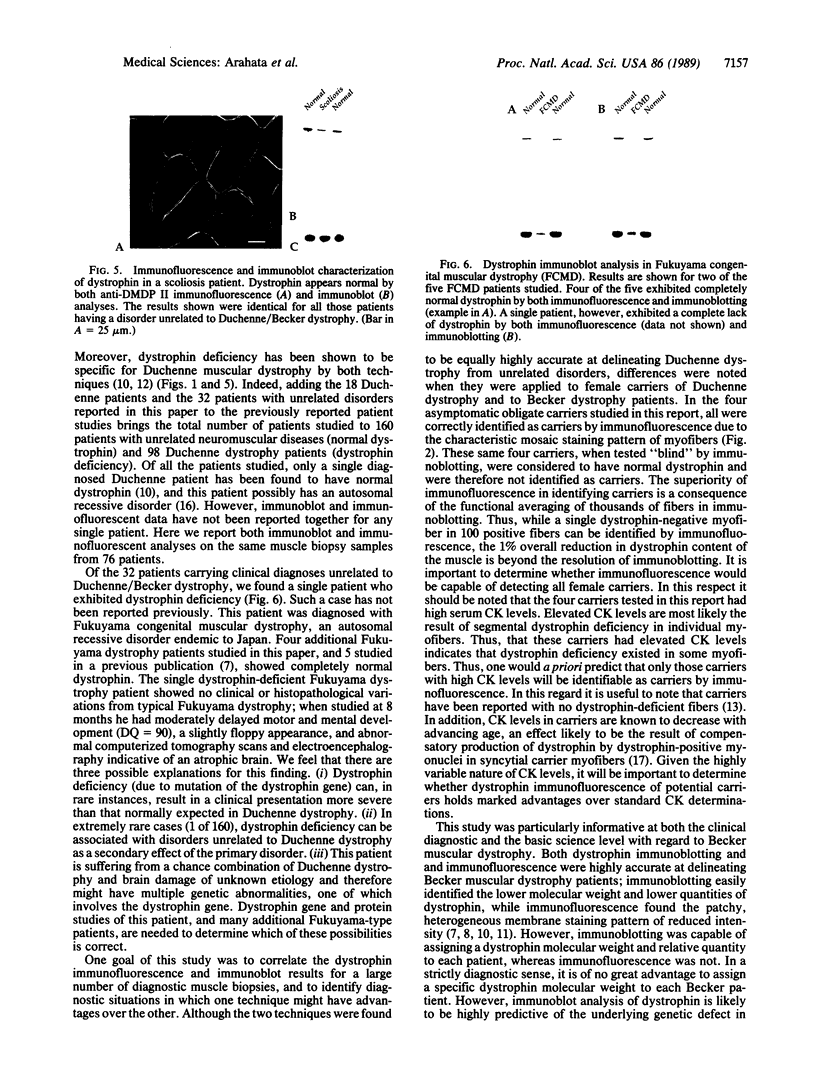
Immunoblot characterization and immunofluorescence localization of dystrophin are presented for 76 human patients with various neuromuscular diseases. Normal dystrophin (shown by immunoblotting) was invariably visualized as a continuous, peripheral membrane immunostaining of myofibers. Biochemical abnormalities of dystrophin (either lower or higher molecular weight dystrophin) resulted in patchy, discontinuous immunostaining, suggesting that the abnormal dystrophin proteins are not capable of creating a complete membrane cytoskeleton network. There was a very strong correlation of clinical diagnoses with the type of dystrophin abnormality; all Duchenne muscular dystrophy patient muscle contained no detectable dystrophin, Becker muscular dystrophy patient muscle had clearly abnormal dystrophin, and unrelated diseases showed normal dystrophin. However, a single patient of five carrying the diagnosis of Fukuyama dystrophy showed no detectable dystrophin and thus appeared to be a Duchenne dystrophy patient by the biochemical assays. We know of no other case of a patient with a disease thought to be unrelated to Duchenne/Becker dystrophy yet demonstrating dystrophin deficiency. Based on the data presented, we conclude that immunofluorescence is the best technique for the detection of female carriers of Duchenne dystrophy, whereas immunoblotting appears superior for the prognostic diagnosis of Becker muscular dystrophy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Engel A. G. Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984 Aug;16(2):193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- Arahata K., Ishihara T., Kamakura K., Tsukahara T., Ishiura S., Baba C., Matsumoto T., Nonaka I., Sugita H. Mosaic expression of dystrophin in symptomatic carriers of Duchenne's muscular dystrophy. N Engl J Med. 1989 Jan 19;320(3):138–142. doi: 10.1056/NEJM198901193200302. [DOI] [PubMed] [Google Scholar]
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Schmidt B., Samitt C. E., Miranda A. F., Hays A. P., de Oliveira A. B., Chang H. W., Servidei S., Ricci E., Younger D. S. Normal and dystrophin-deficient muscle fibers in carriers of the gene for Duchenne muscular dystrophy. Am J Pathol. 1988 Dec;133(3):440–445. [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Fischbeck K. H., Brown R. H., Johnson M., Medori R., Loike J. D., Harris J. B., Waterston R., Brooke M., Specht L. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988 May 26;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Knudson C. M., Campbell K. P., Kunkel L. M. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987 Dec 24;330(6150):754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989 Jan;2(1):1019–1029. doi: 10.1016/0896-6273(89)90226-2. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Kunkel L. M. Cloning of the Duchenne/Becker muscular dystrophy locus. Adv Hum Genet. 1988;17:61–98. doi: 10.1007/978-1-4613-0987-1_3. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Zatz M., Passos-Bueno M. R., Rapaport D. Estimate of the proportion of Duchenne muscular dystrophy with autosomal recessive inheritance. Am J Med Genet. 1989 Mar;32(3):407–410. doi: 10.1002/ajmg.1320320328. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]