Abstract
Objectives
Inflammatory processes may influence the risk of epithelial ovarian cancer, but available epidemiological evidence is limited and indirect. Circulating C-reactive protein (CRP), a sensitive marker of inflammation, may serve as a direct biological marker of an underlying association.
Methods
The association between ovarian cancer risk and pre-diagnostic circulating CRP was tested in a case–control study nested within three prospective cohorts from Sweden, USA, and Italy. The study included 237 cases and 427 individually matched controls. CRP was measured in stored blood samples by high-sensitivity immunoturbidimetric assay. Odds ratios (OR) and 95% confidence intervals (CI) were calculated by conditional logistic regression.
Results
Overall, CRP was not related to risk of ovarian cancer. However, a marked increase in risk was observed for CRP concentrations > 10 mg/l: OR (95% CI) 4.4 (1.8–10.9), which remained significant after limiting analyses to cases diagnosed more than two or five years after blood donation (OR 3.0 (1.2–8.0) and 3.6 (1.0–13.2), respectively). Risk of mucinous tumors increased with high CRP, but the number of cases in this analysis was small.
Conclusion
Study results offer additional support to the concept that chronic inflammation plays a role in epithelial ovarian cancer.
Keywords: C-reactive protein, Ovarian cancer, Inflammation, Prospective study
Introduction
Epidemiological evidence suggests that inflammatory processes may be involved in the pathogenesis of ovarian cancer [1]. Conditions associated with increased inflammation of the ovaries, such as ovulation [2, 3], pelvic inflammatory disease [4], endometriosis [5], polycystic ovary syndrome [6], and exposure to talc and asbestos [7] have been associated with increased risk of ovarian cancer. In contrast, factors that may prevent both exogenous and endogenous (e.g., retrograde menstrual bleeding) irritants reaching the ovaries and thereby reducing ovarian inflammation such as tubal ligation [8] and hysterectomy [9, 10], or the use of anti-inflammatory drugs [11–13] confer protection. It is believed that inflammatory processes could contribute to both early and late stages of tumorigenesis [14] by causing irreversible DNA damage, genomic instability, epigenetic changes and subsequent inappropriate gene expression, enhanced proliferation of initiated cells, resistance to apoptosis, increase in tumor neovascularization, invasion, and metastasis [15].
C-reactive protein (CRP) is highly sensitive as a marker of inflammation (its concentrations can rise up to 1,000-fold). It is produced primarily by the hepatocytes and released into the circulation in response to tissue injury and inflammation [16]. The increase in circulating CRP is non-specific and may reflect underlying inflammatory processes at a variety of anatomic sites [16]. Blood concentrations exceeding 10–20 mg/l indicate the presence of an acute inflammatory state, while intermediate values denote low-grade chronic inflammation [16]. Recently, several well-designed studies, albeit not large, showed that long before clinical diagnosis (>3–5 years) cancer patients have higher circulating CRP than healthy controls [17–19]. So far, only one multicenter prospective cohort study had accrued a number of cases large enough to explore the association of pre-diagnostic CRP specifically in relation to ovarian cancer [20]. A significant, positive dose-response relationship was observed across the increasing thirds of CRP concentrations and persisted after exclusion of cases diagnosed within five years of follow-up, suggesting that low-grade chronic inflammation may be a factor during the very early stages of cancer growth [20]. These initial observations are intriguing and need independent confirmation and replication.
We extended an existing case–control study nested within three prospective cohorts in Northern Sweden, Italy, and the United States to test the hypothesis that low-grade chronic inflammation, as reflected by increased pre-diagnostic circulating concentrations of CRP, is positively associated with risk of ovarian cancer.
Materials and methods
Study population
Three prospective cohorts: the Northern Sweden Health and Disease Study (NSHDS), the New York University Women’s Health Study (NYUWHS), and the Study of Hormones and Diet in the Etiology of Breast Cancer (ORDET) have long combined their resources to address the role of pre-diagnostic endogenous hormones in gynecological cancers. Detailed description of each cohort [21–23] and the collaborative studies have been reported previously [24]. In brief, at recruitment, all participants in each cohort were drawn a venous blood sample that was stored at −80°C for research purposes. Current users of exogenous steroid hormones (oral contraceptives (OC) or hormone replacement therapy (HRT)) were not eligible for inclusion in the NYUWHS and ORDET cohorts. Demographic, lifestyle, exogenous hormone use, reproductive, and medical history information were collected at the time of recruitment in the cohort and/or through follow-up questionnaires. In the NSHDS, medical history and smoking information was collected at enrollment. A questionnaire on reproductive life and sex hormone use was administered prospectively to 47% of the subjects and a similar questionnaire was sent out retrospectively to all cases and matched controls to complete and update the collected information at baseline (response rate = 95%). Comparison of prospectively and retrospectively collected data showed excellent agreement for parity (100%) and ever use of OC (88%). For a few deceased cases from the NSHDS included in the present study (n = 13), medical records were the only available source of information about reproductive history and hormone use. In the NYUWHS, data on reproductive history were collected at enrollment, whereas data on smoking, OC and HRT were collected during follow-up. In ORDET all data were collected at enrollment.
Cases were cohort members with primary invasive or borderline epithelial ovarian cancer diagnosed after blood donation, who had no preceding invasive cancer diagnosis (except non-melanoma skin cancer), did not use exogenous hormones at the time of blood donation and who were identified within the parent cohort by the date of the last complete follow-up (June 2007 for NSHDS, December 1999 for NYUWHS, and November 2003 for ORDET). Information about borderline tumors was available from the NSHDS and ORDET cohorts. Ten NSHDS cases were excluded: seven cases because they were using exogenous hormones at the time of blood draw and three cases who refused participation. By cohort design such exclusions were not relevant for the NYUWHS and the ORDET. A total of 237 epithelial ovarian cancer cases (including 30 borderline tumors) were included (Table 1). For each case, two controls were selected at random among the appropriate risk sets. The risk set for a given case included all cohort subjects alive and free of cancer who did not report a bilateral ovariectomy, did not use exogenous hormones at blood donation and matched the index case for cohort, menopausal status, age (±six months) and date of blood donation (±three months). Matching for phase of menstrual cycle was possible for the premenopausal ORDET (blood was drawn 20–24 days after the onset of the last menstrual period) and NYUWHS subjects, but not for NSHDS participants. Follicle-stimulating hormone (FSH) was measured in blood samples of all women with missing data on menopausal status at blood donation (n = 16, of which six cases) and those aged 47–55. Women were considered as postmenopausal if the relevant blood draw had occurred at least 12 months after their last menstrual period, or were 60 years or older at recruitment, or had an FSH concentration in excess of 30 IU/l. Women who were less than age 42 or reported regular cycles at blood donation, or had an FSH <13 IU/l were considered premenopausal. The menopausal status could not be defined for six cases and the controls for these sets were not matched for menopausal status at blood donation. Fifteen potentially eligible case–control sets were excluded because of lack of sample for CRP analyses from either the case (n = 12) or both controls (n = 3): one set from both NSHDS and ORDET and 13 from NYUWHS. A total of 427 control subjects were identified and included in the study.
Table 1.
Baseline characteristics of the ovarian cancer cases and controls
| Characteristic | Cases | Controls |
|---|---|---|
| All women | 237 | 427 |
| Parent cohort | ||
| NSHDS, Sweden (borderline) | 132 (23) | 235 (40) |
| NYUWHS, USA | 58 | 99 |
| ORDET, Italy (borderline) | 47 (7) | 93 (14) |
| Lag-time to cancer diagnosis, mean (SD) | 6.5 (4.0) | – |
| Diagnosed within two years (%) | 35 (15%) | – |
| Diagnosed between two and five years (%) | 58 (25%) | – |
| Histology (%) | ||
| Serous | 133 (56%) | – |
| Mucinous | 29 (12%) | – |
| Endometrioid | 29 (12%) | – |
| Clear cell | 15 (6%) | – |
| Undifferentiated | 7 (3%) | – |
| Other | 4 (2%) | – |
| NOS | 19 (8%) | – |
| Missing | 1 (1%) | – |
| Menopausal status (%) | ||
| Premenopausal | 88 (37%) | 157 (37%) |
| Postmenopausal | 143 (60%) | 260 (61%) |
| Peri-menopausal/unmatched | 6 (3%) | 10 (2%) |
| Parity (%) | ||
| Nulliparous | 44 (19%) | 54 (13%) |
| Parous | 156 (66%) | 335 (78%) |
| Unknown | 37 (16%) | 38 (9%) |
| Previous OC use (%) | ||
| Never | 134 (57%) | 233 (55%) |
| Ever | 64 (27%) | 137 (32%) |
| Unknown | 39 (16%) | 57 (13%) |
| Previous HRT use (%)a | ||
| Never | 67 (47%) | 171 (66%) |
| Ever | 45 (31%) | 60 (23%) |
| Unknown | 31 (22%) | 29 (11%) |
| Smoking status (%) | ||
| Non Smokers | 102 (43%) | 212 (50%) |
| Ex-smokers | 24 (10%) | 41 (10%) |
| Current smokers | 62 (26%) | 91 (21%) |
| Unknown | 49 (21%) | 83 (19%) |
| BMI (mean, SD) | 25.5 (4.0) | 25.8 (4.2) |
| BMI (%) | ||
| <25 | 113 (48%) | 186 (44%) |
| 26–29 | 82 (35%) | 147 (34%) |
| 30+ | 27 (11%) | 66 (15%) |
| Unknown | 15 (6%) | 28 (7%) |
| Diabetes (%) | ||
| No | 170 (72%) | 337 (79%) |
| Yes | 11 (5%) | 17 (4%) |
| Unknown | 56 (24%) | 73 (17%) |
Among postmenopausal women
Laboratory analyses
For NSHDS subjects, hormone analyses were carried out on plasma samples in which EDTA was the anticoagulant, whereas serum samples were available in NYUWHS and ORDET. The hormone analyses were performed at the Hormone Laboratory, Umeå University, Sweden. Samples from cases and their matched controls were always analyzed with the same assay and on the same day. Laboratory personnel were unable to distinguish among case and control samples. For quality control, samples from two standard sera at known concentrations (1.1 and 16.5 mg/l) were inserted haphazardly in each batch. In addition, 38 aliquots from a plasma pool prepared from NSHDS subjects and indistinguishable from case–control samples were mixed within case–control sets (20 within NSHDS sets, 12 within NYUWHS sets and 6 within ORDET sets) and 23 NYUWHS serum samples were analyzed in duplicate. CRP was measured by a high sensitivity immunoturbidimetric assay on a Roche Cobas Mira analyzer (Roche Diagnostics GmbH, D-68298 Mannheim, Germany). The sensitivity range for the assay was 0.1–20 mg/l. According to the manufacturer, normal adult concentrations are considered those below 5.0 mg/l. The mean intra- and inter-batch coefficients of variations (CV) were very similar when calculated using measurements in the standard sera aliquots with CRP concentration of 1.10 mg/l and in the blinded plasma pool aliquots, all of them <2.4%. The CV based on standard sera aliquots with CRP concentration of 16.5 mg/l were lower—1.4% and 1.2%, respectively for intra and inter-batch variation. The Pearson correlation calculated on the basis of the 23 serum samples analyzed in duplicate was 0.998.
FSH levels were measured by immunoradiometric assay with reagents from Diagnostic System Laboratories, (Webster, TX). For an FSH concentration of 15 IU/l, the mean intra-batch and the inter-batch coefficients of variation were 4.2% and 12.6%, respectively.
Statistical analysis
CRP data were log2-transformed to reduce departures from the normal distribution.
Subgroup differences (e.g., by parity, diabetes) in CRP and correlations between CRP and covariates of interest (e.g., age and BMI) were examined among controls by using Generalized Linear Models (GLM SAS® procedure) [25] and Pearson partial correlation coefficients, respectively.
Mean CRP concentrations in cases and controls in the whole study population (and by parent cohort) were compared by mixed-effects regression models in which matched set and cohort were entered as random variables and the remaining matching factors (age, menopausal status) were included as fixed variables. Odds ratios (OR) and their 95% confidence intervals (CI) for disease according to CRP concentrations or other characteristics of interest were estimated by conditional logistic regression models. OR for CRP were calculated for tertiles (with cohort-specific cutoff points based on CRP distribution in controls), continuous scale of the variable and for pre-defined categories (initially ≤1, 2–3, 4–5, 6–10, >10 mg/l, which after careful inspection of the data were collapsed to ≤1, 2–10, >10). Likelihood ratio tests were used to assess linear trends in ORs over the tertiles, giving quantitative scores to all levels (1, 2, and 3). All statistical tests and corresponding p-values were two-sided and p-values <0.05 were considered statistically significant. The potentially confounding effects of ages at menarche and menopause, parity, BMI, past use of oral contraceptives (OC) and hormone replacement therapy (HRT), diabetes, smoking, current use of non-steroidal anti-inflammatory drugs (NSAIDs), and vitamins were examined by conducting stratified analyses and by including these factors in conditional logistic regression models. When there were missing data for a covariate, first analyses were run excluding subjects with missing values and the effect of the adjustment was examined. In a second step, an adjustment was made by coding the missing values as a separate category. If the effect of the two adjustments were similar, the latter is reported. Only BMI influenced point estimates by >5% and it was included in all models (as BMI >25, 25–29, 30+, missing). Analyses excluding women with uncertain data on menopausal status (either because of missing baseline questionnaire data or those with equivocal data, including the sets for which the matching for menopausal status did not hold) were also conducted.
Statistical heterogeneity (e.g., by cohort, menopausal status, etc.) between top tertile ORs and on a continuous scale of the variables was examined by Cochran’s Q [26] and I2 statistics [27]. The Q-statistic was calculated as the deviations of logistic beta-coefficients observed in each of the subgroups relative to the overall beta-coefficient [26]. The I2 statistics describes the proportion of total variation in study estimates that is due to heterogeneity [27].
The study was approved by the Ethical Review Boards of Umeå University, Sweden, New York University School of Medicine, USA and Istituto Nazionale Tumori in Milan, Italy.
Results
Selected characteristics of the study population are presented in Table 1. For the cases median time from blood draw to cancer diagnosis was 6.1 years, ranging from 1 month to 17 years. Among invasive tumors, the most common types were serous, 53% (n = 109), endometrioid, 13% (n = 29) and mucinous, 11% (n = 23). Among borderline tumors, 80% (n = 24) were serous and 20% (n = 6) were mucinous. The distribution of histological subtypes was similar between cohorts and in agreement with the reported data [28, 29]. Most of the women (61%) were postmenopausal at blood donation. In comparison with controls, cases reported more frequently nulliparity and ever use of HRT, while previous OC use, BMI, diabetes, and smoking were similar.
CRP concentrations of NSHDS and ORDET subjects were similar, but those of NYUWHS participants were higher (p = 0.001), also after adjustment for age, BMI, and diabetes. Among controls, CRP was directly correlated with age (r = 0.23, p < 0.0001) and BMI (r = 0.51, p < 0.0001). Geometric mean CRP concentrations across BMI categories (BMI ≤25, 25–30 and >30 kg/m2) were 1.02, 2.05, and 3.10 mg/l. Women with diabetes had higher CRP than women with no such diagnosis (3.06 vs. 1.67 mg/l, p < 0.002). CRP levels were similar in pre- and postmenopausal controls and according to parity, past use of OC and HRT, smoking status and current use of NSAID, multivitamins, or other supplements.
About 2% of control women and 7% of all women in the study had CRP levels exceeding 10 mg/l. In comparison with women who had CRP ≤10 mg/l, those with high levels had higher BMI, reported more often diabetes, less frequent use of NSAIDs and the cases had shorter follow-up time (4.4 vs. 6.6 years, p < 0.03). There were no significant differences between the two groups according to age, cohort, OC, HRT or smoking.
Mean CRP concentrations were similar in cases and controls, both overall and separately within each cohort (Table 2). ORs for ovarian cancer for the second and third tertile of CRP concentrations were 0.97 (0.64–1.46) and 1.15 (0.74–1.79). Adjustment for BMI increased risk estimates (e.g., OR for the top tertile of CRP changed from 0.99 (0.66–1.50) to 1.15 (0.74–1.79). The strongest effect of CRP was observed for mucinous tumors, both in tertiles [1.32 (0.31–5.55) and 6.29 (0.77–51.5), p-trend <0.09] (Table 2) and on a continuous scale [1.55 (0.93–2.57)], but neither was statistically significant. In analyses in tertiles, there was a non-significant tendency for a positive association of CRP with risk in the NYUWHS cohort, but no indication for association was apparent when risk was calculated on continuous scale of the variable (OR 1.03 (0.82–1.30)). None of the tests for heterogeneity between cohorts was significant and the I2 statistics on continuous scale CRP was 0%; however, when top tertile estimates were compared, the I2 statistic was 45%. Risk estimates for borderline tumors were higher than those for invasive malignancies in tertile analyses, but none of the heterogeneity tests was significant and all I2 statistics were 0%, clearly indicating lack of heterogeneity. There was evidence for a modest association among postmenopausal women [OR 1.19 (1.01–1.40)] in analyses on a continuous scale. Exclusion of women with uncertain data on menopausal status did not alter any of the risk estimates (data not shown).
Table 2.
Geometric means (95% CI) CRP concentrations (mg/l) in cases and controls in the whole study population and by parent cohort study*
| Characteristic | Cases | Controls | p-value* |
|---|---|---|---|
| All women | 1.40 (1.00–1.95) | 1.34 (0.97–1.85) | 0.53 |
| NSHDS (Sweden) | 1.23 (0.91–1.66) | 1.17 (0.87–1.54) | 0.54 |
| NYUWHS (USA) | 1.92 (1.47–2.51) | 1.87 (0.52–2.30) | 0.87 |
| ORDET (Italy) | 1.57 (1.22–2.00) | 1.53 (1.29–1.82) | 0.87 |
From mixed-effects regression models
In further analyses by predefined CRP categories (≤1, 2–10 and ≥10 mg/l), CRP concentrations above 10 mg/l were associated with several-fold increase in ovarian cancer risk (Table 3). This unexpected, strong association was evident in virtually all subgroups based on tumor characteristics or menopausal status considered and the OR for the category CRP >10 mg/l included unity only for premenopausal women (Table 4).
Table 3.
Odds ratios (95% CI) of ovarian cancer by tertiles of CRP
| Tertiles CRP (mg/l)a | 1 | 2 | 3 | p-trend* |
|---|---|---|---|---|
| NSHDS, Sweden | <0.84 | 0.85–2.06 | ≥2.07 | |
| NYUWHS, USA | <1.17 | 1.18–2.94 | ≥2.95 | |
| Ordet, Italy | <0.91 | 0.92–1.96 | ≥1.97 | |
| All cases | ||||
| Cases/controls | 80/138 | 77/145 | 80/144 | |
| OR (95% CI) | 1.00 | 0.97 (0.64–1.47) | 1.15 (0.74–1.79) | 0.54 |
| Invasive cases | ||||
| Cases/controls | 70/117 | 68/130 | 69/126 | |
| OR (95% CI) | 1.00 | 0.90 (0.58–1.40) | 1.05 (0.65–1.69) | 0.85 |
| Invasive cases, NSHDS | ||||
| Cases/controls | 38/59 | 38/72 | 33/64 | |
| OR (95% CI) | 1.00 | 0.81 (0.45–1.47) | 0.83 (0.42–1.62) | 0.56 |
| Invasive cases, NYUWHS | ||||
| Cases/controls | 19/33 | 16/33 | 23/33 | |
| OR (95% CI) | 1.00 | 1.13 (0.47–2.71) | 1.88 (0.66–5.38) | 0.25 |
| Invasive cases, ORDET | ||||
| Cases/controls | 13/25 | 14/25 | 13/29 | |
| OR (95% CI) | 1.00 | 1.19 (0.38–3.72) | 1.17 (0.44–3.09) | 0.77 |
| Borderline cases | ||||
| Cases/controls | 10/21 | 9/15 | 11/18 | |
| OR (95% CI) | 1.00 | 1.64 (0.44–6.06) | 1.88 (0.47–7.53) | 0.37 |
| Serous cases | ||||
| Cases/controls | 44/77 | 39/77 | 50/82 | |
| OR (95% CI) | 1.00 | 0.98 (0.56–1.71) | 1.36 (0.76–2.44) | 0.29 |
| Invasive serous cases | ||||
| Cases/controls | 34/59 | 34/67 | 41/68 | |
| OR (95% CI) | 1.00 | 0.93 (0.51–1.73) | 1.23 (0.63–2.39) | 0.53 |
| Mucinous cases | ||||
| Cases/controls | 9/17 | 8/21 | 12/15 | |
| OR (95% CI) | 1.00 | 1.32 (0.31–5.55) | 6.29 (0.77–51.49) | 0.09 |
| Invasive mucinous cases | ||||
| Cases/controls | 9/14 | 4/16 | 10/11 | |
| OR (95% CI) | 1.00 | 0.63 (0.13–3.00) | 3.75 (0.29–48.63) | 0.42 |
| Endometrioid cases | ||||
| Cases/controls | 13/19 | 13/15 | 3/18 | |
| OR (95% CI) | 1.00 | 0.91 (0.25–3.27) | 0.23 (0.05–1.11) | 0.07 |
| Premenopausal cases | ||||
| Cases/controls | 41/67 | 27/51 | 20/39 | |
| OR (95% CI) | 1.00 | 0.95 (0.50–1.18) | 0.99 (0.46–2.15) | 0.96 |
| Postmenopausal cases | ||||
| Cases/controls | 36/67 | 49/91 | 58/102 | |
| OR (95% CI) | 1.00 | 1.06 (0.60–1.88) | 1.27 (0.72–2.32) | 0.41 |
| Lag >two years | ||||
| Cases/controls | 71/120 | 67/125 | 64/117 | |
| OR (95% CI) | 1.00 | 0.95 (0.61–1.48) | 1.09 (0.67–1.76) | 0.74 |
| Lag >five years | ||||
| Cases/controls | 54/93 | 42/95 | 48/74 | |
| OR (95% CI) | 1.00 | 0.81 (0.49–1.36) | 1.34 (0.76–2.35) | 0.38 |
All models from conditional logistic regression with adjustment for BMI
Cohort-specific tertiles based on CRP distribution among controls was used
p-value for linear trend over the tertiles, giving quantitative scores to all levels (1, 2, and 3)
Table 4.
Odds ratios (95% CI) of ovarian cancer by pre-defined categories of CRP
| CRP (mg/l) | ≤1 | 2–10 | >10 | p-trend* |
|---|---|---|---|---|
| All cases | ||||
| Cases/controls | 83/155 | 138/263 | 16/9 | |
| OR (95% CI) | 1.00 | 1.10 (0.77–1.59) | 4.39 (1.76–10.90) | 0.04 |
| Invasive cases | ||||
| Cases/controls | 72/131 | 121/233 | 14/9 | |
| OR (95% CI) | 1.00 | 1.03 (0.70–1.52) | 3.71 (1.46–9.44) | 0.12 |
| All cases but mucinous | ||||
| Cases/controls | 73/135 | 121/231 | 14/8 | |
| OR (95% CI) | 1.00 | 1.08 (0.74–1.59) | 4.21 (1.60–11.07) | 0.07 |
| Premenopausal cases | ||||
| Cases/controls | 43/70 | 41/83 | 4/4 | |
| OR (95% CI) | 1.00 | 0.91 (0.50–1.63) | 1.88 (0.43–8.36) | 0.88 |
| Postmenopausal cases | ||||
| Cases/controls | 37/79 | 94/176 | 12/5 | |
| OR (95% CI) | 1.00 | 1.25 (0.77–2.05) | 7.48 (2.20–25.42) | 0.01 |
| Lag >two years | ||||
| Cases/controls | 73/135 | 118/219 | 11/8 | |
| OR (95% CI) | 1.00 | 1.10 (0.75–1.63) | 3.04 (1.16–7.99) | 0.13 |
| Lag >five years | ||||
| Cases/controls | 55/103 | 82/155 | 7/4 | |
| OR (95% CI) | 1.00 | 1.10 (0.71–1.72) | 3.60 (0.99–13.16) | 0.23 |
All models from conditional logistic regression with adjustment for BMI
p-value for linear trend over the tertiles, giving quantitative scores to all levels (1, 2, and 3)
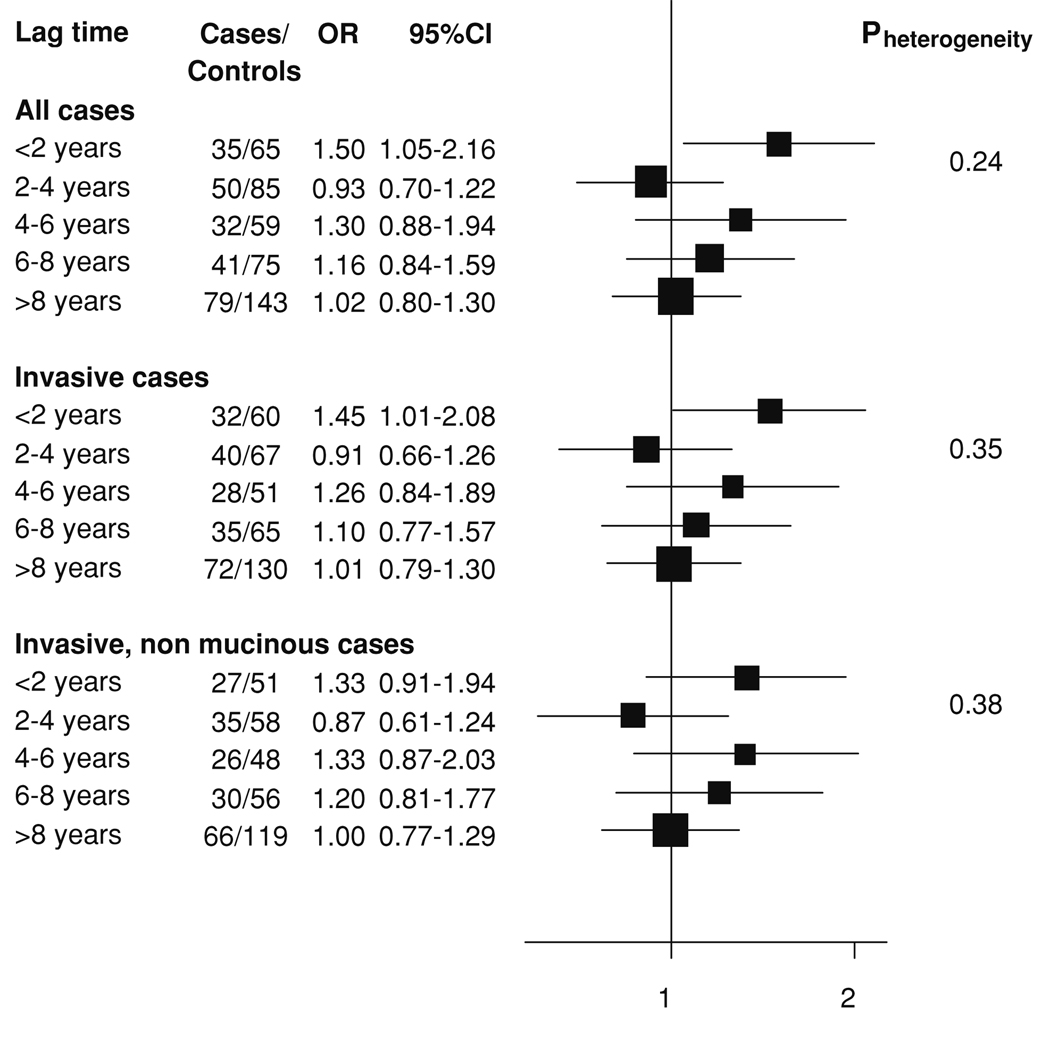
To assess the potential influence of occult cancers undetected at study entry, analyses stratified by lag-time to cancer diagnosis (in two years increments) were conducted (Fig. 1). In women diagnosed within two years of blood donation, CRP was directly associated with risk, but no consistent pattern emerged in women with a lag-time greater than two years. The correlation of CRP with lag-time to diagnosis was −0.15 (p = 0.02) among cases and −0.07 (p = 0.32) when only cases diagnosed two or more years after blood donation were considered. Restricting analyses to women diagnosed either two or five years after blood donation somewhat weakened the effect of CRP >10 mg/l on risk: ORs were reduced from 4.39 (1.76–11.0) to 3.04 (1.16–7.99) for women diagnosed more than two years and to 3.60 (0.99–13.16) for women diagnosed more than five years after blood donation (Table 4), but the indication for a strong direct association remained. Risk associated with CRP concentrations for mucinous tumors remained virtually unchanged after exclusion of cases diagnosed within two years of blood draw [1.54 (0.89–2.70)].
Figure 1.
Relative risk of ovarian cancer for log2 CRP levels by lag-time, adjusted for BMI
Discussion
In this large nested case–control study, we sought to test the hypothesis of a positive association between pre-diagnostic CRP and risk of ovarian cancer. In contrast to the findings of the only other prospective study by McSorley et al. [20], the vast majority of the conducted analyses showed no indication for a relationship. The two studies share a number of similarities, in design, size, study subject characteristics, and type of biological samples analyzed [20]. Minor differences such as inclusion of small proportion of current hormone users in McSorley et al. and of borderline tumors in our study cannot account for the observed difference in results as shown by sensitivity analyses.
Despite the lack of overall association of CRP with ovarian cancer risk, in a few subgroups we observed associations that might warrant future investigation. First, there was a strong suggestion that women with CRP concentrations >10 mg/l at blood donation were at particularly elevated risk. The percentage of women with such high CRP concentrations was small, but in excellent accordance with reported prevalence in apparently healthy, non-users of exogenous hormones general population women of age 45–74 from the US and Europe [30, 31]. Apart from expected differences in presence of overweight/obesity, diabetes, and in use of NSAIDs among controls, women with CRP >10 mg/l reported reproductive and other characteristics very similar to those of women with CRP levels ≤10 mg/l or within the normal range (<5 mg/l). Adjustments for BMI, current smoking, diabetes, NSAID use, or any other covariates did not alter the strong association with risk, ruling out the possibility of a major effect by most traditional ovarian cancer confounders. Tumor characteristics, such as histological sub-type, disease stage, grade, and age at diagnosis were largely similar between cases with high and low CRP concentrations, but the follow- up of cases with CRP >10 mg/l was shorter in comparison with the remaining cases (4.4 vs. 6.6), leaving the possibility that the presence of an undiagnosed tumor could have resulted in increased CRP concentrations. However, despite some reduction of risk estimates after restriction of the analyses to women diagnosed two or five years after blood donation, the effect was still evident.
In cardiovascular epidemiology CRP concentrations exceeding 10 mg/l are not used for risk prediction as they are considered either transient (thus not representative of the usual concentrations) or indicative of the presence of an underlying inflammatory condition not directly relevant to cardiovascular disease risk [32]. Current recommendations are to repeat the CRP measurement after two weeks and initiate a search for an obvious source of infection/inflammation [32]. By the same token, some of the studies on CRP and cancer risk have reported analyses in which subjects with CRP above 10 mg/l were excluded [20, 33]. The underlying rationale for such sensitivity approaches is to increase the study power to detect an existing CRP-disease association by removing subjects with measurements with high random error that do not adequately reflect long-term average level. To our knowledge, so far, no study has reported specifically on the effect of CRP greater than 10 mg/l on cancer risk.
A possible explanation for the observed association is that women with CRP above 10 mg/l are experiencing an unfavorable inflammatory milieu either because of an underlying long-standing inflammatory process of moderate intensity or because they are more susceptible to a strong inflammatory response, which would also lead to increased risk of ovarian cancer. Such an effect would be consistent with the ‘antigenic stimulation theory’ postulating that chronic immune stimulation leads to random pro-oncogenic mutations in actively dividing stem cells [34]. Alternatively, the high CRP levels in some of the cases could reflect ovarian inflammation.
Our results suggest that CRP may be particularly relevant for mucinous tumors, a small sub-type of epithelial ovarian tumors (about 10% of invasive cancers), which differ substantially in terms of epidemiological, pathological, molecular, and clinical characteristics from the other subtypes [35]. Mucinous tumors are usually slow growing and quite large at presentation (mean size about 18 cm [36]), thus the elevated risk associated with CRP in this sub-group could result from the presence of large, but yet undiagnosed tumors. In support of this possibility, there was the negative correlation between lag-time to diagnosis and CRP in cases with mucinous tumors (−0.37, p < 0.09), even though restriction of the analyses to those diagnosed two or more years after blood donation did not alter risk estimates.
In mucinous tumors, patterns of risk factors and somatic genetic alterations across benign, borderline, and invasive subtypes are generally consistent with an adenoma-to-carcinoma developmental sequence, similar to that observed for colon cancer. This is believed to be of no relevance for the high-grade serous tumors, the most frequent sub-type of epithelial ovarian cancer [36]. The only known genetic alteration in mucinous ovarian tumors is a KRAS mutation (in about half of the tumors) [36, 37], a characteristic shared with about 30% of colon tumors [38]. Some studies have shown that pre-diagnostic CRP concentrations are directly associated with risk of colon cancer [39, 40]. Thus, inflammatory processes could be especially relevant in situations of gradual tumor progression, as in colon and mucinous ovarian tumors.
Strengths of our study include its prospective design, the relatively large size (for a study on ovarian cancer), the high quality of the laboratory measurements, as reflected by the very low intra- and inter-batch coefficients of variation and the expected changes in CRP concentrations according to age, BMI, and diabetes. Weaknesses of our study are that only one CRP measurement per subject was available to characterize long-term exposure (resulting in random misclassification and decreased power to detect an existing association) and the large number of subgroup analyses.
In summary, there is a wealth of evidence that chronic inflammation to the ovaries is associated with increased ovarian cancer risk. Recent clinical and prospective data also support a direct association of CRP with either future risk [20] or disease outcome [41, 42]. The observations reported here add further, direct evidence in support to the role of elevated CRP, which does not appear to be the result of an as yet unrecognized, spreading tumor. Of note is the particularly strong association within the restricted sub-group of subjects with markedly elevated CRP. We believe that these findings are noteworthy in that they reveal new, fertile areas for exploration of the undoubtedly important role of inflammation in ovarian cancer.
Acknowledgments
The authors thank Mr Hubert Sjodin, Ms Jasmin Moharer, Ms Helena Schock, and Ms Ann-Marie Åhrén for their excellent technical assistance and Mrs Anika Husing for helpful discussions.
Financial support This investigation was supported by a grant from the Lion’s Cancer Foundation, Umeå University, Umeå, Sweden, the US National Institutes of Health grants CA098661, CA34588 and CA16087, and the US National Institute of Environmental Health Sciences grant ES00260.
Contributor Information
Eva Lundin, Department of Medical Biosciences, Umeå University, Umeå, Sweden.
Laure Dossus, Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Im Neuenheimer Feld, 280, Heidelberg 69120, Germany.
Tess Clendenen, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Vittorio Krogh, Nutritional Epidemiology Unit, National Cancer Institute, Milan, Italy.
Kjell Grankvist, Department of Medical Biosciences, Umeå University, Umeå, Sweden.
Marianne Wulff, Department of Clinical Sciences, Umeå University, Umeå, Sweden.
Sabina Sieri, Nutritional Epidemiology Unit, National Cancer Institute, Milan, Italy.
Alan A. Arslan, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA Department of Obstetrics and Gynecology, New York University School of Medicine, New York, NY, USA.
Per Lenner, Department of Radiation Sciences, Umeå University, Umeå, Sweden.
Franco Berrino, The Etiological and Preventive Epidemiology Unit, National Cancer Institute, Milan, Italy.
Goran Hallmans, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden.
Anne Zeleniuch-Jacquotte, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Paolo Toniolo, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA; Department of Obstetrics and Gynecology, New York University School of Medicine, New York, NY, USA.
Annekatrin Lukanova, Email: a.lukanova@dkfz.de, Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Im Neuenheimer Feld, 280, Heidelberg 69120, Germany.
References
- 1.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 2.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol Cell Endocrinol. 2006;247:4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Risch HA, Howe GR. Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:447–451. [PubMed] [Google Scholar]
- 5.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 6.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88:554–559. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 7.Harlow BL, Hartge PA. A review of perineal talc exposure and risk of ovarian cancer. Regul Toxicol Pharmacol. 1995;21:254–260. doi: 10.1006/rtph.1995.1039. [DOI] [PubMed] [Google Scholar]
- 8.Hankinson SE, Hunter DJ, Colditz GA, et al. Tubal ligation, hysterectomy, and risk of ovarian cancer. A prospective study. JAMA. 1993;270:2813–2818. [PubMed] [Google Scholar]
- 9.Loft A, Lidegaard O, Tabor A. Incidence of ovarian cancer after hysterectomy: a nationwide controlled follow up. Br J Obstet Gynaecol. 1997;104:1296–1301. doi: 10.1111/j.1471-0528.1997.tb10978.x. [DOI] [PubMed] [Google Scholar]
- 10.Green A, Purdie D, Bain C, et al. Tubal sterilisation, hysterectomy and decreased risk of ovarian cancer. Survey of Women’s Health Study Group. Int J Cancer. 1997;71:948–951. doi: 10.1002/(sici)1097-0215(19970611)71:6<948::aid-ijc6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Cramer DW, Harlow BL, Titus-Ernstoff L, Bohlke K, Welch WR, Greenberg ER. Over-the-counter analgesics and risk of ovarian cancer. Lancet. 1998;351:104–107. doi: 10.1016/S0140-6736(97)08064-1. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg L, Palmer JR, Rao RS, et al. A case–control study of analgesic use and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:933–937. [PubMed] [Google Scholar]
- 13.Schildkraut JM, Moorman PG, Halabi S, Calingaert B, Marks JR, Berchuck A. Analgesic drug use and risk of ovarian cancer. Epidemiology. 2006;17:104–107. doi: 10.1097/01.ede.0000190538.55645.f8. [DOI] [PubMed] [Google Scholar]
- 14.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 15.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 17.Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 18.Il′yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 19.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15:381–384. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]
- 20.McSorley MA, Alberg AJ, Allen DS, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gynecol. 2007;109:933–941. doi: 10.1097/01.AOG.0000257126.68803.03. [DOI] [PubMed] [Google Scholar]
- 21.Hallmans G, Agren A, Johansson G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort—evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24. doi: 10.1080/14034950310001432. [DOI] [PubMed] [Google Scholar]
- 22.Toniolo P, Bruning PF, Akhmedkhanov A, et al. Serum insulin-like growth factor-I and breast cancer. Int J Cancer. 2000;88:828–832. doi: 10.1002/1097-0215(20001201)88:5<828::aid-ijc22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Muti P, Bradlow HL, Micheli A, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11:635–640. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Lukanova A, Lundin E, Akhmedkhanov A, et al. Circulating levels of sex steroid hormones and risk of ovarian cancer. Int J Cancer. 2003;104:636–642. doi: 10.1002/ijc.10990. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute. SAS/STATR user’s guide version 6. Cary, NC, USA: SAS Institute Inc.; 1990. [Google Scholar]
- 26.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen VW, Ruiz B, Killeen JL, Cote TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 29.Seidman JD, Russell P, Kurman RJ. Surface epithelial tumors of the ovary. In: Kurman RJ, editor. Blaustein’s pathology of the female genital tract. New York: Springer-Verlag; 2002. pp. 791–904. [Google Scholar]
- 30.Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States: implication for clinical interpretation. Clin Chem. 2003;49:666–669. doi: 10.1373/49.4.666. [DOI] [PubMed] [Google Scholar]
- 31.Imhof A, Frohlich M, Loewel H, et al. Distributions of C-reactive protein measured by high-sensitivity assays in apparently healthy men and women from different populations in Europe. Clin Chem. 2003;49:669–672. doi: 10.1373/49.4.669. [DOI] [PubMed] [Google Scholar]
- 32.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 33.Zhang SM, Lin J, Cook NR, et al. C-reactive protein and risk of breast cancer. J Natl Cancer Inst. 2007;99:890–894. doi: 10.1093/jnci/djk202. [DOI] [PubMed] [Google Scholar]
- 34.Talbot-Smith A, Fritschi L, Divitini ML, Mallon DF, Knuiman MW. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am J Epidemiol. 2003;157:606–612. doi: 10.1093/aje/kwg020. [DOI] [PubMed] [Google Scholar]
- 35.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurman RJ, Shih I. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–160. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gemignani ML, Schlaerth AC, Bogomolniy F, et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90:378–381. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 38.Lea IA, Jackson MA, Li X, Bailey S, Peddada SD, Dunnick JK. Genetic pathways and mutation profiles of human cancers: site- and exposure-specific patterns. Carcinogenesis. 2007;28:1851–1858. doi: 10.1093/carcin/bgm176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 40.Tsilidis K, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systemic review of prospective studies. Int J Cancer. 2008;123:1133–1140. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 41.Kodama J, Miyagi Y, Seki N. Serum C-reactive protein as a prognostic factor in patients with epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1999;82:107–110. doi: 10.1016/s0301-2115(98)00227-9. [DOI] [PubMed] [Google Scholar]
- 42.Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14:710–714. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]