Abstract
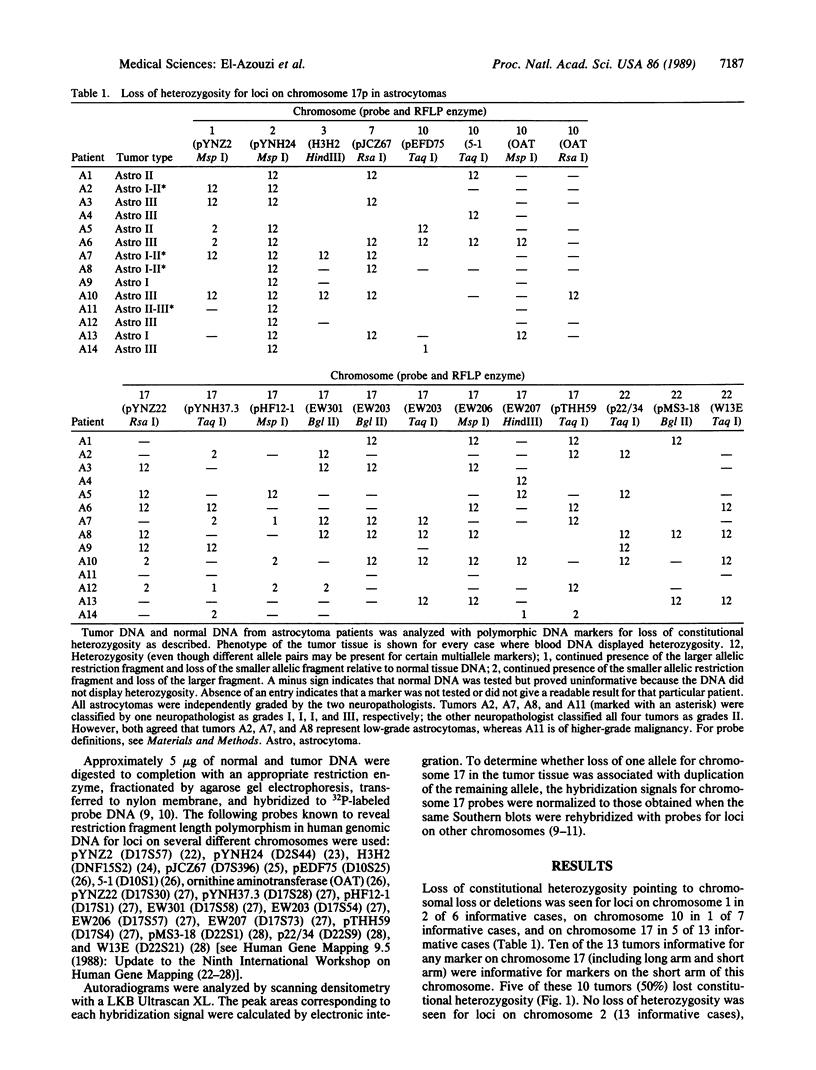
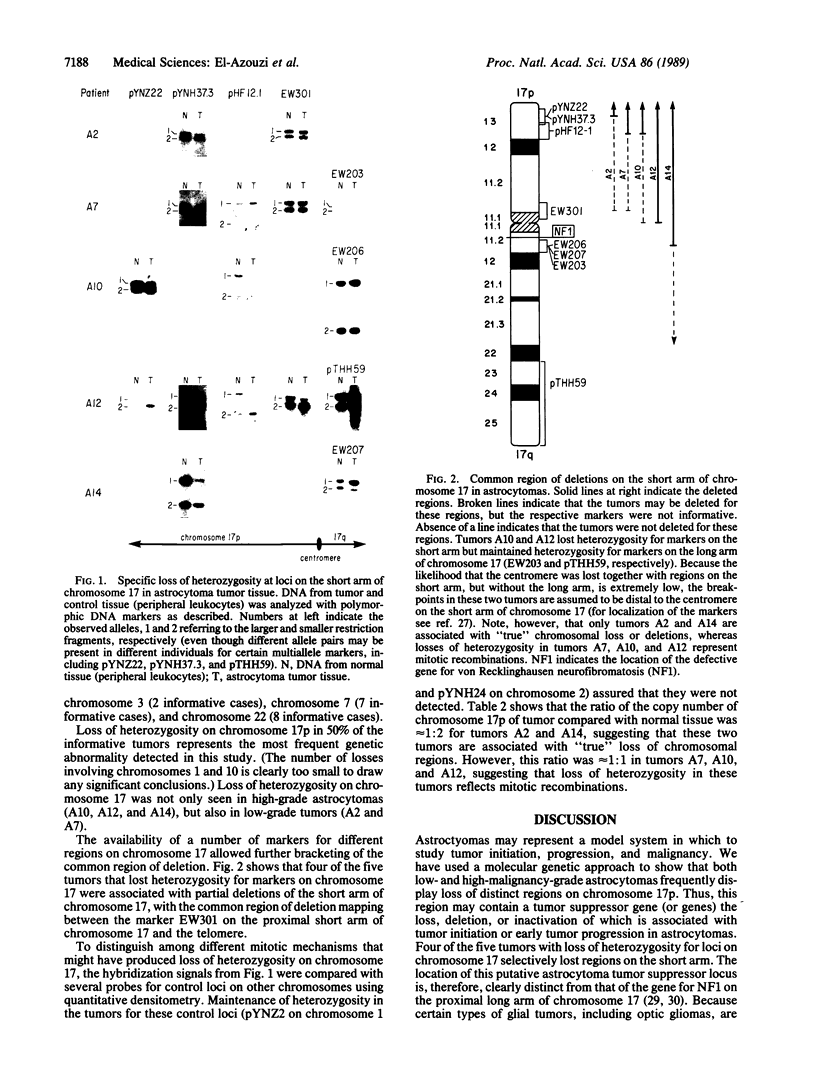
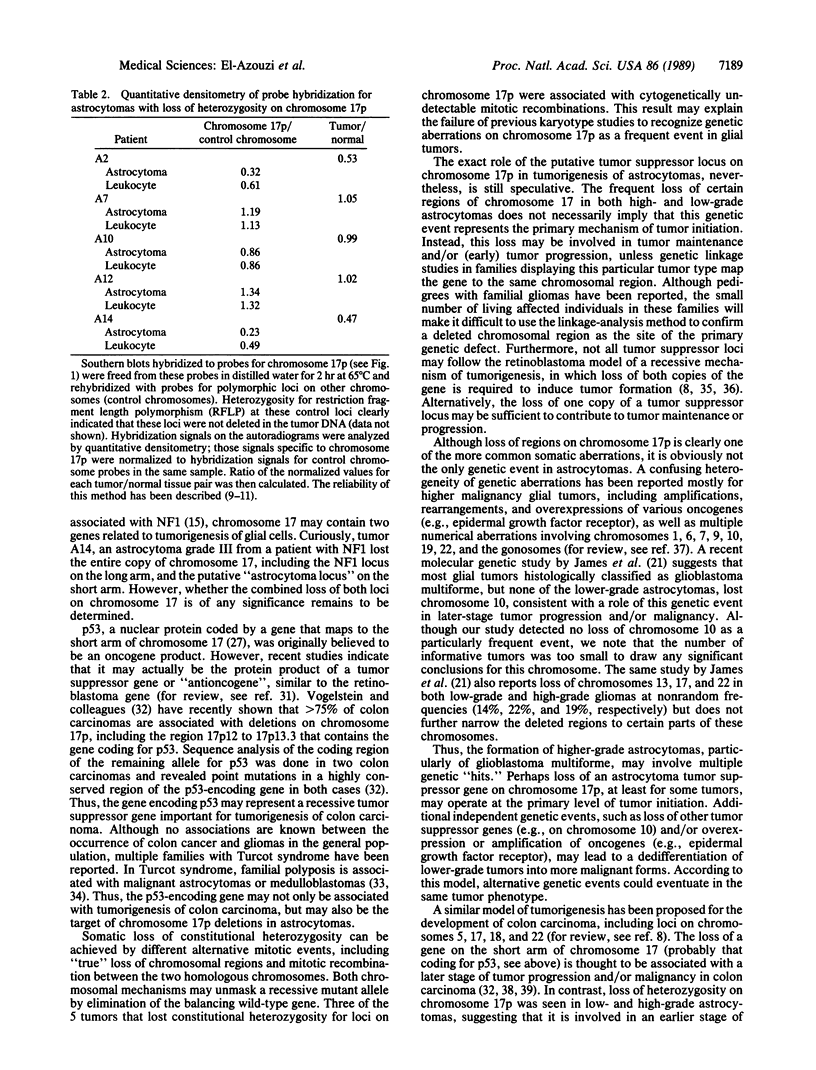
Astrocytomas, including glioblastoma multiforme, represent the most frequent and deadly primary neoplasms of the human nervous system. Despite a number of previous cytogenetic and oncogene studies primarily focusing on malignant astrocytomas, the primary mechanism of tumor initiation has remained obscure. The loss or inactivation of "tumor suppressor" genes are thought to play a fundamental role in the development of many human cancers. Thus, we have analyzed astrocytomas of various histological malignancy grades with polymorphic DNA markers to search for specific chromosomal deletions potentially pointing to loci containing tumor suppressor genes. Loss of constitutional heterozygosity indicating chromosomal loss or deletions was most frequently seen for markers on the short arm of chromosome 17 in 50% of the informative tumors (5 of 10 informative cases) and, to a lesser extent, for markers on chromosomes 1 and 10. Deletions on chromosome 17p were seen in both low-grade and high-grade malignant astrocytomas, suggesting that this chromosome may contain a tumor suppressor gene associated with the early events in tumorigenesis. The common region of deletions on the short arm of chromosome 17 is, therefore, clearly distinct from the gene causing von Recklinghausen neurofibromatosis (NF1), a tumor syndrome associated with glial tumors that maps to the long arm of chromosome 17. The search for progressively smaller deletions on chromosome 17p in astrocytomas may be the way to clone and characterize this locus, thus leading to insights into normal and abnormal growth and differentiation of glial cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer H. E., Glick D. M. The mechanism of activation of porcine pepsinogen. Nature. 1986 Aug 14;322(6080):664–664. doi: 10.1038/322664a0. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Barker D., Wright E., Nguyen K., Cannon L., Fain P., Goldgar D., Bishop D. T., Carey J., Baty B., Kivlin J. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987 May 29;236(4805):1100–1102. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- Baughman F. A., Jr, List C. F., Williams J. R., Muldoon J. P., Segarra J. M., Volkel J. S. The glioma-polyposis syndrome. N Engl J Med. 1969 Dec 11;281(24):1345–1346. doi: 10.1056/NEJM196912112812407. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Burger P. C., Mahaley M. S., Jr, Bullard D. E., Muhlbaier L. H., Bigner D. D. Specific chromosomal abnormalities in malignant human gliomas. Cancer Res. 1988 Jan 15;48(2):405–411. [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Mahaley M. S., Bigner D. D. Patterns of the early, gross chromosomal changes in malignant human gliomas. Hereditas. 1984;101(1):103–113. doi: 10.1111/j.1601-5223.1984.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Burger P. C. Malignant astrocytic neoplasms: classification, pathologic anatomy, and response to treatment. Semin Oncol. 1986 Mar;13(1):16–26. [PubMed] [Google Scholar]
- Caputy A. J., McCullough D. C., Manz H. J., Patterson K., Hammock M. K. A review of the factors influencing the prognosis of medulloblastoma. The importance of cell differentiation. J Neurosurg. 1987 Jan;66(1):80–87. doi: 10.3171/jns.1987.66.1.0080. [DOI] [PubMed] [Google Scholar]
- Daumas-Duport C., Scheithauer B., O'Fallon J., Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988 Nov 15;62(10):2152–2165. doi: 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Goldgar D. E., Green P., Parry D. M., Mulvihill J. J. Multipoint linkage analysis in neurofibromatosis type I: an international collaboration. Am J Hum Genet. 1989 Jan;44(1):6–12. [PMC free article] [PubMed] [Google Scholar]
- Green M. R. When the products of oncogenes and anti-oncogenes meet. Cell. 1989 Jan 13;56(1):1–3. doi: 10.1016/0092-8674(89)90975-6. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Retinoblastoma and the progression of tumor genetics. Trends Genet. 1988 May;4(5):125–128. doi: 10.1016/0168-9525(88)90134-5. [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- James C. D., Carlbom E., Nordenskjold M., Collins V. P., Cavenee W. K. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Emanuel B. Report of the committee on the genetic constitution of chromosome 22. Cytogenet Cell Genet. 1988;49(1-3):104–106. doi: 10.1159/000132661. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. J., Olschwang S., Monpezat J. P., Lefrançois D., Jagelman D., Petrelli N. J., Thomas G., Feinberg A. P. Concerted nonsyntenic allelic loss in human colorectal carcinoma. Science. 1988 Aug 19;241(4868):961–965. doi: 10.1126/science.2841761. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Martuza R. L., Eldridge R. Neurofibromatosis 2 (bilateral acoustic neurofibromatosis). N Engl J Med. 1988 Mar 17;318(11):684–688. doi: 10.1056/NEJM198803173181106. [DOI] [PubMed] [Google Scholar]
- Martuza R. L., Seizinger B. R., Jacoby L. B., Rouleau G. A., Gusella J. F. The molecular biology of human glial tumors. Trends Neurosci. 1988 Jan;11(1):22–27. doi: 10.1016/0166-2236(88)90045-8. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Bishop D. T. Report of the committee on the genetic constitution of chromosome 3. Cytogenet Cell Genet. 1988;49(1-3):48–51. doi: 10.1159/000132648. [DOI] [PubMed] [Google Scholar]
- Packer R. J., Sutton L. N., Rorke L. B., Littman P. A., Sposto R., Rosenstock J. G., Bruce D. A., Schut L. Prognostic importance of cellular differentiation in medulloblastoma of childhood. J Neurosurg. 1984 Aug;61(2):296–301. doi: 10.3171/jns.1984.61.2.0296. [DOI] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Povey S., Falk C. T. Report of the committee on the genetic constitution of chromosome 2. Cytogenet Cell Genet. 1988;49(1-3):46–47. doi: 10.1159/000132647. [DOI] [PubMed] [Google Scholar]
- Rey J. A., Bello M. J., de Campos J. M., Kusak M. E., Ramos C., Benitez J. Chromosomal patterns in human malignant astrocytomas. Cancer Genet Cytogenet. 1987 Dec;29(2):201–221. doi: 10.1016/0165-4608(87)90232-9. [DOI] [PubMed] [Google Scholar]
- Riccardi V. M. Von Recklinghausen neurofibromatosis. N Engl J Med. 1981 Dec 31;305(27):1617–1627. doi: 10.1056/NEJM198112313052704. [DOI] [PubMed] [Google Scholar]
- Rouleau G. A., Wertelecki W., Haines J. L., Hobbs W. J., Trofatter J. A., Seizinger B. R., Martuza R. L., Superneau D. W., Conneally P. M., Gusella J. F. Genetic linkage of bilateral acoustic neurofibromatosis to a DNA marker on chromosome 22. Nature. 1987 Sep 17;329(6136):246–248. doi: 10.1038/329246a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Farmer G. E., Haines J. L., Ozelius L. J., Anderson K., Korf B. R., Parry D. M., Pericak-Vance M. A., Mulvihill J. J., Menon A. Flanking markers for the gene causing von Recklinghausen neurofibromatosis (NF1). Am J Hum Genet. 1989 Jan;44(1):30–32. [PMC free article] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G., Ozelius L. J., Lane A. H., St George-Hyslop P., Huson S., Gusella J. F., Martuza R. L. Common pathogenetic mechanism for three tumor types in bilateral acoustic neurofibromatosis. Science. 1987 Apr 17;236(4799):317–319. doi: 10.1126/science.3105060. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., de la Monte S., Atkins L., Gusella J. F., Martuza R. L. Molecular genetic approach to human meningioma: loss of genes on chromosome 22. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5419–5423. doi: 10.1073/pnas.84.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro W. R. Therapy of adult malignant brain tumors: what have the clinical trials taught us? Semin Oncol. 1986 Mar;13(1):38–45. [PubMed] [Google Scholar]
- Sherman S. L., Bruns G. A. Report of the committee on the genetic constitution of chromosome 1. Cytogenet Cell Genet. 1988;49(1-3):39–45. doi: 10.1159/000132646. [DOI] [PubMed] [Google Scholar]
- Smith M., Simpson N. E. Report of the committee on the genetic constitution of chromosomes 9 and 10. Cytogenet Cell Genet. 1988;49(1-3):71–78. doi: 10.1159/000132653. [DOI] [PubMed] [Google Scholar]
- Solomon E., Barker D. Report of the committee on the genetic constitution of chromosome 17. Cytogenet Cell Genet. 1988;49(1-3):95–98. doi: 10.1159/000132658. [DOI] [PubMed] [Google Scholar]
- TURCOT J., DESPRES J. P., ST PIERRE F. Malignant tumors of the central nervous system associated with familial polyposis of the colon: report of two cases. Dis Colon Rectum. 1959 Sep-Oct;2:465–468. doi: 10.1007/BF02616938. [DOI] [PubMed] [Google Scholar]
- Tsui L. C., Farrall M., Donis-Keller H. Report of the committee on the genetic constitution of chromosomes 7 and 8. Cytogenet Cell Genet. 1988;49(1-3):60–70. doi: 10.1159/000132652. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]



