Abstract
The gap junction protein connexin43 (Cx43) has been proposed to play key roles in bone differentiation and mineralization, but underlying cellular mechanisms are not totally understood. To further explore roles of Cx43 in these processes, we immortalized calvarial osteoblasts from wild-type and Cx43-null mice using human telomerase reverse transcriptase (hTERT). Osteoblastic (MOB) cell lines were generated from three individual wild-type and three individual Cx43-null mouse calvaria. Average population doubling times of the cell lines were higher than of the primary osteoblasts but did not greatly differ with regard to genotype. Modest to high level of Cx45 expression was detected in MOBs of both genotypes. Most of the cell lines expressed osteoblastic markers [Type I collagen, osteopontin, osteocalcin, parathyroid hormone/parathyroid hormone-related peptide receptor (PTH/PTHrP), periostin (OSF-2), osterix (Osx), runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP)], and mineralization was comparable to that of primary osteoblasts. Two MOB cell lines from each genotype with most robust maintenance of osteoblast lineage markers were analyzed in greater detail, revealing that the Cx43-null cell lines showed a significant delay in early differentiation (up to 9 days in culture). Matrix mineralization was markedly delayed in one of the Cx43-null lines and slightly delayed in the other. These findings comparing new and very stable wild-type and Cx43-null osteoblastic cell lines define a role for Cx43 in early differentiation and mineralization stages of osteoblasts and further support the concept that Cx43 plays important role in the cellular processes associated with skeleton function.
Keywords: gap junction, alkaline phosphatase, differentiation, mineralization, human telomerase reverse transcriptase
bone, although seemingly quite rigid, is by no means a quiescent tissue. The dynamic process of bone modeling and remodeling involves coordinated signaling among osteoblasts, osteocytes, and osteoclasts. One mechanism of bone remodeling by which second messenger signals spread throughout the bone cell network involves intercellular gap junction channels. These channels functionally connect osteoblasts, bone lining cells, and osteocytes that are embedded within the bone matrix (12, 19). Gap junction communication is believed to play important roles during embryogenesis, bone remodeling, bone mineralization, and propagation of intercellular signals (8, 10, 30, 48). Previous studies have shown that the gap junction protein connexin43 (Cx43) is the most abundant gap junction protein in bone while other connexins, notably Cx45, are also present (8, 11, 24, 30).
A role for Cx43 in the events leading to normal bone development has been suggested by observations that Cx43-null mice display delayed ossification and osteoblast dysfunction (26, 49) and that mice with osteoblast-specific deletion of Cx43 display reduction of parathyroid hormone-induced bone mass where mineral deposition rate was greatly attenuated compared with wild-type mice (7). In addition, calvarial osteoblasts derived from these mice demonstrate weaker anabolic response to parathyroid hormone (7). Early in vitro studies determined that manipulating Cx43 expression in osteoblasts diminished parathyroid-induced cAMP production (46) and matrix mineralization (37). Furthermore, the human genetic disease oculodentodigital dysplasia (ODDD), which is caused by point mutations in the Gja1 gene encoding Cx43, is characterized by skeletal abnormalities of teeth (microdontia), eyes (micro-cornea/microphthalmia), and digits (syndactyly of hands and feet); mice with mutations similar or corresponding to ODDD also exhibit skeletal abnormalities (14, 22). Moreover, calvarial osteoblasts harvested from transgenic mice harboring the Cx43 mutation G60S (transgenic Gja1Jrt/+ ODDD mouse model) also exhibited reduced late stage osteoblast differentiation (29). However, the mechanisms underlying the contribution of Cx43 to proper bone differentiation and mineralization have not yet been thoroughly explored. Detailed investigation of the roles played by Cx43-related cellular pathways in skeletal development has been hindered to a great degree because the transgenic Cx43-null mice die at birth because of major cardiovascular malformations. Therefore, to examine these cellular processes we have developed new osteoblast cell models from wild-type and Cx43-null mice.
Primary osteoblasts reach replicative senescence after a few passages, and isolated osteoblasts lose many of their phenotypic markers after a few successive passages (13, 32). To overcome these problems, previous studies have isolated osteoblasts from osteosarcomas (27) or immortalized the cells using viral gene Simian virus 40 T antigen driven by either the bone morphogenetic protein-2 (BMP-2) (20) or osteocalcin promoter (5) or spontaneously immortalized using 3T3 subculture schedule (transferred every 3 days and inoculated at the same cell density) (41). In recent years a novel technique has been developed to immortalize cells that uses expression of human telomerase reverse transcriptase (hTERT) to extend the cellular lifespan of primary cells, which would normally undergo cellular senescence (18). Cells that express endogenous telomerase have limited number of times to divide before they senesce. However, in cells where hTERT is constitutively overexpressed, the progressive shortening of telomeres is prevented, thereby allowing cells to ultimately proliferate and divide indefinitely (3). One unique feature of this technique is that it produces cells that are not only capable of extended proliferation but also possess the same genotype and tissue markers of their parental tissue (3, 42). In this study we report the successful use of hTERT transfection to establish mouse osteoblastic cell lines derived from wild-type C57BL/6J mice and Cx43-null littermates and demonstrate with these new cell models that absence of Cx43 causes considerable delay specifically in early differentiation and mineralization stages. Therefore, we conclude that Cx43 presence at early phases of osteoblast development/maturation is essential for proper osteoblast differentiation and mineralization.
MATERIALS AND METHODS
Osteoblast isolation and establishment of immortalized cell line.
Following a protocol modified from Lecanda and coworkers (26), we isolated osteoblasts from calvaria of wild-type and Cx43-null embryonic (E19–20) mice obtained from in-house mating of Cx43 heterozygous mice (C57BL/6J-Gja1tm1Kdr) (35). All animal procedures and experimental protocols were approved by the Institute for Animal Studies of the Albert Einstein College of Medicine in accordance with National Institutes of Health guidelines. Briefly, pregnant Cx43 heterozygous females were deeply anesthetized with isoflurane and euthanized by decapitation, and the E19–20 pups were delivered by cesarean section and euthanized by decapitation. The periosteum and endosteum of individual calvaria were carefully removed, cleaned, and thoroughly diced into small pieces, pooled for each pup, and digested in 1× PBS containing 4 mg/ml of collagenase Type II (Worthington Biochemical, Lakewood, NJ) at 37°C for 10 min. Initial digestions were discarded. Supernatant from the second and third sequential digestions at 37°C were collected. Cells were then collected by centrifugation, resuspended in minimal essential α-medium (α-MEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin-streptomycin (Invitrogen), and seeded in culture dishes. Genotyping of mice corresponding to the osteoblast cultures was determined from tail DNA (9). Medium was changed every 3 days, and the cells were cultured for 10 to 14 days until confluence. At this point the cells were immortalized using hTERT. hTERT cDNA was PCR amplified from its original construct hTERT-pGRN145 (ATCC, Manassas, VA) and subcloned into pcDNA3.1 vector (Invitrogen). Briefly, osteoblast cultures were transfected with 4 μg of nonlinearized pcDNA 3.1 plasmid containing hTERT cDNA using Optifect reagent (Invitrogen). After overnight incubation, the transfection mixture was replaced with normal growth media. Selection of hTERT-expressing cells was then achieved by successive splitting bimonthly for 7 mo in culture, thus eradicating cells that did not continue to divide in prolonged culture. Consequently, mouse osteoblast (MOB) cell lines were established from six pups, designated MOB-A, -B, -C and 43KO-MOB-A, -B, -C.
Control cells and culture conditions.
MC3T3-E1 (subclone 4) (41, 47) was purchased from ATCC. Primary mouse osteoblasts (wild-type, termed PMOB and Cx43-null, termed 43KO-PMOB) were isolated as described above. Primary cultures were pooled after genotyping. All the experiments involving primary osteoblasts were performed using cells from the first passage. Both MC3T3-E1 and primary osteoblasts were cultured in α-MEM + 10% FBS + 1% penicillin-streptomycin, and HeLa cells were cultured in DMEM (Invitrogen) + 10% FBS + 1% penicillin-streptomycin at 37°C with 5% CO2.
RT-PCR and qPCR.
Reverse transcription-polymerase chain reaction (RT-PCR) was used to screen the MOBs and 43KO-MOBs for expression of the key osteoblastic markers osteopontin, Type I collagen, parathyroid hormone/parathyroid hormone-related peptide receptor (PTH/PTHrP), periostin (OSF-2), osteocalcin (23), osterix (Osx), and runt-related transcription factor 2 (Runx2) (2). Wild-type calvarial tissue and MC3T3-E1, PMOB, 43KO-PMOB, and HeLa cells were used as controls. Cell lines (seeding density: 1.5 × 103 cells/cm2) and primary osteoblasts (seeding density: 2.0 × 104 cells/cm2) were plated in 60-mm dishes and cultured for 10 days. Total RNA of confluent cell cultures (10 days) was extracted using TRIzol reagent (Invitrogen), and RT-PCR was performed as previously described (45). The primers used are listed in supplemental Table 1 (see AJP-Cell Physiology website for supplemental material).
Table 1.
Overall phenotypic comparison of MOBs and 43KO-MOBs
| MOB-A | MOB-B | MOB-C | 43KO-MOB-A | 43KO-MOB-B | 43KO-MOB-C | |
|---|---|---|---|---|---|---|
| Osteopontin | + | + | + | + | + | + |
| OSF-2 | + | + | + | + | + | + |
| PTH/PTHrP | + | + | + | + | + | + |
| Osteocalcin | No | + | + | No | + | + |
| Type I collagen | + | + | + | + | + | + |
| Runx2 | + | + | + | Low | + | + |
| Osx | No | + | + | No | + | + |
| Alkaline phosphatase at 2 wk | Low | + | + | No | + | + |
| Matrix mineralization at 2 wk | No | + | + | No | + | + |
| Cuboidal cell morphlogy at day 8 | No | No | + | No | + | No |
| Cuboidal + stellate cell morphlogy at day 8 | + | + | No | + | No | + |
| Lucifer yellow dye transfer | + | + | + | No | No | No |
OSF-2, periostin; PTH/PTHrP, parathyroid hormone/parathyroid hormone-related peptide receptor; Osx, osterix; Runx2, runt-related transcription factor; MOB, mouse osteoblast cell line; 43KO-MOB, connexin43-null osteoblastic cell line; +, positive expression
Quantitative PCR (qPCR) was used to compare levels of osteopontin (44), osteocalcin, Type I collagen (28), Runx2, and Osx (2) mRNA in wild-type and Cx43-null cells at earlier time points, 6 and 9 days in culture. For this, wild-type and Cx43-null primary osteoblasts and hTERT cell lines were plated as described above and harvested after 6 and 9 days in culture. The qPCR was performed using SYBR GREEN Master Mix (Applied Biosystems) in an Applied Biosystems 7300 Real-Time PCR System (Forester City, CA), according to the manufacturer's instructions. Briefly, 2 μg of total RNA was reverse transcribed into cDNA using oligo-dT12–18 priming, 10 mM dNTP mix, and Superscript II reverse transcriptase (Invitrogen). Amplification was carried out for 40 cycles with annealing temperature of 60°C in 25 μl final volume. Finally, a dissociation profile of the PCR product(s) was obtained by a temperature gradient running from 60°C to 95°C. The primers used for qPCR are listed in supplemental Table 2. Two technical replicas for each experiment with total of three biological replicas for cell lines and three technical replicas for primary osteoblasts were amplified. Relative gene expression levels of the mRNA analyzed were calculated using the ΔΔCT method, where values obtained for the gene of interest are first normalized to those of the reference gene, β-actin, and subsequently to those of their respective controls (wild-type primary osteoblasts or hTERT-immortalized cells).
Western blot analysis.
Cells were plated in 60-mm dishes and cultured for 10 days. Samples were lysed as described previously (43). Samples were prepared and loaded onto 7.5 or 10% SDS-PAGE gels for separation and electrophoretically transferred to nitrocellulose membranes (Whatman, Dassel, Germany). The membranes were probed with polyclonal antibodies to goat anti-human telomerase, 1:250 (Abcam); rabbit anti-Cx43, 1:20,000 (courtesy of Dr. Elliot L. Hertzberg, Columbia University); and rabbit anti-Cx45, 1:1,000 (Invitrogen), followed by secondary antibody incubation with horseradish peroxidase-conjugated anti-goat IgG and anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Protein bands were detected using the Amersham ECL detection kit (GE Healthcare, Buckinghamshire, UK) and exposed to Kodak X-ray film (Fisher Scientific, Pittsburgh, PA).
Alkaline phosphatase staining and quantification of alkaline phosphatase concentration.
Cells were cultured in six-well tissue culture plates with α-MEM + 10% FBS for 1, 2, or 3 wk. To evaluate alkaline phosphatase (ALP) expression in MOBs and 43KO-MOBs under short-term culture condition, cells were seeded at 104 cells/cm2 and cultured for 3 days. Cells were then fixed and stained for ALP using naphthol AS-BI phosphate and fast red violet LB salt (Sigma, St. Louis, MO) as described (43). ALP concentration in cells was quantified using the SensoLyte pNPP (p-nitrophenyl phosphate) Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA). Samples were washed, lysed with 1× lysis buffer, and collected according to the manufacturer's instruction at 3, 6, 9, 12, and 15 days after seeding. Supernatant from the samples was assayed for ALP concentration; average OD values resulting from dephosphorylation of pNPP by phosphatase were acquired at 405 nm wavelength using a FLUOstar Omega Plate Reader (BMG Labtech, Cary, NC). ALP concentrations from cell lysates of each set of experiments were determined from standard curves. The quantified ALP concentrations were then normalized to respective cellular protein levels.
von Kossa staining.
Cells were seeded in six-well tissue culture plates and grown for 1, 2, or 3 wk in α-MEM supplemented with 10% FBS + 25 μg/ml ascorbic acid (AA, Sigma) + 2 mM β-glycerol phosphate (β-GP, Sigma). von Kossa staining was performed using the methods previously described (4). Briefly, cells were fixed, rinsed with water, serially dehydrated in 70%, 95% and 100% EtOH, and rehydrated from 100% to 95% to 80% EtOH to water. Next, cells were incubated with 2% silver nitrate solution (Sigma) on a UV transilluminator (UVP, Upland, CA) and subsequently incubated with 5% sodium thiosulfate (Sigma) to remove unreacted silver. Cells were then counterstained with nuclear fast red (Sigma), rinsed, dehydrated in 95%, 100% EtOH, and air dried.
Quantification of in vitro osteoblast mineralization.
Cells were seeded in 96-well tissue culture plates and grown for 1, 2, or 3 wk in α-MEM + 10% FBS supplemented with or without 25 μg/ml AA + 2 mM β-GP. The in vitro mineralization was determined using OsteoImage Mineralization Assay Kit (Lonza, Walkersville, MD). Cells were fixed in 4% paraformaldehyde and washed, and the hydroxyapatite (HA) nodule formation in cell cultures was quantified from the fluorescence emitted by the specific binding of the OsteoImage staining reagent to HA (excitation 492 nm/emission 520 nm) measured using a FLUOstar Omega Plate Reader.
Cell growth measurement.
Cell lines (seeding density: 2 × 103 cells/dish) were plated in 35-mm dishes, and primary osteoblasts (seeding density: 5 × 103 cells/well) were plated in 24-well culture plates. Cells were cultured in α-MEM + 10% FBS, after 2, 4, 6, 8, and 10 days in culture they were harvested in Trypsin-EDTA, and numbers of viable cells at each of these time points were counted using a Hemacytometer (Hausser Scientific, Horsham, PA) and 0.4% Trypan blue solution (Sigma). The population doubling times (Td) of the cells were calculated at 90% confluency time point (day 6) using the equation Td = (t − t0) [ln2/ln(N − N0)] where N is number of cells at time t, and N0 is seeding cell density at time t0 (17).
Immunofluorescence microscopy.
Both MOBs and 43KO-MOBs were fixed with 4% formaldehyde, permeabilized with 0.4% Triton-X100, and blocked with 2% donkey serum (Jackson Immunoresearch, West Grove, PA) as previously described (43). The cells were then incubated with primary antibody against Cx43 (Sigma), and secondary antibody conjugated to Alexa 488 (Invitrogen). The coverslips were mounted on slides, examined on a Nikon Eclipse TE300 microscope, and photographed using a SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Lucifer yellow microinjections.
To evaluate the extent of gap junction-mediated coupling among MOBs, 43KO-MOBs, PMOB, and 43KO-PMOB, we used the dye-injection technique performed in cultures of similar confluency. For this, single cells were impaled with a microelectrode (25 MΩ when filled with 3 M KCl) filled with the gap junction permeant fluorophore Lucifer yellow (LY, 5% wt in 150 mM LiCl), and the dye was injected by iontophoresis (continuous current of 0.1 μA) for 5 min using an electrometer (model 3100; A-M Systems). After removal of the microelectrode from the injected cells, images were immediately acquired using a CoolSNAP-HQ2 CCD camera (Photometrics, Tucson, AZ) attached to a Nikon inverted microscope with ×10 dry objective (numerical aperature 0.3) and FITC filter sets. Fluorescent cells surrounding the LY-injected cell were counted, and the values were then normalized by the total number of cells within the injected region of interest (ROI, with average cell density of 125 ± 13 cells) and expressed as percentage of coupled cells per ROI ± SE; 5 injections were performed in each of two different cultures of each MOB and 43KO-MOB cell line.
Statistical analysis.
Data were analyzed from three to six sets of independent experiments, and P values were obtained using one-way ANOVA analysis followed by Tukey's multiple comparison test or t-tests (GraphPad Prism 5, La Jolla, CA). P < 0.05 was considered statistically significant.
RESULTS
Establishment of wild-type and Cx43-null mouse calvarial osteoblastic cell lines using hTERT.
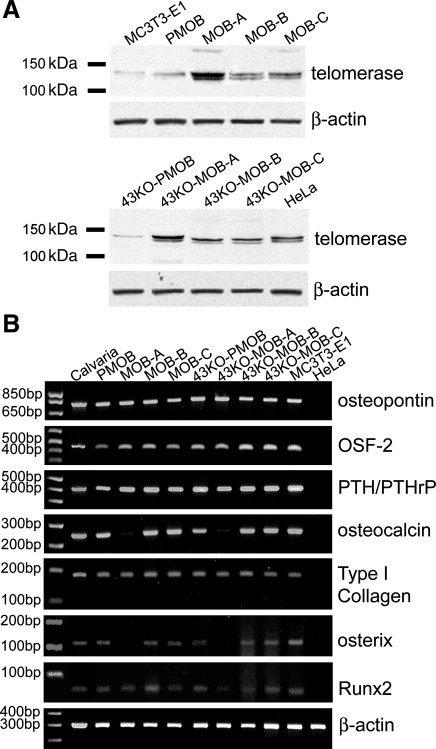
The immortalization with hTERT in the MOB and 43KO-MOB mouse calvarial osteoblastic cell lines was confirmed by determining the expression of telomerase after 10 passages. As shown in Fig. 1A all MOBs and 43KO-MOBs expressed moderate to high amounts of telomerase compared with that of HeLa cells, a positive control. On the other hand, MC3T3-E1, PMOB, and 43KO-PMOB osteoblasts minimally expressed telomerase, consistent with the fact that MC3T3-E1 was spontaneously immortalized by a subculture protocol and that primary osteoblasts still express detectable amounts of telomerase before they reach their replicative senescence.
Fig. 1.
Expression of telomerase and osteoblastic markers in primary calvarial osteoblasts and human telomerase reverse transcriptase (hTERT)-immortalized cell lines. A: telomerase expression in hTERT-immortalized mouse wild-type (MOB) and connexin43 (Cx43)-null (43KO-MOB) osteoblastic cell lines detected by Western blotting. Equal amounts of protein from HeLa, MOBs, 43KO-MOBs, MC3T3-E1, primary mouse wild-type osteoblasts (PMOB), and Cx43-null osteoblasts (43KO-PMOB) were used. HeLa cells were used as positive control. B: RT-PCR analysis of osteoblastic markers (osteopontin, OSF-2, PTH/PTHrP-R, osteocalcin, and type I collagen), two transcription factors associated with bone formation and mineralization [runt-related transcription factor 2 (Runx2) and osterix (Osx)] and β-actin (loading control) in MOBs and 43KO-MOBs. The expression of the osteoblastic markers and bone formation and mineralization associated transcription factors from 10-day culture of the hTERT-immortalized cells were compared with those of wild-type calvarial tissue, primary wild-type and Cx43-null osteoblasts, MC3T3E-1, and HeLa (negative control).
It has been well recognized from earlier studies (13, 32) that primary osteoblasts tend to lose their specific phenotypic features after isolation and culture. Cells immortalized with hTERT, however, have been shown to have an extended life span while maintaining the morphological characteristics and phenotypic markers of the cells of origin (3). Therefore, to confirm that the osteoblast phenotype was maintained in immortalized MOBs and 43KO-MOBs, we determined whether these cells expressed osteoblast-specific markers. As shown in Fig. 1B, all MOBs and 43KO-MOBs expressed mRNA for the bone matrix proteins osteopontin, type I collagen, periostin (OSF-2), and PTH/PTHrP, similar to wild-type calvarial tissue and PMOB, 43KO-PMOB, and MC3T3-E1 cells. Moreover, with the exception of MOB-A and 43KO-MOB-A, all other MOB and 43KO-MOB cell lines expressed osteocalcin, a late osteoblastic marker (Fig. 1B). In addition, we further investigated whether several other transcription factors, such as Runx2 and Osx, which play crucial roles in bone formation and mineralization (31), were also expressed by the hTERT-immortalized cells. As shown in Fig. 1B, except for 43KO-MOB-A, all MOBs and 43KO-MOBs expressed Runx2. Likewise, most of the MOBs and 43KO-MOBs aside from MOB-A and 43KO-MOB-A, expressed Osx (Fig. 1B).
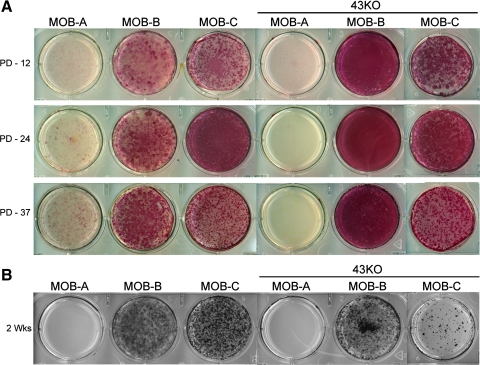
Further evidence that the hTERT-immortalized cells display osteoblast phenotypic characteristics was provided by the demonstration that these cells express ALP, a key osteogenic marker, and are capable to form mineralized extracellular matrix after 2 wk in culture. As shown in Fig. 2A, ALP expression in MOB-B, MOB -C and in the 43KO-MOB-B and 43KO-MOB-C was consistently maintained after 12, 24, and 37 population doublings. However, ALP expression was minimal in MOB-A and 43KO-MOB-A. Formation of mineralized extracellular matrix in the hTERT-immortalized cells was analyzed after 2 wk in culture by von Kossa staining. As shown in Fig. 2B, the majority of the MOBs and Cx43KO-MOBs formed numerous mineralized nodules, except for MOB-A and 43KO-MOB-A. High levels of mineralized nodule formation were observed in MOB-B, MOB-C, and 43KO-MOB-B, while 43KO-MOB-C exhibited a lesser degree of nodule formation.
Fig. 2.
Assessment of osteoblast phenotypic characteristics in hTERT-immortalized MOB and 43KO-MOB mouse cell lines using the key osteogenic marker alkaline phosphatase (ALP) after 12, 24, and 37 population doubling (PD-12, PD-24, PD-37) (A), and the capability to form mineralized extracellular matrix, as determined by von Kossa staining (B). MOBs, 43KO-MOBs, MC3T3-E1 (seeded at 1.5 × 103 cells/cm2), PMOB, and 43KO-PMOB (seeded at 2.0 × 104 cells/cm2) were seeded in six-well plates and stained 2 wk after being plated.
Growth rate and morphology of hTERT-immortalized wild-type and Cx43-null mouse calvarial osteoblastic cell lines.
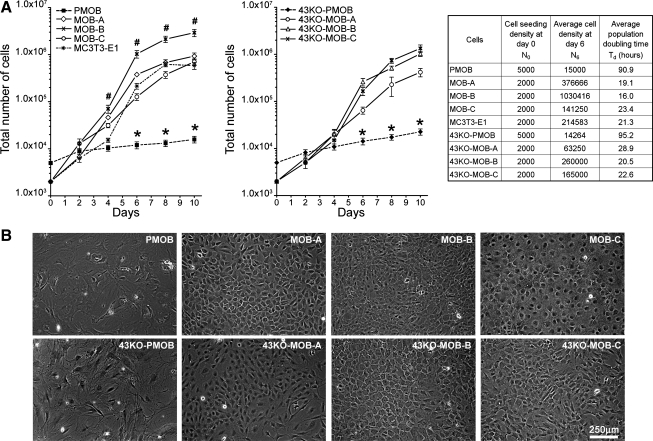
The proliferation capacity of the MOBs and 43KO-MOBs was similar to that of the MC3T3-E1 cells and much higher than that of the PMOB and Cx43-PMOB. As shown in Fig. 3A, the number of cells in hTERT-immortalized MOB cultures (initially seeded at 2×103 cells/dish) markedly increased after day 4 compared with that of PMOB and 43KO-PMOB (seeded at 5 × 103 cells/well). At the end of the 10-day experiments the average number of cells in the hTERT-immortalized MOB cultures was about 40-fold higher than in PMOB and 43KO-PMOB cultures, with the exception of the MOB-B cultures, where the average number of cells was about 70-fold higher than that in primary cells. Further growth kinetics revealed that the average population doubling time (Td) for all hTERT-immortalized cell lines and MC3T3-E1 was between 19 and 28 h, whereas the average Td for primary cells was around 107 h, which is approximately four- to fivefold slower compared with the cell lines (Fig. 3A, right).
Fig. 3.
A: growth kinetics for hTERT-immortalized MOB and 43KO-MOB mouse osteoblastic cell lines. MOBs, 43KO-MOBs, and MC3T3-E1 cells were seeded at 2 × 103 cells/dish and PMOB and 43KO-PMOB mouse osteoblasts were seeded at 5 × 103 cells/well in six-well plates and grown in α-MEM supplemented with 10% FBS for 2, 4, 6, 8, and 10 days. All data are presented as means ± SE, n = 5; *P < 0.01, #P < 0.05. P values were obtained using one-way ANOVA followed by Tukey's multiple comparison test. Population doubling times were calculated at the 90% cell confluency time point (day 6). B: morphological appearance of hTERT-immortalized cell lines observed by phase contrast microscopy. MOBs, 43KO-MOBs, and MC3T3-E1 were seeded at 2 × 103 cells/well, and PMOB and 43KO-PMOB were seeded at 5 × 103 cells/well in six-well plates and cultured for 8 days in α-MEM supplemented with 10% FBS.
Morphological analysis of MOBs and 43KO-MOBs revealed that these cells have stellate morphology in low confluence cultures, and some of them attained cuboidal appearance once they reached confluence. At 8 days in culture the MOB-A, MOB-B, and 43KO-MOB-C showed a mixture of stellate and cuboidal profiles, while MOB-C and 43KO-MOB-B exhibited a more cuboidal morphology. In addition, MOB-B cells that have the fastest population doubling time appeared smaller than the rest of the MOBs and 43KO-MOBs (Fig. 3B). In general, the morphology of MOBs and 43KO-MOBs was similar to that of the well-studied MC3T3-E1 cells (41).
Gap junction expression and function in hTERT-immortalized wild-type and Cx43-null mouse calvarial osteoblastic cell lines.
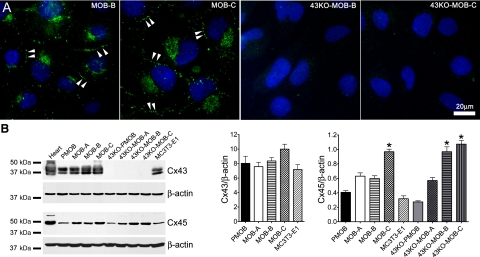
Gap junction channels provide an intercellular pathway for signaling among bone cells and as such, play a key role in the coordination of cellular activity in the skeleton. Cx43 is the dominant gap junction protein in osteoblasts. As shown in Fig. 4A, Cx43 in MOB cells was localized at the cell-cell appositional membranes (double arrowheads) and perinuclear area, whereas no staining was detected in 43KO-MOB cells. Western blot analysis of Cx43 expression in these cells demonstrate that this protein is detected in moderate to high levels only in MOBs (Fig. 4B), corroborating the immunofluorescence microscopy findings (Fig. 4A). As shown in the bar histograms of Fig. 4B, the average levels of Cx43 expression in MOBs were similar to those observed in PMOB and MC3T3-E1 cells. Besides Cx43, osteoblasts have also been shown to express Cx45 (8, 24, 30). Previous studies have shown upregulation of Cx45 in Cx43-null calvarial osteoblasts (26, 49). However, our Western blot analysis demonstrated that there was no association between decreased Cx43 expression and either lower or higher Cx45 expression. We did not detect significant differences in Cx45 expression in the primary wild-type and Cx43-null osteoblasts and in the hTERT-immortalized osteoblasts Cx45 expression was variable, regardless of the genotype (Fig. 4B). The densitometric analysis of Cx45 demonstrated that MOB-C, 43KO-MOB-B, and 43KO-MOB-C expressed significantly higher levels of Cx45 compared with PMOB, 43KO-PMOB, and MC3T3-E1 cells (Fig. 4B). These data revealed no correlation between level of Cx45 expression and genotype or differentiation behavior among the hTERT-immortalized wild-type and Cx43-null osteoblasts.
Fig. 4.
A: assessment of Cx43 distribution in some of the hTERT-immortalized MOB and 43KO-MOB mouse osteoblastic cell lines. MOB-B, MOB-C, 43KO-MOB-B, and 43KO-MOB-C were seeded at 1.5 × 103 cells/cm2 for 6 days and immunostained for Cx43, and cellular distribution of Cx43 was analyzed by epifluorescence microscopy. Double arrowheads, punctate Cx43 distribution at cell-cell appositional membrane. B: Western blot quantification of Cx43 and Cx45 expression levels in MOBs and 43KO-MOBs. Equal amounts of protein from mouse heart (positive control), MOBs, 43KO-MOBs, primary mouse wild-type (PMOB), Cx43-null (43KO-PMOB) osteoblasts, and MC3T3-E1 were used. Western blot analysis was performed using antibodies against Cx43, Cx45, and β-actin. Bar graphs correspond to average Cx43 and Cx45 expression levels determined by densitometric analysis of the Cx43 and Cx45 bands from three independent experiments, using the Scion NIH Image software. All acquired data were normalized with respect to β-actin (internal controls) and expressed as means ± SE, N = 3; *P < 0.05. P values were obtained using one-way ANOVA followed by Tukey's multiple comparison test.
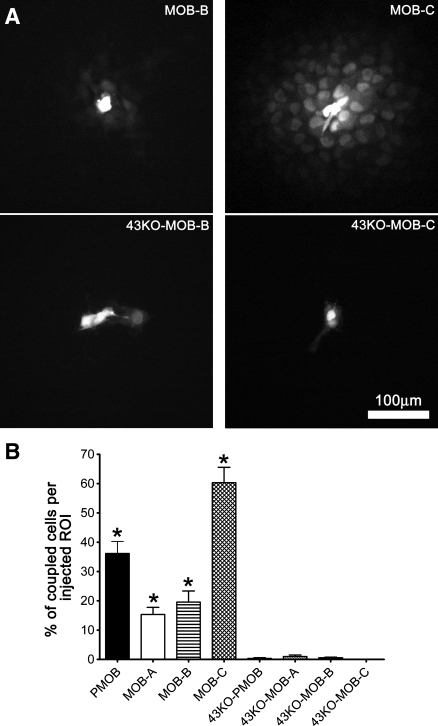
To evaluate the extent of junctional coupling in MOBs and 43KO-MOBs we performed LY microinjection studies (Fig. 5A). As shown in Fig. 5B, the percentage of cells to which LY spread from the injected cell in MOBs and PMOB ranged from 15% to 60% (about 15.33 ± 2.43% in MOB-A, 19.57 ± 3.79% in MOB-B, 60.33 ± 5.29% in MOB-C, and 36.17 ± 4.11% in PMOB) with MOB-C showing the highest degree of dye coupling. In contrast to the marked coupling among MOBs, there was little or no coupling among 43KO-MOBs and 43KO-PMOB (about 1 ± 0.52% in 43KO-MOB-A, 0.33 ± 0.33% in 43KO-PMOB, 0.5 ± 0.28% in 43KO-MOB-B, and no coupling in 43KO-MOB-C), consistent with the absence of Cx43 in these cells and the low permeability of Cx45 channels to LY (25, 40).
Fig. 5.
Degree of gap junction coupling in the hTERT-immortalized osteoblastic cell lines. A: Lucifer yellow (LY) spread in MOB and 43KO-MOB cell lines. For the fields illustrated, LY spread to 19, 60, 0.5, and 0% of cells in the regions of interest in MOB-B, MOB-C, 43KO-MOB-B, and 43KO-MOB-C, respectively. B: overall quantification of the degree of dye coupling in the hTERT-immortalized osteoblasts, primary wild-type, and Cx43-null osteoblasts cultures. Values correspond to means ± SE, N = 5; *P < 0.0001. P values were obtained using one-way ANOVA followed by Tukey's multiple comparison test.
Among all MOBs and 43KO-MOBs, several of the cell lines met most of the key characteristics of osteoblasts as summarized in Table 1. The MOB-B, MOB-C, 43KO-MOB-B, and 43KO-MOB-C not only abundantly displayed all the osteoblastic markers that we analyzed but also differentiated and mineralized. In contrast, MOB-A and 43KO-MOB-A possessed most of the phenotypic osteoblast markers, except osteocalcin and osterix, and barely reached the differentiated phase and hardly mineralized. Coincidently or not, these two cell lines also expressed more telomerase than all other MOB cell lines. It is not yet fully defined whether hTERT overexpression alters osteoblast gene expression. It has already been reported, however, that in hTERT-immortalized human umbilical vein endothelial cell lines the expression of PI3K/Akt-related genes, which have been shown to activate various growth and survival factors associated with anti-apoptosis, is enhanced in the cells overexpressing hTERT (42). We cannot, therefore, dismiss a possible association between hTERT overexpression and inability of our MOB-A and 43KO-MOB-A cells to express ALP and to mineralize. Future studies, such as those using unbiased cDNA microarray methodology, will likely provide definitive demonstration of whether and to what extent hTERT overexpression impacts osteoblast gene expression. We have thus selected the hTERT-immortalized wild-type MOB-B and MOB-C and the Cx43-null 43KO-MOB-B and 43KO-MOB-C cell lines to further evaluate whether Cx43 alters the ability of osteoblasts to differentiate and form mineralized extracellular matrix.
Contribution of Cx43 to osteoblast differentiation and mineralization.
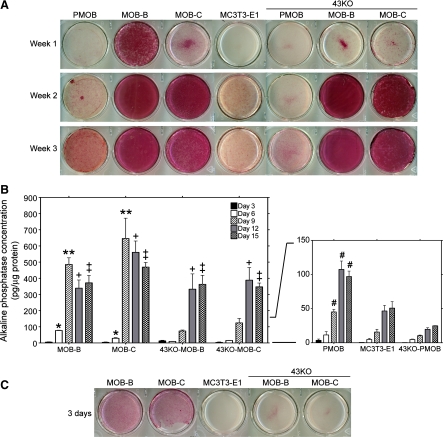
The gap junction protein Cx43 has been suggested to play key roles in bone differentiation and mineralization, but little is still known of the underlying cellular mechanisms. Increase in activity of ALP has been shown to strongly correlate with differentiated phenotype of osteoblasts (33). Bearing this in mind, we compared the expression of ALP in MOBs and 43KO-MOBs cultured for up to 3 wk in α-MEM + 10% FBS. As shown in Fig. 6A, ALP expression was detected in all the selected hTERT-immortalized osteoblasts as early as 1 wk in culture: while MOB-B and MOB-C displayed high to moderate expression, lower ALP expression was observed in 43KO-MOB-B and 43KO-MOB-C. ALP expression increased significantly with time in culture, reaching a plateau after 2 wk. Under these conditions, ALP expression in the selected wild-type and Cx43-null hTERT-immortalized osteoblasts was much more pronounced than that of MC3T3-E1 and primary wild-type and Cx43-null osteoblasts. To better investigate and characterize the role played by Cx43 on the earlier phases of osteoblast differentiation, we quantified ALP concentration between days 3 to 15 at 3-day intervals using pNPP ALP Assay Kit (AnaSpec). As shown in Fig. 6B, as early as day 6 (white bars) and until it peaked at day 9 (hatched bars), the ALP concentration in the two wild-type MOBs was significantly higher than that in both Cx43-null cell lines at the same time points. In Cx43-null cell lines the ALP concentration peaked later, at day 12 (gray bars) and day 15 (checkered bars); once the ALP concentration reached its peak values, the values were not different from those observed in the wild-type MOBs at the same time points. Interestingly, although primary wild-type and Cx43-null osteoblasts were seeded at a 10-fold higher density than the MOBs and 43KO-MOBs, the ALP concentration in the primary osteoblasts was significantly lower than in the hTERT-immortalized osteoblasts at all time points, regardless of genotype. Comparison between primary wild-type and Cx43-null osteoblasts showed that from day 9 to day 15 the ALP concentration in PMOB was considerably higher than that in 43KO-PMOB. In contrast to the 43KO-PMOB that expressed significantly less ALP than PMOB at all time points analyzed, the overall ALP concentration in the hTERT-immortalized Cx43-null cell lines was markedly lower than in their wild-type counterparts only at the early stage of differentiation, attaining levels that were comparable to those of the wild-type cell lines by day 12. Moreover, when the hTERT-immortalized cells were seeded in high density (104 cells/cm2), the wild-type MOBs demonstrated the ability to rapidly differentiate in short-term culture conditions as early as 3 days in culture, whereas the Cx43-null MOBs showed significantly delayed differentiation (Fig. 6C). It should be noted that no ALP expression was observed in the widely used MC3T3-E1 cells at the same time point.
Fig. 6.
Differentiation of the hTERT-immortalized MOB and 43KO-MOB mouse osteoblastic cell lines. The hTERT-immortalized cells and MC3T3-E1 cells (seeded at 1.5 × 103 cells/cm2) and PMOB and 43KO-PMOB mouse osteoblasts (seeded at 2.0 × 104 cells/cm2) were seeded in six-well plates and cultured in α-MEM + 10% FBS for 1, 2, and 3 wk. Note, PMOB and 43KO-PMOB were seeded 10-fold more densely than MOBs, 43KO-MOBs, and MC3T3-E1 to attain a comparable cell density at the time of alkaline phosphatase (ALP) expression analysis. A: ALP expression in selected MOBs and 43KO-MOBs was compared with those of MC3T3-E1, PMOB, and 43KO-PMOB. B: quantification of ALP concentration in selected MOBs and 43KO-MOBs using pNPP ALP Assay Kit. ALP concentration and protein content were measured at day 3, 6, 9, 12, and 15. ALP concentration was calculated from standard curves generated for each set of experiments. Values of ALP concentration were then normalized to respective cellular protein content. Data are expressed as means ± SE, N = 3; *P < 0.01 (MOB-B and MOB-C vs. all other cells at day 6), **P < 0.001 (MOB-B and -C vs. all other cells at day 9), +P < 0.05 (MOB-B and -C, 43KO-MOB-B and -C vs. PMOB, MC3T3-E1, 43KO-PMOB at day 12), ‡P < 0.05 (MOB-B and -C, 43KO-MOB-B and -C vs. PMOB, MC3T3-E1, 43KO-PMOB at day 15), #P < 0.001 (PMOB vs. 43KO-PMOB from day 9 to day 15). P values were obtained using one-way ANOVA followed by Tukey's multiple comparison test. C: ALP expression in hTERT-immortalized cells under short-term cell culture condition. MOBs, 43KO-MOBs, and MC3T3-E1 were seeded at 104 cells/cm2 and cultured for 3 days in α-MEM + 10% FBS.
Osteoblast differentiation is manifested by the formation of mineralized nodules composed of inorganic calcium HA and organic components such as type I collagen, osteocalcin, and osteopontin. To verify the extent of mineralization by the selected hTERT-immortalized osteoblasts, we used the widely established von Kossa staining. For these mineralization time-course studies, all cell lines and primary wild-type and Cx43-null osteoblasts were cultured in regular growth medium with 25 μg/ml AA and 2 mM β-GP for up to 3 wk to stimulate osteoblast differentiation in culture (4). The mineralization capacities of the selected MOBs, 43KO-MOBs, primary wild-type and Cx43-null osteoblasts, and MC3T3-E1 cells are shown in Fig. 7A. In the hTERT-immortalized and primary wild-type osteoblasts, most of the mineralization took place after 2 wk in culture, except for the primary Cx43-null osteoblasts where slight mineralization occurred only after 2 wk in culture. We also noted markedly more mineralized nodules in primary wild-type osteoblasts than in primary Cx43-null osteoblasts after 2 wk in culture, as was previously reported by Lecanda and coworkers (26).
Fig. 7.
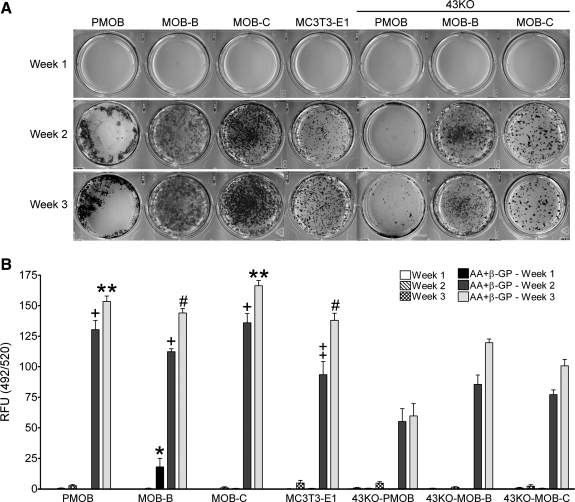
Assessment of matrix mineralization in the hTERT-immortalized MOB and 43KO-MOB mouse osteoblastic cell lines. The hTERT-immortalized cells and MC3T3-E1 cells (seeded at 1.5 × 103 cells/cm2), and PMOB and 43KO-PMOB mouse osteoblasts (seeded 2.0 × 104 cells/cm2) were seeded in six-well plates and cultured in α-MEM + 10% FBS supplemented with or without 25 μg/ml AA and 2 mM β-GP for 1, 2, and 3 wk. A: von Kossa staining in MOBs and 43KO-MOBs treated with AA + β-GP was compared with those of MC3T3-E1, PMOB, and 43KO-PMOB at 1, 2 and 3 wk in culture. B: quantification of in vitro osteoblast mineralization in the hTERT-immortalized cells, MC3T3-E1, PMOB, and 43KO-PMOB cells at 1, 2, and 3 wk in culture using OsteoImage Mineralization Assay Kit. The degree of HA nodule formation was measured as the fluorescence corresponding to the specific binding of the OsteoImage staining reagent to hydroxyapatite (HA) and expressed as relative fluorescence units (RFU; 492 nm excitation/520 nm emission wavelengths). Note, PMOB and 43KO-PMOB were seeded 10-fold more densely than MOBs, 43KO-MOBs, and MC3T3-E1 to attain a comparable cell density at the time of matrix mineralization analysis. All data are presented as means ± SE, N = 3; *P < 0.0001 (MOB-B vs. all other cells at week 1), +P < 0.05 (PMOB, MOB-B, and -C vs. 43KO-PMOB, 43KO-MOB-B, and -C at week 2), ‡P < 0.05 (MC3T3-E1 vs. 43KO-PMOB at week 2), **P < 0.001 (PMOB, MOB-C vs. 43KO-PMOB, 43KO-MOB-B, and -C at week 3), #P < 0.001 (MOB-B, MC3T3-E1 vs. 43KO-PMOB, 43KO-MOB-C at week 3). P values were obtained using one-way ANOVA followed by Tukey's multiple comparison test.
Although the von Kossa method has been widely used to assess in vitro mineralization, it is not adequate to assess bone formation as shown by previous reports (4). Thus we further quantified mineralized nodule formation in selected MOBs and 43KO-MOBs using the OsteoImage Mineralization Assay Kit (Lonza) to detect the HA portion of bone-like nodules deposited by cells. Cells were cultured with or without AA and β-GP for 1, 2, and 3 wk for this analysis. The amount of HA deposited in the cell cultures was determined by measuring the level of fluorescence resultant from the specific binding of the OsteoImage staining reagent to HA, expressed as relative fluorescence units (RFU) as shown in Fig. 7B. There was little or no HA staining in any of the cells when they were cultured without AA and β-GP (Fig. 7B, white and patterned bars). However, similar to von Kossa evaluation of mineralization, when cells were cultured in the presence of AA and β-GP, we observed abundant HA-positive staining at 2 (dark gray bars) and 3 wk (light gray bars) in culture. In MOB-B, significant HA staining was detected as early as 1 wk (Fig. 7B, black bars). By week 2, the HA fraction of bone-like nodules in primary wild-type osteoblasts in MOB cell lines and MC3T3-E1 cells was substantially higher than in primary Cx43-null osteoblasts and in Cx43-null cell lines (Fig. 7B, dark gray bars). By week 3, only primary Cx43-null osteoblasts and 43KO-MOB-C exhibited significantly lower HA deposition compared with primary wild-type osteoblasts and MOB cell lines. This detailed analysis of HA nodule formation further complements our von Kossa observation that matrix mineralization was higher at 2 and 3 wk in culture in wild-type compared with Cx43-null osteoblasts.
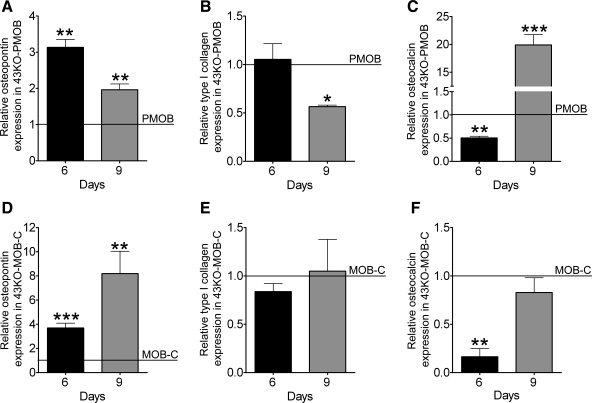
Findings from these studies clearly indicate that there is an early critical time window for osteoblast differentiation that depends on the presence of Cx43, without which osteoblast differentiation is delayed and mineralization is ultimately hindered. Temporal expression patterns of some of the key osteoblastic markers were examined to further evaluate the impact of Cx43 absence at early stages of osteoblast differentiation. For this we used the MOB-C and 43KO-MOB-C cell lines, which are phenotypically most similar to their respective primary cell counterparts, and we determined by qPCR the mRNA expression levels of osteopontin, type I collagen, and osteocalcin in wild-type and Cx43-null cultures at 6 and 9 days after plating the cells. Because of differences in population doubling time, primary osteoblasts were seeded at a 10-fold higher density than those of the hTERT-immortalized cells. For comparison, mRNA expression levels in Cx43-null cells were normalized by those of their respective wild types. As shown in Fig. 8A, osteopontin mRNA expression in Cx43-null PMOB was significantly higher than in primary wild-type PMOB at both time points (day 6 and 9), which is in accordance with previous observation of Lecanda and coworkers (26). Similarly, in the hTERT-immortalized cells, osteopontin expression in 43KO-MOB-C compared with MOB-C was markedly increased at both days 6 and 9 (Fig. 8D). At day 6, type I collagen expression in 43KO-PMOB was comparable to that in PMOB but was considerably lower at day 9 (Fig. 8B). In hTERT-immortalized cells, type I collagen expression in 43KO-MOB-C compared with MOB-C was slightly lower at day 6 (Fig. 8E, black bar) and reached comparable expression levels by day 9 (Fig. 8E, gray bar). Similarly to that shown in previous studies (26), we observed that at day 6 the osteocalcin expression in 43KO-PMOB compared with PMOB was significantly lower (Fig. 8C, black bar). However, at day 9 the osteocalcin expression in 43KO-PMOB is markedly higher than in PMOB (Fig. 8C, gray bar), which clearly indicates that differentiation in primary wild-type osteoblasts is occurring much earlier than in primary Cx43-null osteoblasts. Likewise, at day 6 the osteocalcin expression in the hTERT-immortalized 43KO-MOB-C cells was significantly lower than in MOB-C cells (Fig. 8F, black bar), but at day 9 the expression levels in both cell lines were comparable (Fig. 8F, gray bar). The observation that expression levels of osteocalcin in primary Cx43-null cells relative to wild-type levels were much higher than those in 43KO-MOB-C relative to MOB-C at day 9 indicates that the differentiation gap between primary wild-type and Cx43-null osteoblasts is larger than that between the hTERT-immortalized cells. Therefore, when compared with the primary Cx43-null osteoblasts, differentiation of the 43KO-MOB-C is less delayed, in agreement with our reported differences between these Cx43-null cells regarding ALP expression and mineralization (Figs. 6 and 7).
Fig. 8.
Impact of Cx43 deletion on expression of key osteoblastic differentiation markers. MOB and 43KO-MOB mouse osteoblastic cell lines (seeded at 1.5×103 cells/cm2) and PMOB and 43KO-PMOB mouse osteoblasts (seeded 2.0 × 104 cells/cm2) were plated in 35-mm dishes and cultured in α-MEM + 10% FBS for 6 and 9 days. Quantitative real-time PCR analysis of osteopontin (A, D), type I collagen (B, E), and osteocalcin (C, F) mRNA expression in Cx43-null cells relative to wild-type cells at 6 and 9 days after being plated. The ΔΔCT method was used for analysis, where the value obtained for each gene of interest is first normalized to that of the reference gene (β-actin) and then to MOB-C for 43KO-MOB-C, and to PMOB for 43KO-PMOB (indicated by horizontal lines). All data are presented as means ± SE, N = 3 for hTERT-immortalized cells; *P < 0.05, **P < 0.005, ***P < 0.0005. P values were obtained using t-tests.
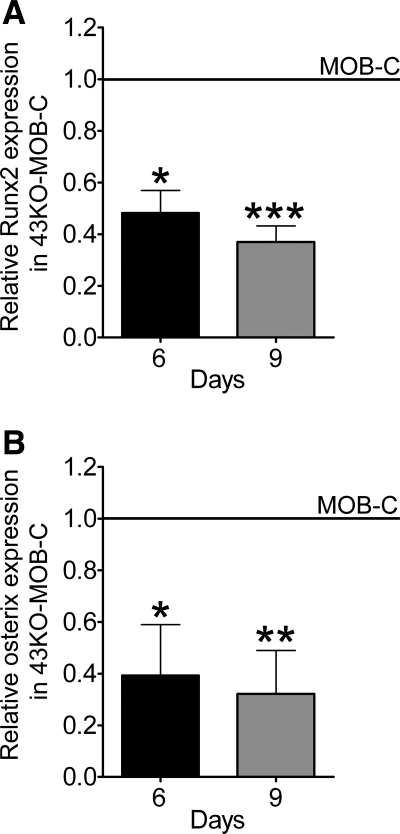
Besides osteocalcin, type I collagen and osteopontin, Runx2 and Osx are also markers of osteoblast differentiation and known to play key roles in bone formation and mineralization. Consistent with our findings of early delayed osteoblast differentiation in the absence of Cx43, we observed that expression levels of Runx2 and Osx at day 6 and 9 in hTERT-immortalized 43KO-MOB-C were significantly lower than in MOB-C cultures (Fig. 9).
Fig. 9.
Effect of Cx43 deletion on expression levels of Runx2 and osterix. Wild-type MOB and 43KO-MOB mouse osteoblastic cell lines (seeded at 1.5 × 103 cells/cm2) were plated in 35-mm dishes and cultured in α-MEM + 10% FBS for 6 and 9 days. Quantitative real-time PCR analysis of Runx2 (A) and osterix (B) mRNA expression in 43KO-MOB-C relative to MOB-C at 6 and 9 days after platting. The ΔΔCT method was used for data analysis where the value obtained for each gene of interest is first normalized to the reference gene (β-actin) and then to that obtained for MOB-C (indicated by horizontal lines). All data are presented as means ± SE, N = 3; *P < 0.05, **P < 0.005, ***P < 0.0005. P values were obtained using t-tests.
DISCUSSION
In this study we report the generation of new osteoblastic cell models from wild-type (MOB) and Cx43-null (43KO-MOB) mice using hTERT immortalization, and we used two cell lines from each genotype showing comparable phenotypic expression of osteoblast markers to investigate the effect of Cx43 on osteoblast differentiation and mineralization. Culture models consisting of either primary osteoblasts or osteoblastic cell lines (such as MC3T3-E1) have been widely used to study the processes involved in osteoblast differentiation and mineralization. Typically, after initial plating, osteoblasts undergo three distinct phases: the proliferative phase (associated with low expression of terminal differentiation markers), the phase of synthesis and maturation of collagen and extracellular matrix, and the phase of matrix mineralization (39, 47). Once matrix synthesis begins, osteoblast markers are activated in a temporal sequence starting with such early genes as those encoding alkaline phosphatase and parathyroid hormone and its receptors (PTH/PTHrP-R), followed by late osteoblast maturation markers such as osteocalcin (15, 39, 47) and transcription factors such as Runx2 and Osx that are crucial for bone formation and mineralization (2, 31). Although existing cell models become phenotypically heterogeneous as a result of prolonged passaging (13, 32, 47), our cell lines exhibited retention of osteoblast lineage markers extending through many population doublings.
Previous studies in primary cultures have compared Cx43-null and wild-type osteoblasts with regard to both general properties (such as cell growth) and bone cell-specific parameters (such as mineralization). In our studies, average population Td of primary osteoblasts was lower than of immortalized cells, but in neither case was there a difference with respect to genotype. These findings support a previous study where no marked differences between the proliferation rates of wild-type and Cx43-null osteoblasts were observed (26). However, in a study using calvarial explants the cell outgrowth from Cx43-null tissue was significantly slower than the wild-type during the first week in culture but became markedly faster than in wild-type explants after the third week (49). The difference in findings between cell cultures (where differences in growth rate have not been detected) and explants (where variable growth that differs between genotype has been described) might simply be due to geometric constrains on growth in the explant conditions; alternatively a more complex phenomenon may be involved, such as a downstream effect of Cx43 ablation as the authors proposed (49).
With regard to osteoblast phenotype, the hTERT-immortalized wild-type and Cx43-null cells expressed well the early marker ALP, consistent with studies using it as a indicator of osteoblast differentiation (1, 33). However, when the time course of ALP expression was quantified, there was a significant delay in differentiation during the first week of culture in the hTERT Cx43-null cell lines. By 12 days in culture, all hTERT cell lines exhibited similar ALP expression regardless of genotype. This pattern of delayed differentiation observed in our selected hTERT Cx43-null osteoblast lines is somewhat similar to that previously reported for Cx43-null calvarial osteoblasts (26), but it differs from the similar differentiation seen in calvarial osteoblasts derived from transgenic mice (Gja1Jrt/+) expressing one of the dysfunctional Cx43 mutations causing ODDD (29). Our findings also contrast with those showing increased ALP expression in osteoblasts cultured from explants of Cx43-null calvaria (49). As mentioned above, the extent to which these results can be compared with those of cell lines may not be straightforward.
With respect to matrix mineralization, we found a high but variable deposition of matrix in hTERT-immortalized cell lines that started as early as 2 wk and gradually progressed through the third week in culture in the presence of AA and β-GP. This finding of high mineralization is further indication of a high degree of differentiation of the hTERT-immortalized compared with primary cells. Detailed quantification of HA deposition revealed that typical mineralization observed in primary and hTERT-immortalized wild-type osteoblasts was subtly but significantly delayed in one of the hTERT-immortalized Cx43-null and greatly hindered in the other Cx43-null cell line. Similarly, our primary Cx43-null osteoblasts and those from previous reports (26, 49) exhibited a prominent delay in mineralization compared with wild-type osteoblasts.
Our findings that ALP expression in the hTERT-immortalized Cx43-null osteoblasts was significantly delayed during the first week but became equal to that of wild-type osteoblasts by the second week in culture indicated that presence of Cx43 is required at early phases of osteoblast differentiation. Further evidence for the existence of such an early critical time window was provided by our analysis of osteopontin, type I collagen, osteocalcin, Runx2, and Osx expression at days 6 and 9 in culture. Taken together, the findings obtained from our wild-type and Cx43-null cell models not only demonstrate that MOB-C and 43KO-MOB-C cell lines phenotypically resemble their primary wild-type and Cx43-null counterparts but also show that Cx43 plays an essential role in early stages of osteoblast differentiation and also in the mineralization process.
Regarding the other main osteoblast gap junction protein Cx45, it might in principle play a role in skeleton formation. However, Cx45 protein levels are reportedly higher in Cx43-null mouse calvarial osteoblasts than in wild-type (26, 49) yet skeletal formation is abnormal, thus indicating lack of complete compensation for Cx43 absence. In our study, primary wild-type and Cx43-null osteoblasts, hTERT-immortalized osteoblasts, and MC3T3-E1 presented variable yet comparable levels of Cx45 expression, demonstrating a lack of consistent upregulation of Cx45 expression in either the primary or hTERT-immortalized Cx43-null osteoblasts. Moreover, regardless of the level of Cx45 expression, both hTERT wild-type and Cx43-null cell lines displayed similar degree of differentiation and matrix mineralization.
Cx43-null mice and also those expressing missense mutations in the Cx43 gene (causing ODDD) display profound developmental abnormalities in the skeleton as well as osteoblast dysfunction (26, 29). Our studies with primary and immortalized Cx43-null osteoblasts provide further mechanistic insight, showing that there is a narrow window during which absence of Cx43 impairs osteoblast differentiation and mineralization. These new hTERT-immortalized wild-type and Cx43-null osteoblast cell lines now allow detailed temporal dissection of alterations in the transcriptional programs responsible for the Cx43-associated delay in differentiation and mineralization. Additional roles for Cx43 in bone physiology include Ca2+ signaling (21) and prostaglandin E2 release in response to fluid flow (6, 36), response to mechanical loading (16), and conditioning bone cell responses to hormonal stimulation (7, 8, 11, 37). However, the evidence linking Cx43 to these functions is primarily from studies of Cx43 knockdown using antisense oligonucleotides, which can interact with a broad spectrum of cellular proteins, resulting in off-target or downstream effects (38), or the use of chemical inhibitors that can affect other pore-forming channels (34). The Cx43-null and wild-type hTERT-immortalized cell lines described here should provide a complementary tool to clarify these and other potential roles for Cx43 in osteoblast function in normal and pathological conditions.
GRANTS
This work was supported by the following National Institutes of Health grants F32HL-082130, NS-041282, S10RR-020949, AR-057139, and DK-081435.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGEMENTS
The authors are indebted to Dr. Robert Majeska (The City College of New York) and Dr. Linda Bonewald (University of Missouri, Kansas City School of Dentistry) for helpful discussions. The assistance of Aisha Cordero with animal breeding is greatly appreciated.
REFERENCES
- 1.Aubin JE, Turksen K, Heersche JNM. Osteoblastic cell lineage. In: Cellular and Molecular Biology of Bone, edited by Noda M. San Diego, CA: Academic, 1993, p. 1–45 [Google Scholar]
- 2.Baek WY, Lee MA, Jung JW, Kim SY, Akiyama H, de Crombrugghe B, Kim JE. Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J Bone Miner Res 24: 1055–1065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349–352, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int 72: 537–547, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Chen H, Feng JQ, Windle JJ, Koop BA, Harris MA, Bonewald LF, Boyce BF, Wonzney JM, Mundy GR, Harris SE. Osteoblastic cell line derived from a transgenic mouse containing the osteocalcin promoter driving SV40 T-antigen. Mol Cell Differ 3: 193–212, 1995 [Google Scholar]
- 6.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell 16: 3100–3106, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung DJ, Castro CHM, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 119: 4187–4198, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys 473: 188–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MVL, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev 32: 45–56, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Donahue HJ. Gap junctions and biophysical regulation of bone cell differentiation. Bone 26: 417–422, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Donahue HJ, McLeod KJ, Rubin CT, Andersen J, Grine EA, Hertzberg EL, Brink PR. Cell-to-cell communication in osteoblastic networks: cell line-dependent hormonal regulation of gap junction function. J Bone Miner Res 10: 881–889, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Doty SB. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int 33: 509–512, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Ecarot-Charrier B, Glorieux FH, van der Rest M, Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol 96: 639–643, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JEI, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, Nieman BJ, Peterson K, Qu D, Roscoe W, Shao Q, Tong D, Veitch GIL, Voronina I, Vukobradovic I, Wood GA, Zhu Y, Zirngibl RA, Aubin JE, Bai D, Bruneau BG, Grynpas M, Henderson JE, Henkelman RM, McKerlie C, Sled JG, Stanford WL, Laird DW, Kidder GM, Adamson SL, Rossant J. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132: 4375–4386, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3–E1 cells. J Bone Miner Res 7: 235–246, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). J Bone Miner Res 23: 879–886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haag M, Van Linthout S, Schroder SE, Freymann U, Ringe J, Tschope C, Sittinger M. Endomyocardial biopsy derived adherent proliferating cells: a potential cell source for cardiac tissue engineering. J Cell Biochem 109: 564–575, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Harley CB. Telomerase is not an oncogene. Oncogene 21: 494–502, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Jeansonne BG, Feagin FF, McMinn RW, Shoemaker RL, Rehm WS. Cell-to-cell communication of osteoblasts. J Dent Res 58: 1415–1423, 1979 [DOI] [PubMed] [Google Scholar]
- 20.Jikko A, Harris SE, Chen D, Mendrick DL, Damsky CH. Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. J Bone Miner Res 14: 1075–1083, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen NR, Henriksen Z, Brot C, Eriksen EF, Sorensen OH, Civitelli R, Steinberg TH. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J Bone Miner Res 15: 1024–1032, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci USA 104: 20512–20516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. J Bone Miner Res 16: 1622–1633, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH. Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol 130: 987–995, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell 9: 2249–2258, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol 151: 931–944, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majeska RJ, Rodan GA. Alkaline phosphatase inhibition by parathyroid hormone and isoproterenol in a clonal rat osteosarcoma cell line. Possible mediation by cyclic AMP. Calcif Tissue Int 34: 59–66, 1982 [DOI] [PubMed] [Google Scholar]
- 28.Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T. BMP2 Regulates Osterix through Msx2 and Runx2 during Osteoblast Differentiation. J Biol Chem 283: 29119–29125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLachlan E, Plante I, Shao Q, Tong D, Kidder GM, Bernier SM, Laird DW. ODDD-Linked Cx43 mutants reduce endogenous Cx43 expression and function in osteoblasts and inhibit late stage differentiation. J Bone Miner Res 23: 928–938, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Minkoff R, Rundus VR, Parker SB, Hertzberg EL, Laing JG, Beyer EC. Gap junction proteins exhibit early and specific expression during intramembranous bone formation in the developing chick mandible. Anat Embryol (Berl) 190: 231–241, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Nijweide PJ, van Iperen-van Gent A, Kawilarang-de Haas EW, van der Plas A, Wassenaar AM. Bone formation and calcification by isolated osteoblastlike cells. J Cell Biol 93: 318–323, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143: 420–430, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Intern 45: 259–264, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science 267: 1831–1834, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Saunders MM, You J, Trosko JE, Yamasaki H, Li Z, Donahue HJ, Jacobs CR. Gap junctions and fluid flow response in MC3T3–E1 cells. Am J Physiol Cell Physiol 281: C1917–C1925, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Schiller PC, D'Ippolito G, Balkan W, Roos BA, Howard GA. Gap-junctional communication mediates parathyroid hormone stimulation of mineralization in osteoblastic cultures. Bone 28: 38–44, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Stein CA. Phosphorothioate antisense oligodeoxynucleotides: questions of specificity. Trends Biotechnol 14: 147–149, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J 4: 3111–3123, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD, Wang HZ, Warlow PM, Westphale EM, Laing JG. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J 13: 744–750, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol 96: 191–198, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takano H, Murasawa S, Asahara T. Functional and gene expression analysis of hTERT overexpressed endothelial cells. Biologics 2: 547–554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thi MM, Kojima T, Cowin SC, Weinbaum S, Spray DC. Fluid shear stress remodels expression and function of junctional proteins in cultured bone cells. Am J Physiol Cell Physiol 284: C389–C403, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Mackie EJ, Pagel CN. Osteopontin and skeletal muscle myoblasts: Association with muscle regeneration and regulation of myoblast function in vitro. Intern J Biochem Cell Biol 40: 2303–2314, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Urban M, Rozental R, Spray DC. A simple RT-PCR-based strategy for screening connexin identity. Braz J Med Biol Res 32: 1029–1037, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Vander Molen MA, Rubin CT, McLeod KJ, McCauley LK, Donahue HJ. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J Biol Chem 271: 12165–12171, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3–E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res 14: 893–903, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Warner A. Gap junctions in development–a perspective. Semin Cell Biol 3: 81–91, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Weimann M, Gramsch B, Winterhager E, Schirrmacher K. Growth and differentiation of osteoblast-like cells from calvaria of connexin43 deficient mice. Materialwissenschaft und Werkstofftechnik 35: 962–967, 2004 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.