Abstract
Risk covariates of neuropsychological ability (NA) at treatment entry and neuropsychological recovery (NR) across 15 months were examined and replicated in 2 samples (Ns = 952 and 774) from Project MATCH, a multisite study of alcoholism treatments. NA at treatment entry was associated with age, education, and other covariates. Statistically significant mean increases in NA over time had small effect sizes, suggesting limited clinical significance of NR in the samples as a whole. However, initial NA and a combination of risk factors in direct and mediated pathways predicted a large proportion of individual differences in NR. Statistically significant but modest differential treatment effects on NR suggest that addiction treatments may need to be modified or developed to facilitate this important aspect of recovery.
About 50%–80% of persons diagnosed with alcohol use disorders display deficits on neuropsychological tests, indicating subtle to profound impairment of attention, cognitive flexibility, episodic and working memory, abstract reasoning, and other cognitive abilities (Bates & Convit, 1999; Rourke & Løberg, 1996). A sizable minority of these adults display impairments as clinically severe as those seen in persons with traumatic brain injury (Bates, 1997; Donovan, Kivlahan, Kadden, & Hill, 2001; Victor & Adams, 1985). More severely impaired clients may benefit from cognitive rehabilitation in addition to addiction treatment (Allen, Goldstein, & Seaton, 1997). Yet, deficit severity may be underestimated by treatment providers because of its insidious onset (Knight & Longmore, 1994) and the difficulty in detecting impairments in conversation or structured interviews (Fals-Stewart, 1997). Evidence suggests that negative characteristics often attributed to clients, such as inattention, low motivation, and minimization or denial of problem severity, may arise from cognitive deficits that are distinct from other psychological or psychosocial disruptions that are attributable to the primary use disorder (Fals- Stewart, Shanahan, & Brown, 1995; Goldman, 1995).
Although thorough neuropsychological assessment of all clients entering addiction treatment may not be feasible, risk factors have been identified that account for up to 57% of the true variance in executive function, memory, verbal ability, and information processing speed of clients with substance use disorders at treatment entry (Bates, Labouvie, & Voelbel, 2002; Fals-Stewart & Bates, 2003; Hesselbrock, Weidenman, & Reed, 1985; Malloy, Noel, Rogers, Longabaugh, & Beattie, 1989). Older age, lower education, health problems, psychiatric diagnoses, familial alcoholism, and duration of heavy drinking, for example, have been inversely related to neuropsychological ability. Treatment providers can use information about risk factors in their initial functional analysis of client strengths and weaknesses to help identify those clients most at risk for cognitive compromise and in need of neuropsychological assessment.
Following the planning phase of addiction treatment, however, it is not clear whether risk covariates are informative about meaningful individual differences in neuropsychological recovery over time. This is an important question in view of evidence that alcohol-related impairment is often not permanent and that spontaneous, time-dependent neuropsychological recovery may follow abstinence or greatly reduced drinking (Parsons, 1998; Rourke & Grant, 1999). Questions concerning the need for cognitive rehabilitation, long-term employment options, and other psychosocial outcomes could be more adequately addressed if the likelihood and extent of expected cognitive improvement were more predictable. There is some evidence that age, severity of depressive symptoms, and chronicity of use are negatively associated with neuropsychological recovery (Rourke & Grant, 1999; Schafer et al., 1991). Yet, the literature is not straightforward, and interpretation is problematic because of methodological difficulties in controlling for practice effects and inconsistencies in the neuropsychological tests and time points used in different studies. Moreover, applied questions regarding clinically significant versus statistically significant improvements in neuropsychological functioning, and whether spontaneous recovery of function varies in clients exposed to different addiction treatment approaches, have received very little attention.
In this study, we used data from Project MATCH, a clinical trial of three alcohol treatments, to examine hypotheses about whether risk factors associated with cognitive deficits at treatment entry predict cognitive recovery over 15 months. A structural equation modeling approach to hypothesis testing was used, and the magnitude and prediction of recovery over time was considered in relation to the effect size (ES) of statistically significant mean changes and path coefficients. We predicted that risk factors for impairment identified in the previous literature would be associated with poorer neuropsychological ability in clients at treatment entry and with less cognitive recovery at 15 months. Improvement over time in depressive symptoms and medical test results, in addition to decreases in alcohol consumption, were expected to facilitate greater recovery. We also examined whether changes in depressive symptoms and medical problems mediated the influence of alcohol consumption on recovery of cognitive ability. Finally, we explored differential changes in cognitive status in clients exposed to cognitive– behavioral coping skills therapy (CBT; Kadden et al., 1995), motivational enhancement therapy (MET; Miller, Zweben, DiClemente, & Rychtarik, 1995), and twelve-step facilitation (TSF; Nowinski, Baker, & Carroll, 1992) treatment approaches.
Method
Participants
Data were from 1,726 participants (952 outpatient, 774 aftercare clients) of Project MATCH, a large, national multisite clinical trial that assessed differences in treatment outcomes between three alcohol treatments: CBT, MET, and TSF. Two separate populations of clients were included: 952 outpatient participants who were actively seeking treatment for an ongoing use disorder and 774 aftercare participants who were enrolled in the study after completion of an inpatient or intensive day treatment (Project MATCH Research Group, 1997). Details of the inclusion and exclusion criteria are available in many publications (e.g., Project MATCH Research Group, 1997). In summary, participants were required to (a) have a Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; American Psychiatric Association, 1987) diagnosis of alcohol abuse or dependence; (b) indicate alcohol as the primary drug of abuse; (c) have maintained active drinking during the 3 months prior to treatment entry; (d) be 18 years of age or older; (e) have a minimum of a sixth-grade reading level; and (f) have an absence of legal, probation, or parole requirements. Exclusion criteria were current drug dependence other than alcohol, intravenous drug use during the 6 months prior to the study, and symptoms of acute psychosis and severe organic impairment. If necessary, participants were detoxified prior to treatment entry. Demographic characteristics are described in Table 1.
Table 1.
Demographic Characteristics of the Project MATCH Participants
| Outpatient | Aftercare | Total Sample | |
|---|---|---|---|
| N | 952 | 774 | 1,726 |
| Age (years) | 38.88 ± 10.72 | 41.91 ± 11.11 | 40.24 ± 10.99 |
| Education (years) | 13.44 ± 2.15 | 13.08 ± 2.05 | 13.28 ± 2.11 |
| Gender (%) | |||
| Men | 72 | 80 | 76 |
| Women | 28 | 20 | 24 |
| Race-ethnicity (%) | |||
| White | 82 | 81 | 82 |
| African-American | 6 | 15 | 10 |
| Hispanic-Latino | 12 | 4 | 8 |
Measures
Neuropsychological Tests
Assessments included the Vocabulary and Abstraction subtests of the Shipley Institute of Living Scale (SILS; Zachary, 1986), Parts A and B of the Trail Making Test (TMT-A, TMT-B; Reitan & Wolfson, 1985), and the Symbol Digit Modalities Test (SDMT; Smith, 1982). Although the battery was brief, the tests are reliable, valid, and sensitive to cerebral dysfunction and brain damage (Lezak, 1995; Spreen & Strauss, 1998). These tests assess the following important areas of functioning impaired in samples with substance use disorders: abstraction, cognitive flexibility, working memory, and psychomotor processing speed (Knight & Longmore, 1994; Nixon, 1995). Raw SILS and SDMT accuracy scores and TMT time (in seconds) scores were analyzed.
Psychopathology
Diagnoses of mood, anxiety, and antisocial personality disorder (ASPD) were obtained with the Computerized Diagnostic Interview Schedule (Robins, Helzer, Cottler, & Goldring, 1989) and coded as 0 or 1 to indicate absence or presence of disorder, respectively. The psychiatric severity composite score was from the Addiction Severity Index (McLellan, Luborski, Woody, & O’Brien, 1980). Depression symptoms were assessed with the Beck Depression Inventory (BDI; Beck, Rush, Shaw, & Emery, 1979).
Medical Problems
A composite score was constructed on the basis of the sum of abnormal results from five blood and urine tests used to detect signs of liver, blood, kidney, and connective tissue disease (1 for each abnormal test result, 0 for each normal result). Abnormal test results suggest physiological dysfunction or medical illness that may interfere with cognitive ability and recovery of ability (Lehman, Pilich, & Andrews, 1993; Marsano, 1994).
Family history of alcoholism
Familial alcoholism history was determined from the family history section of the Addiction Severity Index (McLellan et al., 1992) and coded as 1 for a positive family history and 0 for a negative family history in all first-degree relatives.
Quantity of Alcohol Consumed
Drinking data were collected using the Form 90 (Miller, 1996; Miller & Del Boca, 1994). The total number of alcoholic drinks consumed in the 3 months prior to treatment entry was the baseline measure, and the total number of alcoholic drinks consumed between baseline and the 15-month evaluation was the follow-up measure.
Procedure
Participants who met all screening criteria and provided informed consent were scheduled for three intake assessments during which neuropsychological tests and risk covariate measures were administered. Participants then were randomly assigned to enter one of three 12-week treatments for alcohol use disorders. The Form 90 data were administered at all five follow-up assessments. Neuropsychological test, medical test, and BDI data were used from the 15-month follow-up (see Project MATCH Research Group, 1997, for detailed procedures.)
Data Analysis
Mplus (Muthén & Muthén, 1998) was used to simultaneously estimate model parameters in both outpatient and aftercare samples from raw data with a maximum likelihood approach with missing data assumed to be missing at random (Little & Rubin, 1987). A full information covariance matrix, in which all participants were included, was analyzed to minimize bias (Allison, 2002; Kline, 1998; Naehri, Laaksonen, Hietala, Ahonen, & Lyyti, 2001). All outpatients and all but 1 aftercare participant completed the initial neuropsychological assessment; 827 (87%) outpatient and 663 (86%) aftercare participants also completed the follow-up assessment. Comparison of participants who did and did not complete the follow-up assessment revealed no significant differences on the intake neuropsychological measures or selected demographic variables. The largest source of missing data was a medical test result, which was missing for 392 and 357 participants at treatment entry and 15 months, respectively.
Confirmatory factor analysis was used to examine three alternative measurement models of neuropsychological ability on the basis of conceptual models and a consideration of the common measures in this and earlier studies (Bates et al., 2002; Fals-Stewart & Bates, 2003). The fit of an inclusive one-factor model of general cognitive ability (SILS Vocabulary, SILS Abstraction, SDMT, TMT-A, and TMT-B) was contrasted with the fit of a one-factor model of executive cognitive ability (SILS Abstraction, SDMT, and TMT-B), and a two-factor model of executive ability (SILS Abstraction, TMT-B) and psychomotor speed (SDMT, TMT A).
We examined whether the neuropsychological measurement model was invariant over time (treatment entry, 15 months) and across outpatient and aftercare samples. Because increases in performance due to practice are test specific, practice effects were reflected in the model as changes in the intercepts of individual tests over time. Therefore, the contribution of practice to increased performance at 15 months was examined by testing for significant decreases in model fit when intercepts were constrained to be equal across time. Changes in performance due to recovery of underlying ability were reflected in changes in means of the latent ability factors. Cognitive recovery was thus defined by significant changes (increases) in the mean of the latent ability factors across time while constraining factor loadings and intercepts to be equal across time.
Structural equation path modeling was then used to determine the amount of true variance in neuropsychological ability at treatment entry and in change in ability over time that was associated with risk factors. Taking individual differences in risk covariates into account, we explored differences in initial cognitive ability and changes in ability over time in the three treatment approaches. Treatments (TSF, CBT, and MET) were dummy coded, with TSF designated as the reference group to which initial ability and recovery in CBT and MET were compared. Thus, by controlling for the associations of multiple risk correlates and by accounting for measurement error and test-specific variance in performance, the structural equation modeling approach potentially allows results to generalize beyond the specific tests that are used to assess ability within a given neuropsychological domain (e.g., executive function).
Results and Discussion
Table 2 shows mean scores and standard deviations for each neuropsychological test. Mean scores, standard deviations and frequencies for the other measures are provided in Tables 3 and 4.
Table 2.
Mean and Standard Deviations of Raw Neuropsychological Test Scores
| Outpatient | Aftercare | Total Sample | ||||
|---|---|---|---|---|---|---|
| Test | Mean | SD | Mean | SD | Mean | SD |
| Treatment Entry | N=952 | N=773 | N=1725 | |||
| SILS-Abstraction | 26.40 | 8.63 | 23.60 | 9.25 | 25.15 | 9.02 |
| SILS-Vocabulary | 30.72 | 5.25 | 29.63 | 5.25 | 30.23 | 5.27 |
| Trail Making, Part A | 30.79 | 11.50 | 37.21 | 19.15 | 33.67 | 15.73 |
| Trail Making, Part B | 72.53 | 33.39 | 87.86 | 47.73 | 79.42 | 41.17 |
| SDMT | 50.56 | 9.70 | 45.99 | 10.78 | 48.52 | 10.45 |
| 15 Months | N=827 | N=660 | N=1487 | |||
| SILS-Abstraction | 27.59 | 8.53 | 24.29 | 9.72 | 26.13 | 9.22 |
| SILS-Vocabulary | 30.94 | 5.48 | 29.61 | 5.56 | 30.35 | 5.56 |
| Trail Making, Part A | 27.97 | 10.39 | 36.01 | 17.02 | 31.54 | 14.30 |
| Trail Making, Part B | 68.08 | 31.12 | 83.74 | 41.76 | 75.05 | 37.06 |
| SDMT | 51.91 | 10.05 | 46.23 | 11.00 | 49.39 | 10.85 |
Note. SILS = Shipley Institute of Living Scales; SDMT = Symbol Digit Modalities Test
Table 3.
Risk Factor Predictors of Neuropsychological Ability
| Outpatient | Aftercare | |||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| # of Standard Drinks Consumed (3 Mo. Before Treatment Entry) |
952 | 1056.87 | 815.86 | 774 | 1845.72 | 1434.72 |
| # of Standard Drinks Consumed (Treatment Entry to 15 Months) |
830 | 989.42 | 1318.18 | 618 | 916.17 | 1707.74 |
| Medical Test Results (At Treatment Entry) |
943 | 1.81 | 1.02 | 391 | 1.92 | 1.05 |
| Medical Test Results (At 15 Months) |
745 | 0.71 | 1.03 | 624 | 0.89 | 1.16 |
| BDI Score (At Treatment Entry) |
896 | 9.84 | 7.97 | 722 | 10.57 | 8.56 |
| BDI Score (At 15 months) |
825 | 7.09 | 7.65 | 680 | 8.97 | 9.13 |
Note. # of Standard Drinks Consumed was derived from the Form 90. Medical Test Results are the sum of abnormal results from 5 tests used to detect signs of liver, blood, kidney, and connective tissue disease. BDI = Deck Depression Inventory.
Table 4.
Baseline Prevalence of Psychiatric Disorders and Family History of Alcohol Use Disorders
| Outpatient N=882 |
Aftercare N=750 |
Total Sample N=1632 |
|
|---|---|---|---|
| Mood Disorder (%) | 30.57 | 36.05 | 33.02 |
| Anxiety Disorder (%) | 34.14 | 45.61 | 39.28 |
| ASPD (%) | 9.56 | 15.76 | 12.34 |
| Positive Family History (%)a | 78.89 | 74.29 | 76.83 |
Note. Mood, anxiety and antisocial personality disorder (ASPD) diagnoses were obtained with the Computerized Diagnostic Interview Schedule. Positive history of familial alcoholism (1st degree relatives) was obtained from the family history section of the Addictions Severity Index.
Outpatient group, n = 939; aftercare group, n = 761; total sample, N = 1,700.
Neuropsychological Measurement Model
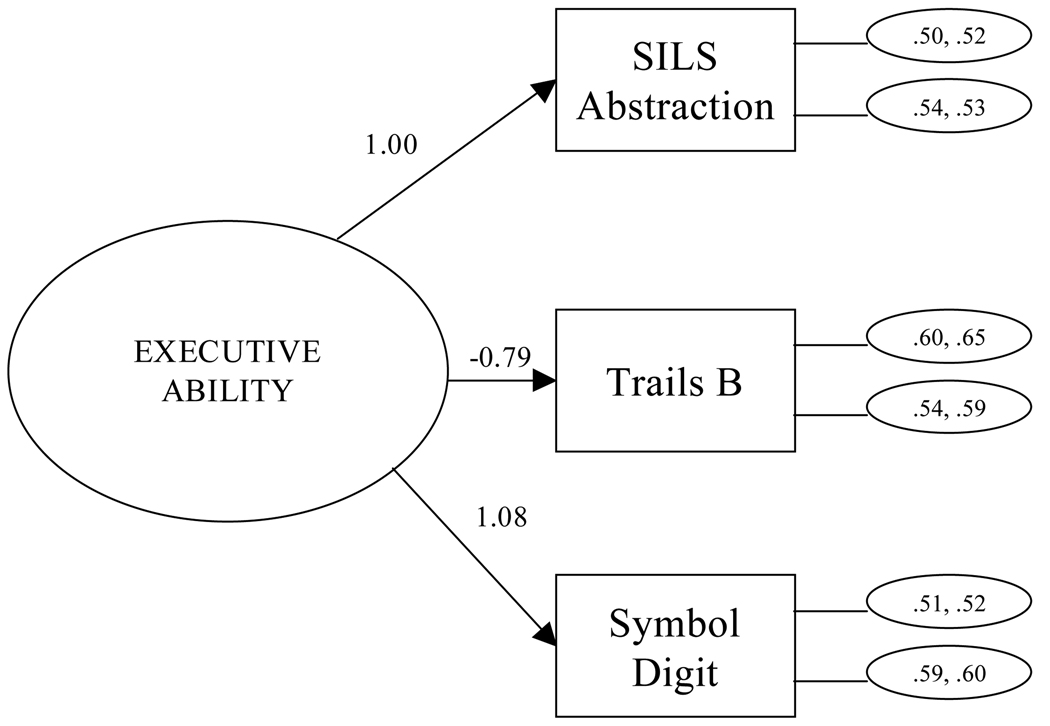
As can be seen from the model fit statistics in Table 5, both the one-factor general ability model that included all five tests and the two-factor model (executive, psychomotor speed) fit the data poorly. The alternative one-factor model of executive cognitive ability that included SILS Abstraction, TMT-B, and the SDMT provided a close fit to the data across time and samples. The chi-square statistic was significant, likely due to the large sample size, in view of all other fit indices that exceeded the cutoffs recommended as indicating close fit (Hu & Bentler, 1999; Yu & Muthén, 2002). Figure 1 shows the measurement model for outpatient and aftercare samples at baseline and 15 months. The latent executive function factor can be interpreted as supporting abstract reasoning, working memory, set shifting, cognitive flexibility, and the initiation and regulation of action. Executive functions are necessary for abstraction and novel problem solving in the intellectual arena and are also crucial to self-regulation and social problem solving (Damasio & Anderson, 2003; Stuss & Benson, 1986).
Table 5.
Goodness of Fit Statistics for three measurement models and one structural model
| Model | 1-Factor: General Cognitive |
2-Factor: Executive Function and Speed |
1-Factor: Executive Function |
Risk Factor Path Model |
|---|---|---|---|---|
| χ2 | 420.611 | 1183.303 | 54.459 | 559.966 |
| Df | 18 | 36 | 22 | 246 |
| P | 0.0000 | 0.0000 | 0.0001 | 0.0000 |
| RMSEA | 0.16 | 0.19 | 0.04 | 0.04 |
| CI | 0.15–0.18 | 0.18–0.20 | 0.03–0.06 | 0.03 – 0.04 |
| SRMR | 0.08 | 0.09 | 0.05 | 0.04 |
| CFI | 0.86 | 0.85 | 0.99 | 0.96 |
| TLI | 0.84 | 0.78 | 0.99 | 0.95 |
Note. df = degrees of freedom, RMSEA = root-mean-squared error of approximation, CI = 90% confidence interval of the RMSEA, SRMR = standardized root-mean-squared residual, CFI = comparative fit index, TLI = Tucker-Lewis Index
Figure 1.
Measurement Model: Baseline and 15 Months. Residuals for outpatient sample (baseline, 15-months) are in the top ovals connected to each test on the right side of the diagram; aftercare sample residuals are in the bottom ovals.
Constraining the test intercepts to be equal across time did not cause a significant decrease in model fit (p > .05), suggesting the absence of practice effects on performance across 15 months. Yet, within this constrained model, the outpatient sample had significantly higher latent ability levels than the aftercare sample at both assessment times (treatment entry: 0.000 [outpatient], −0.952 [aftercare]; 15 months: 0.342 [outpatient], −0.784 [aftercare]; p <.05). This finding replicates and extends the greater severity of deficit previously noted in the aftercare sample at treatment entry (Donovan et al., 2001).
The increase in the latent means across 15 months is statistically significant in both samples (p < .05). These significant latent mean increases, combined with the lack of significant change in each test’s intercept, support the idea that temporal improvements in executive ability were due to recovery of function, not practice effects. At the same time, the ESs for recovery are small. Although there is significant debate within the field as to how to best derive clinical significance of neuropsychological data (e.g., Jacobson, Roberts, Berns, & McGlinchey, 1999), researchers commonly use approaches such as measuring ES or using cutoff values based on confidence intervals. Unfortunately, established measures of clinical significance use manifest, rather than latent, variables. Despite this complication, the small ESs found here suggest that at the level of the group mean, changes in the latent executive ability factor do not represent clinically significant increases over time.
Risk Factor Correlates of Latent Neuropsychological Abilities
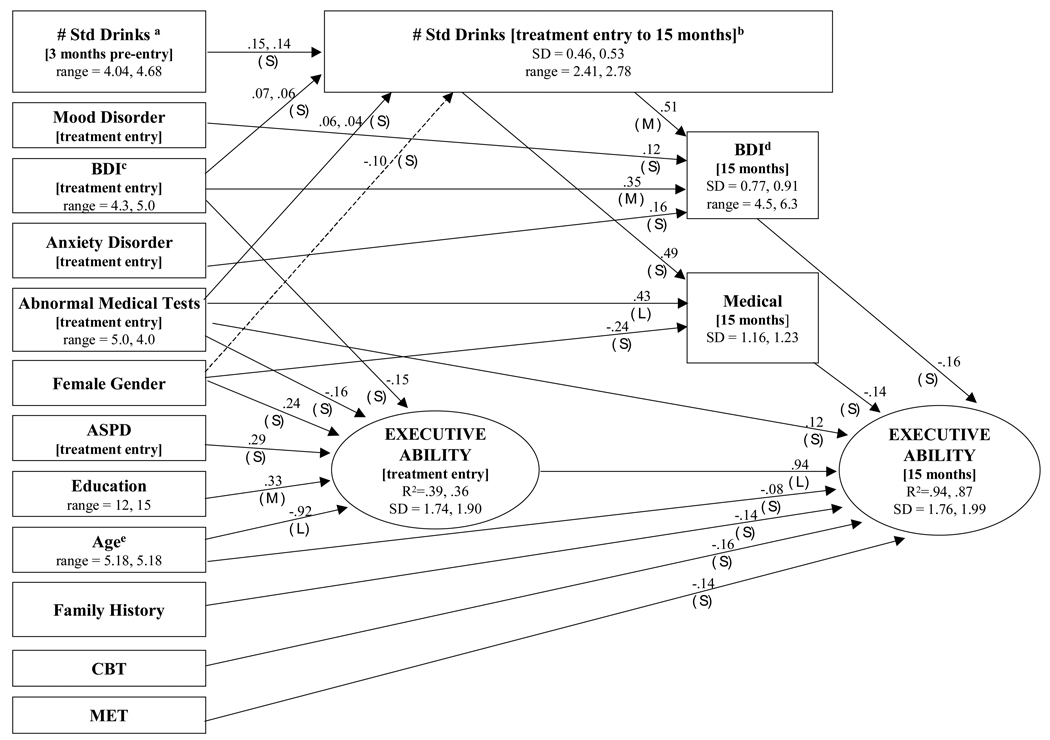
The initial model included hypothesized direct and indirect paths from the risk covariates to the latent neuropsychological ability factor at baseline and 15-month follow-up, and then nonsignificant and small paths were sequentially eliminated until there was a significant decrease in model fit according to the chi-square difference test. As shown in Table 5, the final path model yielded a significant chi-square, but other fit indices suggest a close agreement to the data, with the entire 90% confidence interval for the root-mean-square error of approximation falling below recommended cutoffs for close fit. Figure 2 shows the final path model, including unstandardized path coefficients and an indication of the magnitude of estimated ESs (expressed as proportion of variance) for all significant paths. Standard deviations of the latent factors and continuous manifest variables are provided because the effect sizes of unstandardized path coefficients need to be interpreted with respect to the standard deviations of the variables. For ease of interpretation, ESs < .10 were coded as small, ESs = .10 –.24 were coded as medium, and ESs = .25 and greater were coded as large (Murphy & Myors, 2004). With one exception (gender effect on standard drinks following treatment entry in aftercare), all paths were replicated across samples. With three exceptions (paths with dual coefficients), the absolute magnitude of the coefficients was replicated across samples (constraining the path coefficients to be equal across samples did not cause a significant increase in chi-square). Replication of the pattern and magnitude of the paths across independent samples supports the reliability of the results.
Figure 2.
Risk factor Path Model. All significant paths were replicated across outpatient and aftercare samples with the exception of the path from female gender to alcohol consumption (dashed line). When unstandardized outpatient and aftercare path coefficients or standard deviations differed, the outpatient value was listed first. For dichotomous variables, the unstandardized path coefficients represent the difference between the ability means of the two groups. The point ranges of continuous variables are shown in parentheses; standard deviations (SD) of the latent factors and continuous manifest variables are provided because the effect sizes of unstandardized path coefficients need to be interpreted with respect to the standard deviations of the variables. For the readers’ convenience, paths with estimated effect sizes (ES, unique proportion of variance explained) <.10 were coded as small (S), ES = .10 – .24 as medium (M), and ES = .25 and up as large (L) (Murphy & Myors, 2004). Note that several continuous variables were rescaled to facilitate iterative estimation: # Std Drinksa = square root (# Std Drinks/300); # Std Drinksb (treatment entry to 15 months) = square root (# Std Drinks (treatment entry to 15 months/1800); BDIc = BDI/10; BDId = BDI/10; and Agee = Age/11.
Executive Ability at Baseline
Younger age and higher education were the strongest predictors of executive ability. Depression symptoms, abnormal medical test results, male gender, and the absence of ASPD1 were associated with poorer ability in both groups, although their unique ESs were much smaller. As anticipated, because of random assignment, no differences in initial level of executive ability were observed between participants in the three alcohol treatments. The present results generally replicate, in a sample of persons with primary alcohol use disorders, previous research using clients with primary drug use disorders or alcohol and drug use disorders that has shown that similar risk covariates account for a notable proportion of true variance in executive ability at addiction treatment entry (e.g., Bates et al., 2002; Fals-Stewart & Bates, 2003).
Mediators and Executive Recovery at 15 Months
Baseline medical test results,2 older age, and a positive family history status directly predicted less neuropsychological recovery, although their unique ESs were modest. The influence of several other of the risk covariates on recovery was mediated through their influence on cognitive status at treatment entry. Intensity of alcohol consumption between baseline and 15 months in both groups was predicted by alcohol use in the 3 months prior to treatment, depression symptoms at baseline, and abnormal baseline medical test results. This suggests that severity of alcohol dependence, depressive symptoms, and physical health problems are associated with a poorer drinking prognosis for individuals entering alcohol treatment. Greater alcohol consumption across 15 months was a moderately strong predictor of less improvement in medical problems and depressive symptoms, and the influence of alcohol consumption on cognitive recovery was fully mediated by these effects. These findings underscore the importance of treating comorbid health problems and depression in alcohol-treatment clients.
Finally, participants assigned to MET and CBT showed significantly less improvement in latent executive ability compared with those assigned to TSF. Our aim in including treatment modality in the model was exploratory, as the three treatments were neither designed nor modified to affect cognitive recovery. Given the higher rate of abstinence among those in the TSF condition relative to the other treatments (Project MATCH Research Group, 1998), the techniques of TSF may have potentially contributed to cognitive recovery by increasing the likelihood of sustained abstinence and/or by providing a social environment conducive to cognitive rehabilitation. TSF incorporated the principles of Alcoholics Anonymous (AA) and encouraged clients to attend daily AA meetings, reinforcing the inherent structure of AA with additional guidance tailored to an individual client’s specific needs and challenges. One may speculate that aspects of TSF have a subtle yet positive effect on cognition because TSF attempts to enhance motivation by breaking complex, long-term goals into small manageable subgoals, allowing clients to accumulate a history of successes. This technique has been recommended in enhancing treatment adherence among cognitively impaired individuals who may have motivational deficits related to their organic cognitive deficits (Heinssen, 1996; Jeffrey, 1981). The ESs of the statistically significant negative paths from CBT and MET to cognitive ability at 15 months were small, however, suggesting that enhancement of cognitive recovery directly related to participation in TSF was modest at best.
Baseline ability and risk factors together predicted 94% of the variance in executive ability at the 15-month follow-up in the outpatient sample and 87% in the aftercare sample. Baseline ability uniquely accounted for 62% of the variance in executive ability at 15 months. As noted above, the ESs for individual risk covariates and treatments were small (each accounting uniquely for <1% of the variance), yet collectively they accounted for large proportions of the variance in executive ability at 15 months (32% in outpatient group, 25% in aftercare group). Thus, the overall pattern of findings suggests that although the average level of change in executive functioning in the samples was small, multiple risk factors in combination contributed to individual differences in cognitive recovery. That is, risk factors for impairment assessed at treatment entry were indirectly informative about the likelihood of cognitive improvement via relations to baseline ability. Over time, the influence of these factors combined with familial alcoholism history, age, treatment approach, and less than normative improvement in depression and medical problems to influence differential recovery.
It is important to note that these results are limited to executive cognitive ability and may not generalize to other cognitive domains such as memory or verbal abilities. Additional limitations include missing data at follow-up and assessed risk covariates that are not comprehensive. In addition, speculations regarding the advantages of TSF should be considered very cautiously. In view of the small ES of increases in the latent ability mean, the present findings question the extent of normative cognitive recovery that might be expected to occur in a spontaneous fashion following alcohol treatment without cognitive rehabilitation. It is possible that some previous studies overestimated the extent of spontaneous recovery in substance use disordered samples by focusing primarily on the statistical significance of increases in test performance. The modest average recovery found here and the small size of unique treatment effects on recovery point to the need for addiction treatments that are specifically modified or designed to promote cognitive recovery in clients with moderate to severe cognitive impairment. In an earlier study, Fals-Stewart and Lucente (1994) found that patients who received cognitive rehabilitation while in long-term residential substance abuse treatment exhibited an accelerated rate of cognitive recovery early in treatment and better long-term outcomes, suggesting that cognitive rehabilitation may enhance this important aspect of recovery from alcohol and other drug use disorders.
Acknowledgments
This study was supported by National Institute of Alcohol Abuse and Alcoholism Grants P50 AA 08747, AA 11594, and K02 AA 00325.
We thank Frances Del Boca for her help with the medical test and neuropsychological data sets and J. Scott Tonigan for his assistance with the risk covariate data sets from Project MATCH.
Footnotes
Preliminary results of this study were presented at the 24th Annual Scientific Meeting of the Research Society on Alcoholism, Montreal, Quebec, Canada, June 2001, and at the 25th Annual Scientific Meeting of the Research Society on Alcoholism, San Francisco, California, June 2002.
The positive association between ASPD and baseline cognitive ability (ES = small) was unanticipated and may have been due to differential ASPD-subtype representation (Gorton, Swirsky-Sacchetti, Sobel, Samuel, & Gordon, 1999) or concurrent control of risk covariates (Waldstein, Malloy, Stout, & Longabaugh, 1996).
A nonpredicted positive path between abnormal baseline medical tests and higher cognitive ability at 15 months was significant (ES = small). The emergence of this path in the model most likely indicates that changes in medical test results over time had more influence on executive ability than either baseline or 15-month values alone.
Contributor Information
Marsha E. Bates, Center of Alcohol Studies, Rutgers, The State University of New Jersey
Danielle Barry, Center of Alcohol Studies, Rutgers, The State University of New Jersey.
Erich W. Labouvie, Center of Alcohol Studies, Rutgers, The State University of New Jersey
Jennifer F. Buckman, Center of Alcohol Studies, Rutgers, The State University of New Jersey
William Fals-Stewart, Research Institute on Addictions, State University of New York at Buffalo.
Gerald Voelbel, Psychology Department, Rutgers, The State University of New Jersey.
References
- Allen DN, Goldstein G, Seaton BE. Cognitive rehabilitation of chronic alcohol abusers. Neuropsychology Review. 1997;7(1):21–39. doi: 10.1007/BF02876971. [DOI] [PubMed] [Google Scholar]
- Allison PD. Missing data. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: Author; 1987. rev. [Google Scholar]
- Bates ME. Stability of neuropsychological assessments early in alcoholism treatment. Journal of Studies on Alcohol. 1997;58:617–621. doi: 10.15288/jsa.1997.58.617. [DOI] [PubMed] [Google Scholar]
- Bates M, Convit A. Neuropsychology and neuroimaging of alcohol and illicit drug abuse. In: Calev A, editor. The Assessment of Neuropsychological Functions in Psychiatric Disorders. Washington, DC: American Psychiatric Press; 1999. pp. 373–445. [Google Scholar]
- Bates ME, Labouvie EW, Voelbel GT. Individual differences in latent neuropsychological abilities at addictions treatment entry. Psychology of Addictive Behaviors. 2002;16:35–46. doi: 10.1037//0893-164x.16.1.35. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Damasio AR, Anderson SW. The frontal lobes. In: Heilman K, Valenstein E, editors. Clinical neuropsychology. 4th edition. New York: Oxford University Press; 2003. pp. 404–446. [Google Scholar]
- Donovan DM, Kivlahan DR, Kadden RM, Hill D. Project MATCH Monograph Series: Vol. 8. Project match hypotheses: Results and causal chain analyses. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 2001. Cognitive impairment as a patient-treatment matching hypothesis; pp. 62–81. [Google Scholar]
- Fals-Stewart W. Ability of counselors to detect cognitive impairment among substance-abusing patients: An examination of diagnostic efficiency. Experimental and Clinical Psychopharmacology. 1997;5(1):39–50. doi: 10.1037//1064-1297.5.1.39. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Bates ME. The neuropsychological test performance of drug-abusing patients: An examination of latent cognitive abilities and associated risk factors. Experimental and Clinical Psychopharmacology. 2003;11:34–45. doi: 10.1037//1064-1297.11.1.34. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Lucente S. The effect of cognitive rehabilitation on the neuropsychological status of patients in drug abuse treatment who display neurocognitive impairment. Rehabilitation Psychology. 1994;39:75–94. [Google Scholar]
- Fals-Stewart W, Shanahan T, Brown L. Treating alcoholism and substance abuse: A neuropsychiatric perspective. Psychotherapy in Private Practice. 1995;14:1–21. [Google Scholar]
- Goldman M. Recovery of cognitive functioning in alcoholics - the relationship to treatment. Alcohol Health & Research World. 1995;19:148–154. [PMC free article] [PubMed] [Google Scholar]
- Gorton GE, Swirsky-Sacchetti T, Sobel R, Samuel S, Gordon A. Neuropsychological functions in personality disorder. In: Calev A, editor. Assessment of neuropsychological functions in psychiatric disorders. Washington, DC: American Psychiatric Press; 1999. pp. 233–280. [Google Scholar]
- Heinssen RK. The cognitive exoskeleton: Environmental interventions. In: Corrigan PW, Yudofsky SC, editors. Cognitive Rehabilitation for Neuropsychiatric Disorders. Washington, DC: American Psychiatric Press; 1996. pp. 395–423. [Google Scholar]
- Hesselbrock MN, Weidenman MA, Reed HB. Effect of age, sex, drinking history and antisocial personality on neuropsychology of alcoholics. Journal of Studies on Alcohol. 1985;46(4):313–320. doi: 10.15288/jsa.1985.46.313. [DOI] [PubMed] [Google Scholar]
- Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining the clinical significance of treatment effects: Description, application, and alternatives. Journal of Consulting and Clinical Psychology. 1999;67:300–307. doi: 10.1037//0022-006x.67.3.300. [DOI] [PubMed] [Google Scholar]
- Jeffrey DL. Cognitive clarity: Key to motivation in rehabilitation. Journal of Rehabilitation. 1981;47:33–35. [PubMed] [Google Scholar]
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Project MATCH Monograph Series: Vol. 3. Cognitive Behavioral Coping Skills Therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- Kline RB. Principles and practices of structural equation modeling. New York: Guilford Press; 1998. [Google Scholar]
- Knight RG, Longmore BE. Clinical neuropsychology of alcoholism. Hillsdale, NJ: Erlbaum; 1994. [Google Scholar]
- Lehman LB, Pilich A, Andrews N. Neurological disorders resulting from alcoholism. Alcohol Health and Research World. 1993;17(4):305–309. [Google Scholar]; Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Little R, Rubin D. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- Malloy P, Noel N, Rogers S, Longabaugh R, Beattie M. Risk factors for neuropsychological impairment in alcoholics: Antisocial personality, age, years of drinking and gender. Journal of Studies on Alcohol. 1989;50:422–426. doi: 10.15288/jsa.1989.50.422. [DOI] [PubMed] [Google Scholar]
- Marsano L. Alcohol and malnutrition. Addictions Nursing. 1994;6:62–71. [Google Scholar]
- McLellan AT, Luborski L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. The Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom GP, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller WR. Project MATCH Monograph Series: Vol. 5, Manual for Form 90: A structured assessment interview for drinking and related behaviors. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1996. [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. Journal of Studies on Alcohol. 1994 Suppl. 12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Project MATCH Monograph Series: Vol. 2. Motivational Enhancement Therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- Murphy KR, Myors B. Statistical power analysis. 2nd. Ed. Mahwah, NJ: Erlbaum; 2004. [Google Scholar]
- Muthén L, Muthén B. Mplus: The comprehensive modeling program for applied researchers: User’s guide. Los Angeles: Muthén & Muthén; 1998. [Google Scholar]
- Naehri V, Laaksonen S, Hietala R, Ahonen T, Lyyti H. Treating missing data in a clinical neuropsychological dataset – data imputation. Clinical Neuropsychologist. 2001;15:380–392. doi: 10.1076/clin.15.3.380.10266. [DOI] [PubMed] [Google Scholar]
- Nixon SJ. Assessing cognitive impairment. Alcohol Health and Research World. 1995;19:97–103. [PMC free article] [PubMed] [Google Scholar]
- Nowinski J, Baker S, Carroll K. Project MATCH Monograph Series: Vol. 1. Twelve Step Facilitation Therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1992. [Google Scholar]
- Parsons OA. Neurocognitive deficits in alcoholics and social drinkers: A continuum? Alcoholism: Clinical and Experimental Research. 1998;22:954–961. [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project match three-year drinking outcomes. Alcoholism: Clinical & Experimental Research. 1998;22:1300–1311. doi: 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological battery: Theory and clinical implications. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Robins L, Helzer J, Cottler L, Goldring E. NIMH Diagnostic Interview Schedule: version III revised (DIS-III-R), question by question specifications. St. Louis: Washington University; 1989. [Google Scholar]
- Rourke SB, Grant I. The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: A 2 year follow-up study. Journal of the International Neuropsychological Society. 1999;5:234–246. doi: 10.1017/s1355617799533067. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Løberg T. The neurobehavioral correlates of alcoholism. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. New York: Oxford University Press; 1996. pp. 423–485. [Google Scholar]
- Schafer K, Butters N, Smith T, Irwin M, Brown S, Hanger P, Grant I, Schuckit M. Cognitive performance of alcoholics: A longitudinal evaluation of the role of drinking history, depression, liver function, nutrition, and family history. Alcoholism: Clinical and Experimental Research. 1991;15:653–660. doi: 10.1111/j.1530-0277.1991.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test (SDMT). Manual. Los Angeles: Western Psychological Services; 1982. Revised. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms and commentary. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and related neurological disorders due to alcoholism and malnutrition. Philadelphia: F.A. Davis; 1989. [Google Scholar]
- Waldstein SR, Malloy PF, Stout R, Longabaugh R. Predictors of neuropsychological impairment in alcoholics: Antisocial versus nonantisocial subtypes. Addictive Behaviors. 1996;21:21–27. doi: 10.1016/0306-4603(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Yu C-Y, Muthén B. Evaluation of model fit indices for latent variable models with categorical and continuous variables. 2002 Unpublished manuscript. [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]