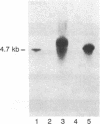
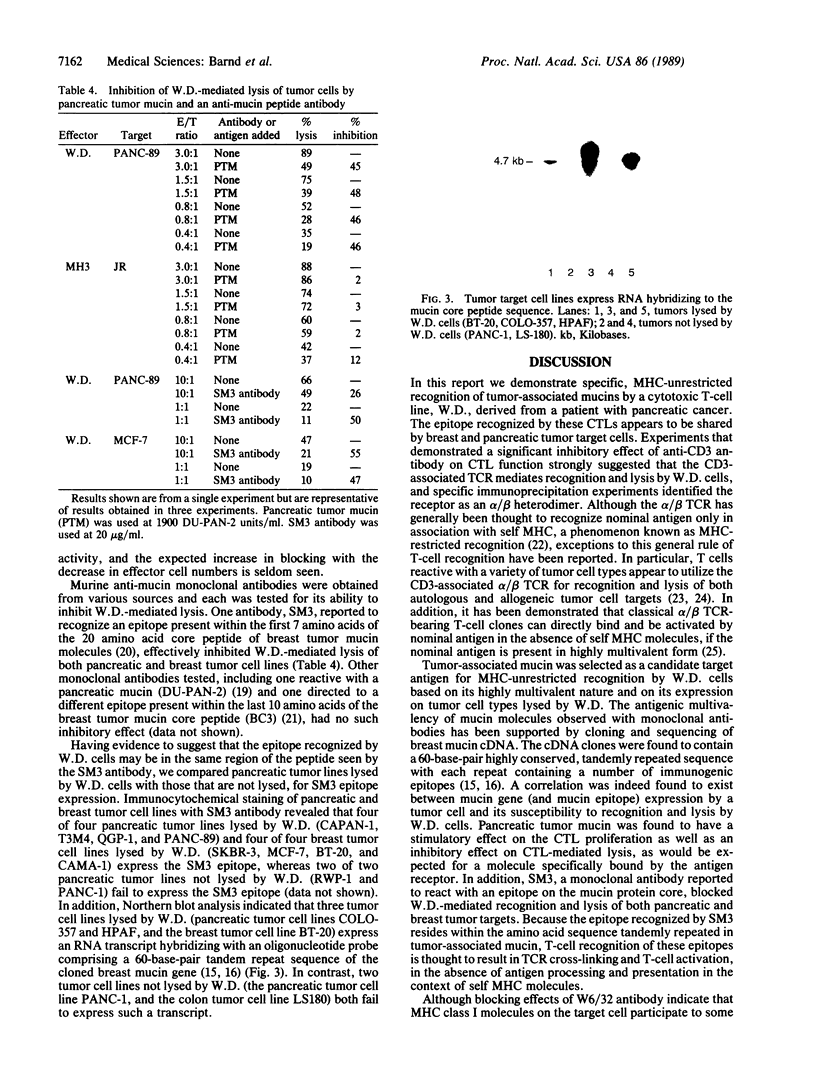
Abstract
We have previously reported the establishment of cytotoxic T-cell lines from pancreatic cancer patients, by continuously stimulating tumor-draining lymph node cells with allogeneic pancreatic tumor cell lines. After the preliminary characterization of their phenotype and tumor specificity, detailed studies performed with one of the cell lines, W.D., show that it recognizes a specific antigen, a large and heavily glycosylated mucin molecule, expressed on pancreatic and breast tumors and tumor cell lines. Although this recognition appears major histocompatibility complex (MHC)-unrestricted, the antigen receptor used by the cytotoxic T cell is the alpha/beta heterodimer, typically found on MHC-restricted T cells. The target antigen is atypical, however, in its ability to directly bind and activate the T cells in the absence of self MHC, presumably by abundant and regularly repeated antigenic epitopes. These findings are important because they demonstrate a specific T-cell response against a human tumor-associated antigen. In addition to pancreatic and breast tumors, various mucin molecules are known to be produced by other tumors of epithelial cell origin and could be expected to stimulate similar T-cell-mediated immune responses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anichini A., Fossati G., Parmiani G. Clonal analysis of cytotoxic T-lymphocyte response to autologous human metastatic melanoma. Int J Cancer. 1985 May 15;35(5):683–689. doi: 10.1002/ijc.2910350518. [DOI] [PubMed] [Google Scholar]
- Barnd D. L., Kerr L. A., Metzgar R. S., Finn O. J. Human tumor-specific cytotoxic T cell lines generated from tumor-draining lymph node infiltrate. Transplant Proc. 1988 Apr;20(2):339–341. [PubMed] [Google Scholar]
- Burchell J., Gendler S., Taylor-Papadimitriou J., Girling A., Lewis A., Millis R., Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987 Oct 15;47(20):5476–5482. [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Carossino A. M., Giordani R., Selli C., Talini G., Reali E., Novelli A., Pistoia V., Ferrarini M. Characterization of cells from invaded lymph nodes in patients with solid tumors. Lymphokine requirement for tumor-specific lymphoproliferative response. J Exp Med. 1987 Aug 1;166(2):303–318. doi: 10.1084/jem.166.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V., Bourge J. F., Guglielmi P., Mathieu-Mahul D., Degos L., Bensussan A. Human T cell clones use a CD3-associated surface antigen recognition structure to exhibit both NK-like and allogeneic cytotoxic reactivity. J Immunol. 1987 May 1;138(9):2831–2836. [PubMed] [Google Scholar]
- Frankel A. E., Ring D. B., Tringale F., Hsieh-Ma S. T. Tissue distribution of breast cancer-associated antigens defined by monoclonal antibodies. J Biol Response Mod. 1985 Jun;4(3):273–286. [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Hersey P., MacDonald M., Schibeci S., Burns C. Clonal analysis of cytotoxic T lymphocytes (CTL) against autologous melanoma. Classification based on phenotype, specificity and inhibition by monoclonal antibodies to T cell structures. Cancer Immunol Immunother. 1986;22(1):15–23. doi: 10.1007/BF00205711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannides C. G., Itoh K., Fox F. E., Pahwa R., Good R. A., Platsoucas C. D. Identification of a second T-cell antigen receptor in human and mouse by an anti-peptide gamma-chain-specific monoclonal antibody. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4244–4248. doi: 10.1073/pnas.84.12.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lan M. S., Finn O. J., Fernsten P. D., Metzgar R. S. Isolation and properties of a human pancreatic adenocarcinoma-associated antigen, DU-PAN-2. Cancer Res. 1985 Jan;45(1):305–310. [PubMed] [Google Scholar]
- Lan M. S., Khorrami A., Kaufman B., Metzgar R. S. Molecular characterization of a mucin-type antigen associated with human pancreatic cancer. The DU-PAN-2 antigen. J Biol Chem. 1987 Sep 15;262(26):12863–12870. [PubMed] [Google Scholar]
- Metzgar R. S., Gaillard M. T., Levine S. J., Tuck F. L., Bossen E. H., Borowitz M. J. Antigens of human pancreatic adenocarcinoma cells defined by murine monoclonal antibodies. Cancer Res. 1982 Feb;42(2):601–608. [PubMed] [Google Scholar]
- Metzgar R. S., Rodriguez N., Finn O. J., Lan M. S., Daasch V. N., Fernsten P. D., Meyers W. C., Sindelar W. F., Sandler R. S., Seigler H. F. Detection of a pancreatic cancer-associated antigen (DU-PAN-2 antigen) in serum and ascites of patients with adenocarcinoma. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5242–5246. doi: 10.1073/pnas.81.16.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli C., Metzgar R. S., Chedid M., Ward F., Finn O. J. Long-term culture and characterization of alloreactive T-cell infiltrates from renal needle biopsies. Hum Immunol. 1985 Nov;14(3):295–304. doi: 10.1016/0198-8859(85)90237-x. [DOI] [PubMed] [Google Scholar]
- Miceli M. C., Barry T. S., Finn O. J. Human allograft-derived T-cell lines: donor class I- and class II-directed cytotoxicity and repertoire stability in sequential biopsies. Hum Immunol. 1988 Jul;22(3):185–198. doi: 10.1016/0198-8859(88)90028-6. [DOI] [PubMed] [Google Scholar]
- Mitchell M. S., Kan-Mitchell J., Kempf R. A., Harel W., Shau H. Y., Lind S. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res. 1988 Oct 15;48(20):5883–5893. [PubMed] [Google Scholar]
- Siddiqui J., Abe M., Hayes D., Shani E., Yunis E., Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano R. F., Hemesath T. J., Pratt J. C., Dintzis R. Z., Dintzis H. M., Acuto O., Shin H. S., Reinherz E. L. Direct evidence for the existence of nominal antigen binding sites on T cell surface Ti alpha-beta heterodimers of MHC-restricted T cell clones. Cell. 1986 Oct 24;47(2):161–171. doi: 10.1016/0092-8674(86)90439-3. [DOI] [PubMed] [Google Scholar]
- Slovin S. F., Lackman R. D., Ferrone S., Kiely P. E., Mastrangelo M. J. Cellular immune response to human sarcomas: cytotoxic T cell clones reactive with autologous sarcomas. I. Development, phenotype, and specificity. J Immunol. 1986 Nov 1;137(9):3042–3048. [PubMed] [Google Scholar]
- Xing P. X., Tjandra J. J., Reynolds K., McLaughlin P. J., Purcell D. F., McKenzie I. F. Reactivity of anti-human milk fat globule antibodies with synthetic peptides. J Immunol. 1989 May 15;142(10):3503–3509. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]