Abstract
BACKGROUND
Multiple drug immunosuppression has allowed the near elimination of rejection, but without commensurate improvements in longterm graft survival and at the cost of quality of life. We have suggested that transplantation outcomes can be improved by modifying the timing and dosage of immunosuppression to facilitate natural mechanisms of alloengraftment and acquired tolerance.
STUDY DESIGN
Two therapeutic principles were applied for kidney transplantation: pretransplant recipient conditioning with antilymphoid antibody preparations (Thymoglobulin [Sangstat] or Campath [ILEX Pharmaceuticals]), and minimal posttransplant immunosuppression with tacrolimus monotherapy including “spaced weaning” of maintenance doses when possible. The results in Thymoglobulin- (n = 101) and Campath-pretreated renal transplantation recipients (n = 90) were compared with those in 152 conventionally immunosuppressed recipients in the immediately preceding era.
RESULTS
Spaced weaning was attempted in more than 90% of the kidney transplant recipients after pretreatment with both lymphoid-depleting agents, and is currently in effect in two-thirds of the survivors. Although there was a much higher rate of acute rejection in the Thymoglobulin-pretreated recipients than in either the Campath-pretreated or historic control recipients, patient and graft survival in both lymphoid depletion groups is at least equivalent to that of historic control patients. In the Thymoglobulin-conditioned patients for whom followups are now 24 to 40 months, chronic allograft nephropathy (CAN) progressed at the same rate as in historic control patients. Selected patients on weaning developed donor-specific nonreactivity.
CONCLUSIONS
After lymphoid depletion, kidney transplantation can be readily accomplished under minimal immunosuppression with less dependence on late maintenance immunosuppression and a better quality of life. Campath was the more effective agent for pretreatment. Guidelines for spaced weaning need additional refinement.
Kidney transplantation became a practical clinical service after it was shown that rejections developing under azathioprine were highly reversible with prednisone, and that the reversals often were succeeded by the emergence of variable donor-specific nonreactivity (ie, tolerance).1 In 1966, anti-lymphoid globulin (ALG) was added to azathioprine and prednisone as a steroid-sparing adjunct.2 A short course of antilymphoid globulin was begun preoperatively and continued for several posttransplant days or weeks.2,3 The pre-transplant portion of the course was subsequently deemphasized and omitted because of uncertainty about its value and because the time constraints of cadaveric transplantation made the pretreatment impractical. Instead, antilymphoid globulin usually was started on the day of, or day after, transplantation (induction therapy), and in addition, prednisone or other agents were increasingly instituted at this time in multiple drug regimens designed to eliminate the threat of acute rejection.4
After elucidation of the mechanisms of alloengraftment,5,6 It was apparent that this treatment policy could subvert the seminal mechanism of clonal exhaustion-deletion.7 Consequently, we suggested modifications of immunosuppression in accordance with two principles. The first was lymphoid depletion before rather than after transplantation to reduce the anticipated donor-specific response into a more easily deletable range. The second was avoidance of so much posttransplant immunosuppression that the immune activation-dependent mechanism of clonal exhaustion-deletion would be interdicted. Pretransplant conditioning was done with a single infusion of rabbit antithymocyte globulin (rATG, Thymoglobulin [Genzymel]),8,9 or alternatively, of alemtuzumab (Campath IH [ILEX Pharmaceuticals]).10–14 Minimalistic posttransplant immunosuppression was begun with relatively low doses of tacrolimus monotherapy with the intention of dose weaning after the first few months of highest immunologic risk. Our initial experience15,16 and that reported here suggest that this approach to management can be carried out efficiently and safely.
METHODS
Institutional review process
Modifications in the timing and dosage of conventional immunosuppression were undertaken in July 2001. The primary purpose was to improve the quality-of-life outcomes and patient and graft survival across the full spectrum of all kinds of adult kidney recipients in our clinical practice. The modifications were submitted in this context to the University of Pittsburgh Institutional Review Board (IRB), which judged the changes to be within the boundaries of historically based standard treatment. The treatment protocols were reviewed by the Presbyterian University Hospital Committee on Innovative Practices and the Pharmacy and Therapeutic Practices Committee, with approval by both. All patients provided informed consent. In addition, separate informed consent was obtained with IRB approval for studies of immune variables not routinely assayed in our conventional practice. Safety and efficacy monitoring were assured by formal weekly reviews of all patients.
Patient selection
No adult kidney recipients were denied access to the reforms in management because of high risk factors. Recipients who received previous, simultaneous, or subsequent nonkidney solid organ allografts or bone marrow were removed from this analysis, as were kidney-only transplantations performed in the period of time when Thymoglobulin and Campath pretreatment were both used. The three study populations were compiled during the eras of March 2000 to July 2001 (historic controls, no pretreatment: n = 152); July 2001 to October 2002 (Thymoglobulin pre-treatment: n = 101); and March 2003 to September 2003 (Campath pretreatment: n = 90) (Table 1).
Table 1.
Population Characteristics
| Characteristic | Historic controls | Thymoglobulin pretreatment | Campath pretreatment |
|---|---|---|---|
| n | 152 | 101 | 90 |
| Accrual dates | 3/00 to 7101 | 7/01 to 10/02 | 3/03 to 9/03 |
| Followup, (mo) | 39 to 54 | 23 to 39 | 12 to 18 |
| Recipient age, (y) | 50.6 ± 14.9 | 51.1 ± 14.5 | 50.8 ± 16.6 |
| Recipient gender (M/F), % | 61/39 | 65/35 | 63/37 |
| African-American recipients, % | 14 | 14 | 18 |
| Primary Tx/Re-Tx, % | 78/22 | 85/15 | 87/13 |
| Recipient PRA > 20%, % | 26 | 18 | 19 |
| Donor age, (y) | 35.5 ± 18.0 | 39.4 ± 15.4 | 41.7 ± 16.0 |
| Donor gender (M/F), % | 57/43 | 40/60 | 47/53 |
| African-American donors, % | 10 | 9 | 8 |
| Living/cadaveric donors, % | 20/80 | 46/54 | 39/61 |
| Sibling-sibling | 12 | 14 | 6 |
| Parent-offspring | 1 | 6 | 4 |
| Offspring-parent | 3 | 7 | 5 |
| Other related | 3 | 5 | 4 |
| Nonrelated (incl. spouse) | 11 | 14 | 16 |
| Cadaveric ischemia time, (h) | 27.7 ± 8.69 | 26.4 ± 6.37 | 20.8 ± 7.64 |
| ABDR mismatch | 3.21 ± 1.61 | 3.12 ± 1.72 | 3.64 ± 1.57 |
Significant variations of the lymphoid depletion/minimum immunosuppression populations as compared with the historic controls are highlighted in bold with p ranging from 0.04 to less than 0.001. All significant differences in the populations are attributable to the cadaveric donor subgroup, because live donor characteristics were statistically similar.
ABDR,; PRA, panel reactive antibodies; Tx, transplant; Re-Tx
Differences in donor characteristics reflected nationwide efforts to expand the donor pool by using older deceased donors and more living donors. The large complement of nonrelated live donors (mostly spousal) is noteworthy. The mean histocompatibility match was worst and the mean ischemic time best in the Campath pretreatment series. Differences in recipient demographic factors were not statistically significant.
Immunosuppression
Historic controls
Patients treated between March 2000 and June 2001 received multidrug immunosuppression that included tacrolimus, a 5-day intravenous and oral prednisone taper (200 → 40 mg in 40 mg decrements) followed by oral prednisone (20 mg/d) and often a third agent (usually mycophenolate mofetil or sirolimus). The multiagent immunosuppression (particularly the steroid component) was weaned slowly throughout the first 6 to 12 posttransplant months.
Lymphoid depletion
Lymphoid depletion between July 2001 and October 2002 was done with an infusion of 5 mg/kg rabbit anti-thymocyte globulin (rATG, Thymoglobulin). The Thymoglobulin was administered over several hours before allograft reperfusion. The antibody infusion was accompanied by 1 or 2 g methylprednisolone to prevent cytokine release consequences. The same steroid doses were used when alemtuzumab (anti-CD 52 mAb, Campath I-H) infusion of 30 mg was substituted for Thymoglobulin as the conditioning agent.
Mlnimalistic immunosuppression
The lymphoid depleted patients were started on twice daily tacrolimus (Prograf) on postoperative day 1, with a target 12-hour trough level of 10 ng/mL. In a few cases, sirolimus (or cyclosporine) was substituted for tacrolimus because of nephro- or neurotoxicity. Renal function was monitored primarily with serum creatinine determinations. Suspected rejection was confirmed by biopsy and treated with one or more boluses of methylprednisolone, muronmonab-CD3 (OKT3), or alemtuzumab. Oral steroids or other secondary agents such as sirolimus were added only as necessary.
Spaced weaning
At some time after 3 to 4 months in lymphoid depleted patients who had been stable on tacrolimus monotherapy, the twice daily doses were consolidated to a single daily dose. For example, someone on 2 mg twice a day would be converted to a single 4-mg dose. One and a half or more months later, weaning to every-other-day dosing was begun (ie, 4 mg every other day). Subsequent weaning to three times weekly, twice weekly, and once weekly tacrolimus was instituted on an individualized basis.
Cellular immunologic monitoring
Peripheral blood mononuclear cells were obtained from recipients who had been followed up for 1 year or more. The cells were separated by a standard gradient centrifugation method17 and used fresh for in vitro functional assays.
Leukocyte surface markers
Three- or four-color flow cytometry with appropriate flurochrome conjugated mAb combinations, and isotype-matched nonspecific mAbs (negative controls) were used to determine lymphocyte subsets. Data acquisition and analysis was performed on a Coulter EPICS XL flow cytometer (Beckman Coulter Corp). In conventionally gated cells for lymphocytes, 50,000 CD45+ events were typically collected per sample and analyzed with EXP032 software (Applied Cytometry System).17, 18
Mixed lymphocyte reaction
Conventional unidirectional mixed lymphocyte reaction (MLR) cultures were set up for 6 days using 1 X 105 irradiated stimulator cells (2000R). The degree of [3H] thymidine incorporation was assessed during the final 20 hours of incubation.
Cell-mediated Iymphocytoxicify
The cytolytic activity of recipient lymphocytes toward donor and third party targets was assessed in cell-mediated lymphocytotoxicity. In these assays, effector lymphocytes were incubated with Cr-labeled donor phytohemagglutinin-induced target cells at various E:T ratios, ranging from 10: 1 to 30:1.
Limiting dilution assay
Cytolytic T lymphocyte precursor frequency was analyzed as previously described by Kaminski and colleagues19 using recipient cells as responders and donor cells as stimulators, with the addition on days 4 and 6 of recombinant interleukin-2 to fresh medium to give a final concentration of 10 U/mL. On day 10, a cytotoxic assay was carried out for each culture using 51Cr-labeled phytohemagglutinin-induced blast cells from the original stimulator as the targets. Supernatants harvested from each well were measured for 51Cr along with appropriate positive and negative controls; cytolytic T lymphocyte precursor frequency was considered as minimum or background with frequencies of less than 1 in 300,000.20,21
Antibody monitoring
Lymphocytotoxic crossmatches were negative in all cases. The pre- and posttransplant sera of 92 recipients were screened by ELISA for the presence of IgG anti-HLA class I and class II alloantibodies according to the manufacturer’s instructions (One Lambda Inc). The resulting optical densities were analyzed by LATTM software for Windows (One Lambda Inc).22,23
Pathologic studies
Posttransplant biopsies were not taken on protocol but rather because of clinical evidence (or suspicion) of rejection. But a few biopsies in the historic control patients were obtained in pursuit of unrelated research projects, and some of the lymphoid depleted patients had baseline biopsies before beginning spaced weaning.
Tissues were handled according to hospital procedures.15 Biopsy findings were categorized by the standardized Banff system24 and CAN was scored with a scale of 0 to 3 for each of four kinds of abnormality: glomerulopathy (cg), interstitial fibrosis (ci), tubular atrophy (ct), and chronic vasculopathy (cv).
Data management and statistical analysis
An honest brokering system approved by the University of Pittsburgh IRB was used for data management. Data were extracted, related, reviewed, augmented (where required) for accuracy and completeness, and deidentified for statistical analysis. Differences in means and standard deviations calculated from participant characteristics by treatment group were evaluated for the statistical significance using t-tests and ANOVAs for continuous comparisons and chi-square tests for categorical comparisons. Kaplan-Meier survival curves were generated and evaluated for significance using a log-rank test. Kaplan-Meier comparisons were adjusted for significant population differences to ascertain their effect. A p value <0.05 two sided was considered significant.
RESULTS
Survival and graft function
Survival
Survival of live donor grafts (Fig. 1) was better in both the Thymoglobulin- and Campath-pretreated patients than in the live donor historic controls (p ≤ 0.037). Otherwise, patient and graft survival were not significantly different in either of the lymphoid depletion populations versus the historic controls overall (p = 0.12 to 0.59) or in the subgroups of the respective cadaveric or liver donor recipients (p = 0.25 to 0.61); These findings did not change when survival was adjusted for population differences.
Figure 1.
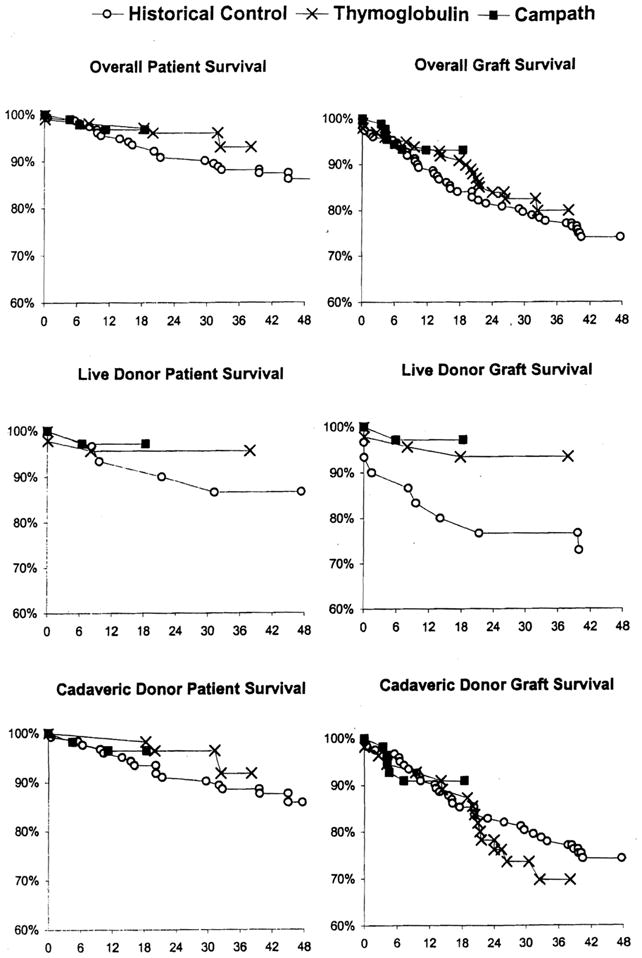
Patient and graft survival in historic control (○○), Thymoglobulin-pretreated (×), and Campath-pretreated (black squares) kidney recipients.
Graft function
Campath-pretreated patients had the best early mean serum creatinine, but there was no difference in the three populations at 1 year (see Table 2). Mean serum creatinine at 2 years and currently in Thymoglobulin-pretreated patients was higher than in historic controls, mostly because of four outliers (see footnote to Table 2).
Table 2.
Mean Creatinine (mg/dL) at Successive Posttransplant Times of Allografts that Still Function
| n | 1 wk | 1 mo | 3 mo | 6 mo | 1 y | 2 y | 3 y | Current |
|---|---|---|---|---|---|---|---|---|
| Historic controls (n = 113) | ||||||||
| Mean | 4.45 | 2.03 | 1.75 | 1.68 | 1.56 | 1.48 | 1.53 | 1.64 |
| SD | 4.13 | 1.47 | 0.89 | 0.59 | 0.57 | 0.56 | 0.79 | 1.02 |
| Thymoglobulin (n = 83) | ||||||||
| Mean | 3.90 | 2.24 | 1.84 | 1.60 | 1.58 | 1.89* | — | 2.08* |
| SD | 4.32 | 1.14 | 1.08 | 0.62 | 0.55 | 0.95 | — | 1.23 |
| Campath (n = 84) | ||||||||
| Mean | 1.95 | 1.74 | 1.54 | 1.47 | 1.59 | — | — | 1.69 |
| SD | 1.60 | 0.71 | 0.58 | 0.54 | 0.75 | — | — | 0.83 |
| p Value | <0.001 | 0.025 | 0.073 | 0.051 | 0.957 | <0.001 | — | — |
Removal of 5% (n = 4) of the outlying results for the Thymoglobulin group results in 2-y and current creatinines of 1.74 ± 0.07 and 1.86 ± 0.86, respectively.
Rate of acute rejection
The three groups showed a markedly different incidence and time to acute rejection (Fig. 2). In the Thymoglobulin-pretreated patients, the onset of rejection was earlier (p < 0.001) and the incidence was higher than in either the Campath or historic control recipients. The incidence of rejection during the first 6 months after Campath pretreatment was 1%. Rejections that occurred after 6 months were frequently associated in both the Thymoglobulin- and Campath-pretreatment groups with attempts to space wean (see below).
Figure 2.
Incidence and time to first acute rejection in historic control (○○), Thymoglobulin-pretreated (×), and Campath-pretreated (black squares) recipients.
Weaning
After Thymoglobulin pretreatment
Spaced weaning was attempted in 91 (90.1 %) of the 101 recipients after a mean of 5.9 ± 1.4 months. Clinical courses were highly variable before and after weaning. The weaning process was uncomplicated in the majority of cases in which it was attempted (Fig. 3A and B). But in 45% of the patients in whom spaced weaning was started, daily therapy was resumed because of acute rejection. If the rejection promptly responded to 1 or 2 boluses of prednisone, less aggressive spaced weaning subsequently was resumed (Fig. 3C).
Figure 3.
Variable preweaning courses and weaning outcomes in Thymoglobulin-pretreated kidney recipients. Solid shade = daily tacrolimus (Tac) dosing, Spaced weaning represented by spikes that indicate frequency and dose. (A) Uncomplicated weaning from daily to once weekly doses of tacrolimus between 4 and 10 posttransplant mo in a cadaver kidney recipient who has been rejection free for more than 3 y. (B) Spaced weaning begun after 7 mo after a difficult rejection at 2½ mo. Tacrolimus doses have been three times per week for the past year in this recipient of a kidney from a live unrelated donor. (C) Reweaning in a patient who developed a mild rejection after 9 mo on one dose of tacrolimus per week. The eventual (and current) dose is three times per week. (D) Irreversible rejection of a cadaver kidney 4 wk after an attempt to reduce tacrolimus doses to every other day. The patient’s original disease was lupus nephritis.
Weaning either could not be successfully done or was never attempted in about one-third of the recipients. In some of these patients and in others in whom spaced weaned was never tried, grafts were lost to nonreversible rejection. In the patient depicted in Figure 3D, the original renal disease was lupus nephritis.
With followups of 24 to 39 months, 68% of the 83 patients with currently surviving grafts are on spaced doses of maintenance immunosuppression. Another 25% are on daily monotherapy. Only 7% are receiving more than one immunosuppressant (Table 3).
Table 3.
Current Immunosuppression for Recipients with Surviving Grafts
| Immunosuppression | Historic controls (n= 113) | Thymoglobulin (n = 83) | Campath (n = 84) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Daily monotherapy | 46 | 41 | 21 | 25 | 12 | 14 |
| Multidrug therapy | 67 | 59 | 6 | 7 | 10 | 12 |
| Spaced dose weaning | — | 56 | 68 | 62 | 74 | |
| Once a week | — | 12 | — | |||
| Twice a week | — | 18 | 6 | |||
| Three times a week | — | 17 | 40 | |||
| Every other day | — | 9 | 16 | |||
After Campath pretreatment
Spaced weaning was attempted in 83 (91.2%) of the 90 patients after a mean of 6.3 ± 1.2 months. The decision to go forward with weaning was more straightforward than it was in the Thymoglobulin-conditioned patients because of the nearly complete absence of preweaning rejection. In addition, the incidence of postweaning rejection (20%) has been lower (Fig. 2).
With followups of 12 to 18 months, 62 (74%) of the 84 Campath-pretreated patients are on spaced weaning, 14% are on daily monotherapy, and only 12% are receiving more than a single agent (Table 3).
Morbidity
In the historic controls, the incidence of symptomatic infections by cytomegalovirus and by BK virus was 4.5% and 3.2%, respectively. The incidence of posttransplant lymphoproliferative disease was 2%, and 9.7% had new onset diabetes. These complications were not seen in the lymphoid depleted patients, except for a 2% incidence of BK virus infection in the Thymoglobulin series and a 2.2% incidence of new onset diabetes in the Campath patients.
Pathology
Acute rejection
Acute cellular rejection was diagnosed and graded for severity according to the Banff 1997 schema of renal allograft pathology.24 Patients were biopsied only when there was a rise in creatinine. Several of these specimens showed intimal arteritis, glomerulitis, and focal or diffuse C4d deposition. The incidence of subclinical rejection and its effect on graft morphology in the nonbiopsied patients cannot be addressed because no protocol biopsies were performed. It is notable that only one rejection episode occurred during the preweaning period after a Campath infusion.
Chronic allograft nephropathy
The numbers of recipients having a baseline biopsy within the first 30 days and later biopsies in the historic control, Thymoglobulin- and Campath-pretreated groups were 58 (38%), 52 (51%), and only 8 (9%), respectively. These frequencies largely reflected the relative incidence of early renal dysfunction from primary graft dysfunction or acute rejection. Although these and subsequent samples were in no sense protocol biopsies, enough histopathology was available to tentatively assess the posttransplant progression of CAN.
Only a minority of the baseline (control) biopsy samples were completely free of findings that contribute to the 12-point maximum CAN score. The baseline CAN scores averaged from 1.3 to 2.0 to 2.6 in the three successively compiled series. The acceptance of kidneys with an increasing incidence and severity of preexisting donor disease reflected nationwide efforts to expand the organ pool, ie, by relaxing the criteria for organ acceptance.
During the first year posttransplantation, CAN progressed from baseline by 2.5 points in recipients pre-treated with Thymoglobulin, and by 3.0 points in the historic control patients. The 1-year increase was only 1.4 points in the recipients pretreated with Campath, but this was based on a very small number of biopsies.
In years 2 and 3 posttransplantation, average CAN progressed an additional 1.7 and 0.34 points, respectively, for the Thymoglobulin patients and 1.5 and 1.2 points, respectively, in the historic controls. In the absence of protocol biopsies, an important caveat to the third year value is that very few samples were available at this time point: 13 in the reference group and 7 in the Thymoglobulin group.
Cellular Immunologic monitoring
Pretreatment with 5 mg/kg Thymoglobulin, as previously reported,14,25 resulted in profound depletion of T cells (CD3+) by postoperative day 1. T cell counts remained below baseline for 3 months, with a gradual return toward baseline values. The CDB population recovered more promptly than the CD4 population, resulting in an inverted CD4/CDB ratio that persisted up to 6 months posttransplant. B cells were not depleted. The T cell depletion was considerably greater and more sustained with Campath, and in addition, B cells were notably depleted.
Approximately 1 year after transplantation, more detailed studies were obtained in eight of the Thymoglobulin-pretreated patients. Seven of the eight were on spaced weaning, with doses of one per week (n = 2), two per week (n = 1), and three per week (n = 4). The eighth patient was on multiple drugs. The mean CD4/CDB ratio, which had been 2.3 before lymphoid depletion, remained less than 1.0 in five of the eight patients between 1.38 and 1.5 in the other three.
At 1 year, all seven of the patients on spaced weaning had donor-specific hypo reactivity demonstrated with MLR; the patient on multiple drugs had global hyporesponsiveness. In six of the seven hyporesponsive patients, kidney function was excellent at the time of testing. The kidney of the seventh patient was failing at the time of testing (serum creatinine 3.7 mg/dL). Biopsy of this allograft revealed recurrent membranous glomerulonephritis similar to that which had destroyed the native kidney, but with no histopathologic evidence of rejection.
In additional studies of four of the seven patients with MLR evidence of donor-specific hyporeactivity, the absence of donor cell killing was confirmed by cell-mediated lymphotoxicity assay, the essential absence with limiting dilution assay of cytolytic T lymphocyte precursor, or both findings. But one of these patients, who was on one dose per week at the time, subsequently developed a mild rejection that was treated with a single dose of prednisone and temporary reinstitution of daily tacrolimus. This patient is presently on three doses of tacrolimus per week.
Antibody monitoring
Serial samples were available from 92 of the 101 Thymoglobulin-pretreated recipients, all of whom had negative conventional crossmatches with their donors. In 59 (64%) of these patients, nonspecific anti-HLA antibodies were never detectable before or after transplantation. In 14 of the other 33, anticlass I antibodies predated transplantation (n = 12) or developed afterward (n = 2). These disappeared in 9 who had adequate subsequent samples, and they were known to persist in only 2 of the others. Similarly, the isolated finding of anticlass II antibodies before or after transplantation was frequently temporary or it waxed and waned. There were only three examples of a class switch or addition.
Combined anticlass I and class II antibodies predated transplantation in five patients and developed de novo after 2,4, and 12 months in the three others. Of interest, four of the five patients with both kinds of preformed antibodies received zero mismatched kidneys. In two cases, the antibodies disappeared after transplantation.
Except for a trend to poorer results in patients with anti class II antibodies (ie, graft losses or death at 8, 21, 24, and 28 months), the preexistence or de novo development of the anti-HLA antibodies did not appear to be associated with clinical outcomes. C4D staining on biopsy samples was not routinely carried out in these patients. So in these patients, a meaningful correlation of the serum antibody and tissue C4D complement deposition was not possible.
DISCUSSION
The efficacy of the multiple drug protocols of immunosuppression in wide use for kidney transplantation has been judged primarily by how well these regimens prevent acute rejection. The extent to which this objective can be accomplished was exemplified by our historic control group. Despite the low incidence of early rejection, however, there was a steady erosion of patient and graft survival that usually was related in some way to chronic rejection, organ-specific drug toxicity, immune depression per se, or combinations of these factors.
With our revised strategy, improvement in the quality of recipient life replaced avoidance of acute rejection as the highest priority. Because the immune activation that can proceed to organ rejection also is the mandatory first step of the seminal tolerance mechanism of clonal exhaustion-deletion, 5–7,26 the greater than 50% rate of early and delayed acute cellular rejection in Thymoglobulin-pretreated patients (Fig. 2) was not necessarily viewed with alarm. Nevertheless, the frequent need for urgent intensification of immunosuppression mandated unusually close physician surveillance. Despite vigilant supervision, there were examples of nonreversible acute rejection that proceeded to graft loss. These cases included, but were not limited to, patients who were on spaced weaning.
The obvious question raised by these observations concerned the risk-to-benefit ratio of the tolerogenic strategy overall, with particular reference to the spaced weaning in the Thymoglobulin series. With followups of 2 to more than 3 years, patient survival, graft survival, and graft function of the Thymoglobulin-pretreated recipients are equivalent overall to the results in the historic controls. In the subgroup of recipients of live donor kidneys, survival parameters are superior to those in the historic controls. Although these results are encouraging, the effect of the high rate of rejection on longterm prognosis in the Thymoglobulin-pretreated recipients cannot yet be definitively evaluated.
Protocol biopsies for histopathologic studies were not systematically obtained in either the historic control or Thymoglobulin-pretreated patients. Instead, most of the biopsies were done to confirm the clinically diagnosed rejections that were most common in the Thymoglobulin cohort. In cases in which multiple biopsies were done, the severity and progression of CAN were similar in the Thymoglobulin and historic control recipients. The lesions under both kinds of immunosuppression were particularly prevalent in kidneys from cadaveric donors. This was not surprising because it is well known that preexisting donor disease and ischemic injury may contribute to CAN27–29 and aggravate drug nephrotoxic-ity.30 It also is well established that any degree of acute rejection (even of the subclinical variety) may contribute to the arteriopathy, fibrosis, tubular atrophy, and glomerular loss that were the criteria for our semiquantitative histopathologic scale of CAN.30,31
Concerns about acute rejection were largely eliminated after Campath was substituted for Thymoglobulin as pre-treatment. With Campath, the incidence of rejection before spaced weaning was 1%, and even afterward, the cumulative total has been only 20%. In addition, the trend of better patient and graft survival relative to historic controls out to 1 to 2 years, are much the same as in the Thymoglobulin-pretreated cohort. The superior performance of Campath may reflect, in part, the benefit of lessons previously accrued with the Thymoglobulin experience. For example, because of rejections associated with too rapid weaning in the Thymoglobulin series, spaced dosing beyond every other day or three times a week is no longer attempted until at least 1 year unless there are specific indications (eg, drug nephrotoxicity or neurotoxicity).
It should be emphasized that it is not yet known whether the exceptionally complete elimination of acute early rejection with Campath will carry a delayed price. In addition to its long biologic effect (6 to 12 months), unbound Campath remains in the circulation for 1 to 2 weeks after infusion (information from ILEX Inc), and theoretically could erode the mechanism of clonal exhaustion-deletion in the same way as multidrug posttransplant therapy.7 If so, clinically silent CAN could develop and not be detected, particularly because the rate of biopsy sampling has been very low with the efficient avoidance of acute rejection.
The most striking benefit of the tolerogenic immunosuppression in both the Thymoglobulin- and Campath-pretreatment series was the improvement in recipient quality of life. This was reflected in a very low incidence of infection and of de novo malignancies, and freedom from new onset insulin-dependent diabetes. The gains were clearly associated with reduced exposure to chronic daily immunosuppression. In both the Thymoglobulin and Campath series, only about 10% of the recipients who still bear functioning grafts are on more than a single immunosuppressive agent. The vast majority of the others are on spaced dose schedules of the monotherapy, including 12 patients in the Thymoglobulin cohort who are on one dose per week. Based on observations in adults, the Thymoglobulin-based strategy was adopted in April 2003 for all pediatric kidney recipients at our center, and more recently, Thymoglobulin was replaced with Campath.
In conclusion, recipient pretreatment by lymphoid depletion combined with minimalistic posttransplant immunosuppression is an acceptable way to manage kidney transplant recipients. Campath currently appears to be the most effective means of pretreatment. But the strategy, which is designed to permit natural mechanisms of tolerogenesis, is neither drug nor organ specific. It is most easily applied for live donor organ transplantation, but can be readily used for transplantation of cadaveric organs. Additional improvements should be possible, including development of better guidelines for the optimal timing and extent of drug weaning.
Acknowledgments
We gratefully acknowledge the contributions of transplant surgeons who are no longer at University of Pittsburgh Medical Center: Velma P Scantlebury, MD, Mark L Jordan, MD, Carlos Vivas, MD, Potdar Santosh, MD, and Ashok Jain, MD. The work would not have been possible without the indispensable help of Dr Jennifer Woodward and of the renal transplant coordinators: Cindy Anderson, Angela Barber, Linda Bonazza, Denise Boris, Cheryl Buzzard, Jareen Flohr, Jami Gandy, Janice Glidewell, Debbie Good, Gerri James, Jackie Lever, Cordie McFeaters, Kim Meyer, Annie Smith, Maureen Vekasy. We finally thank Ms Terry L Mangan for the skillful preparation of the article.
Supported by NIH grant DK 64207-01.
Footnotes
No competing Interests declared.
Part of this work was presented at the IS Ravdin Lecture in the Basic Sciences at the American College of Surgeons 90th Annual Clinical Congress, New Orleans, LA, October 2004.
Author Contributions
Study conception and design: Starzl
Acquisition of data: Shapiro, Basu, Tan, Khan, Randhawa, Murase, Zeevi, Girnita, Metes, Demetris
Analysis and interpretation of data: Gray, Bass, Ness
Drafting of manuscript: Shapiro
Critical revision: Starzl
Statistical expertise: Bass, Ness
Supervision: Starzl, Fung, Marcos
References
- 1.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Porter KA, Iwasaki Y, et al. The use of antilymphocyte globulin in human renal homotransplantation. In: Wolstenholme GEW, O’Connor M, editors. Antilymphocytic Serum. London: J and A Churchill Limited; 1967. pp. 4–34. [Google Scholar]
- 4.Starzl TE, Murase N, Demetris AJ, et al. Lessons of organ-induced tolerance learned from historical clinical experience. Transplantation. 2004;77:926–929. doi: 10.1097/01.tp.0000117780.74133.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. New Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. NATURE Reviews: Immunology. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Préville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a non-human primate model. Transplantation. 2001;71:460–468. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Mueller TF. Thymoglobulin: an immunologic overview. Curr Opin Organ Transplant. 2003;8:305–312. [Google Scholar]
- 10.Hale G, Waldmann H, Dyer M. Specificity of monoclonal antibody Campath-l. Bone Marrow Transplant. 1988;3:237–239. [PubMed] [Google Scholar]
- 11.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. Erratum in: Lancet 1998;352:408. [DOI] [PubMed] [Google Scholar]
- 12.Stuart FP, Leventhal JR, Kaufman DB, et al. Alemtuzumab facilitates prednisone free immunosuppression in kidney transplant recipients with no early rejection. Am J Transplant. 2002;2(suppl 3):397. [Google Scholar]
- 13.Knechtle SJ, Pirsch JDH, Fechner J, Jr, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro R, Jordan M, Basu A, et al. Kidney transplantation under a tolerogenic regimen of recipient pre-treatment and low-dose postoperative immunosuppression, with subsequent weaning. Ann Surg. 2003;238:520–527. doi: 10.1097/01.sla.0000089853.11184.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest. 1968;(Suppl 97):7. [PubMed] [Google Scholar]
- 18.Metes D, Logar A, Rudert WA, et al. Four-color flow cytometric analysis of peripheral blood donor cell chimerism. Hum Immunol. 2003;64:787–795. doi: 10.1016/s0198-8859(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminski E, Hows J, Goldman J, Batchelor R. Optimising a limiting dilution culture system for quantitating frequencies of alloreactive cytotoxic T lymphocyte precursors. Cell Immunol. 1991;137:88–95. doi: 10.1016/0008-8749(91)90059-k. [DOI] [PubMed] [Google Scholar]
- 20.Fussell ST, Donnellan M, Cooley MA, Farrell C. Cytotoxic T lymphocyte precursor frequency does not correlate with either the incidence or severity of graft-versus-host disease after matched unrelated donor bone marrow transplantation. Transplantation. 1994;57:673–676. doi: 10.1097/00007890-199403150-00008. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski E, Hows J, Man S, et al. Prediction of graft versus host disease by frequency analysis of cytotoxic T cells after unrelated donor bone marrow transplantation. Transplantation. 1989;48:608–613. [PubMed] [Google Scholar]
- 22.Suciu-Foca N, Reed E, D’Agati VD, et al. Soluble HLA antigens, anti-HLA antibodies, and antiidiotypic antibodies in the circulation of renal transplant recipients. Transplantation. 1991;51:593–601. doi: 10.1097/00007890-199103000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Monteiro F, Buelow R, Mineiro C, et al. Identification of patients at high risk of graft loss by pre- and posttransplant monitoring of anti-HLA class I IgG antibodies by enzyme-linked immunosorbent assay. Transplantation. 1997;63:542–546. doi: 10.1097/00007890-199702270-00010. [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Sob K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.McCurry K, Iacano A, Zeevi A, et al. Early outcomes in human lung transplantation utilizing thymoglobulin or campath 1H for recipient pretreatment followed by posttransplant tacrolimus near-mono therapy. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2004.09.040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 27.Isoniemi H, Taskinen E, Hayry R. Histological chronic allograft damage index accurately predicts chronic renal allograft rejection. Transplantation. 1994;58:1195–1198. [PubMed] [Google Scholar]
- 28.Tullius SG, Tilney NL. Both akkiabtugeb-dependent and -independent factors influence chronic allograft rejection. Transplantation. 1995;59:313. [PubMed] [Google Scholar]
- 29.Halloran RF, Melk A, Barth C. Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol. 1999;10:167–181. doi: 10.1681/ASN.V101167. [DOI] [PubMed] [Google Scholar]
- 30.Nankivell BJ, Borrows RJ, Fung CL-S, et al. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004;78:557–565. doi: 10.1097/01.tp.0000128636.70499.6e. [DOI] [PubMed] [Google Scholar]
- 31.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]




