Abstract
Formyl peptide receptor-induced chemotaxis of neutrophils depends on the release of ATP and autocrine feedback through purinergic receptors. Here, we show that adrenergic receptor signaling requires similar purinergic feedback mechanisms. Real-time RT-PCR analysis revealed that human embryonic kidney (HEK)-293 cells express several subtypes of adrenergic (α1-, α2-, and β-receptors), adenosine (P1), and nucleotide receptors (P2). Stimulation of Gq-coupled α1-receptors caused release of cellular ATP and MAPK activation, which was blocked by inhibiting P2 receptors with suramin. Stimulation of Gi-coupled α2-receptors induced weak ATP release, while Gs-coupled β-receptors caused accumulation of extracellular ADP and adenosine. β-Receptors triggered intracellular cAMP signaling, which was blocked by scavenging extracellular adenosine with adenosine deaminase or by inhibiting A2a adenosine receptors with SCH58261. These findings suggest that adrenergic receptors require purinergic receptors to elicit downstream signaling responses in HEK-293 cells. We evaluated the physiological relevance of these findings using mouse aorta tissue rings. Stimulation of α1-receptors induced ATP release and tissue contraction, which was reduced by removing extracellular ATP with apyrase or in the absence of P2Y2 receptors in aorta rings from P2Y2 receptor knockout mice. We conclude that, like formyl peptide receptors, adrenergic receptors require purinergic feedback mechanisms to control complex physiological processes such as smooth muscle contraction and regulation of vascular tone.
Keywords: phenylephrine; isoproterenol; mitogen-activated protein kinase; adenosine 3′,5′-cyclic monophosphate; P2Y2 knockout mice
atp release from mammalian cells is a newly discovered physiologic process that controls cellular functions in an autocrine and paracrine fashion (5). ATP release was first observed in peripheral and central neurons (10). Later, it was discovered that other cell types also release ATP in response to mechanical stress, hypoxia, and stimulation by various chemical agents (5, 8). Extracellular nucleotides act through purinergic receptors that comprise seven distinct P2X receptor subtypes (P2X1–7), which act as ion channels, and eight P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–14) that are G protein-coupled receptors (GPCRs) (47).
We reported previously that stimulation of formyl peptide receptors (FPR) with chemoattractants results in the release of ATP from human neutrophils and that ATP release is required to drive chemotaxis through purinergic feedback mechanisms that involve P2Y and adenosine receptors (15). FPR and other chemotaxis receptors require P2Y2 receptors that amplify chemotactic signals and facilitate gradient sensing. Chemotaxis receptors are members of the GPCR receptor family and couple to Gi subunits of the heterotrimeric G proteins that associate with GPCRs (33, 37, 54). The purpose of the present study was to investigate whether neutrophil chemotaxis receptors are unique with regard to their need for purinergic signaling or whether similar purinergic signaling mechanisms are a more general feature of GPCR-type receptors.
In the vascular system, ATP released from nerves and endothelial cells controls vascular tone through complex paracrine purinergic signaling mechanisms (7). P2Y1 and P2Y2, the main nucleotide receptors in vascular endothelial cells, mediate vasodilation by the release of nitric oxide (43). In contrast to endothelial cells, vascular smooth muscle cells express multiple other purinergic receptor subtypes that trigger smooth muscle contraction (31, 50). Adrenergic receptors are archetypical GPCRs and recognize the endogenous catecholamines epinephrine and norepinephrine that control vascular tone and a wide spectrum of other physiologic functions (6, 49). Because of their wide-ranging effects, adrenergic receptors are therapeutic targets for diseases ranging from heart failure to shock (2, 29, 41, 58). On the basis of molecular structures, pharmacological properties, and their downstream signal pathways, adrenergic receptors are divided into three major subfamilies: α1-, α2-, and β-adrenergic receptors. These three adrenergic receptor (ADR) subtypes couple to different classes of heteromeric G proteins. The α1- and α2-subtypes couple to Gq/11 and Gi/o, respectively, while the β-receptors couple to Gs (24). In the present study, we investigate in human embryonic kidney (HEK)-293 cells whether stimulation of these different ADR subtypes results in ATP release and whether purinergic signaling processes are required for the downstream signaling events and functional responses elicited by adrenergic receptors; in addition, we test the physiologic significance of the findings in HEK-293 cells using mouse aorta ring experiments.
MATERIALS AND METHODS
Materials.
ATP, ATPγS [adenosine 5′-(3-thiotriphosphate)], apyrase, adenosine deaminase, phenylephrine, UK14,304 [5-bromo-N-(2-imidazolin-2-yl)-6-quinoxalinamine], dl-isoproterenol, HCl, Krebs-Henseleit (KH) buffer, dithiothreitol, proteinase inhibitor cocktail, acetylcholine, KCl, chloroacetaldehyde, SCH58261 [2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2, 4]triazolo[1,5-c]pyrimidin-5-amine], MRS1754 [N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1, 3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide], dipyridamole, NH4HCO3, and carbenoxolone were purchased from Sigma-Aldrich (St. Louis, MO). Suramin was from EMD Chemicals (Darmstadt, Germany), sodium dodecyl sulfate (SDS) buffer was from Invitrogen (San Diego, CA), mammalian protein extraction reagent and phosphatase inhibitor cocktail were from Thermo Scientific (Rockford, IL), Na2HPO4 was purchased from J. T. Baker (Phillipsburg, NJ), and 10Panx1 (Trp-Arg-Gln-Ala-Ala-Phe-Val-Asp-Ser-Tyr) was from Tocris Bioscience (Ellisville, MO).
Cells.
HEK-293 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM) from Mediatech Cellgro (Manassas, VA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Cells were kept at 37°C in humidified atmosphere consisting of 95% air and 5% CO2.
Real-time reverse transcriptase polymerase chain reaction analysis.
Real-time reverse transcriptase polymerase chain reactions (RT-PCR) were used to assess adrenergic and purinergic receptor expression patterns in HEK-293 cells. Total RNA was extracted from HEK-293 cells using TRIzol reagent (Invitrogen) and treated with 1 U/μg RNase-free DNAse (Invitrogen), and RNA concentrations were determined using a spectrophotometer. First-strand cDNA was synthesized from 2 μg RNA using Superscript reverse transcriptase (Invitrogen) according to the manufacturer's instructions. PCR was performed using Platinum PCR Supermix (Invitrogen) containing 100 nM sense and anti-sense primers and 100 ng cDNA. Real-time RT-PCR was performed using a QuantiTect SYBR Green PCR kit from Qiagen (Valencia, CA). Primer sets for purinergic receptors were developed in our laboratory (15), and validated predesigned primer sets for all adrenergic receptor subtypes were purchased from Qiagen. Reactions were amplified and subjected to melting curve analysis using a Mastercycler Realplex instrument (Eppendorf; Hamburg, Germany) and the following cycle parameters: 1 step at 95°C (15 min), 40 cycles at 94°C (15 s), 55°C (30 s), and 72°C (30 s). A standard curve was generated by plotting the threshold cycle (Ct) against the log of the initial starting quantity. Adrenergic and purinergic receptor gene expression levels were measured in samples using the comparative Ct method for relative quantification of gene expression, and results were normalized against β-actin mRNA levels.
Quantification of ATP release.
ATP release from HEK-293 cells was determined with a commercially available ATP bioluminescence assay kit HS (Roche Diagnostics; Mannheim, Germany). HEK-293 cells were seeded at a density of 3.5 × 105/well in 24-well tissue culture plates coated with poly-l-lysine. Cells were grown to confluence and maintained in serum-free medium overnight before use. On the day of the experiment, cells were allowed to rest in a volume of 200 μl medium per well for 1 h in a water bath at 37°C on a vibration isolation table to minimize ATP release due to mechanical stimulation. The different reagents used in our experiments were diluted in 200 μl medium resulting in the following concentrations: 10 μM phenylephrine (α1-agonist), 1 μM UK14,304 (α2-agonist), 1 μM isoproterenol (β-agonist), and 10 μM acetylcholine (muscarinic acetylcholine receptor agonist). These final dilutions were obtained by gently adding appropriate volumes of stock solutions. After incubation as indicated, plates were placed on ice and supernatants were removed and centrifuged to eliminate cellular contaminants and debris. Supernatants were boiled for 5 min to inactivate nucleotidases, and 50 μl/well was transferred to a 96-well luminometer plate. Luciferin-luciferase reagent was added, and sequential readings were taken using a Luminoskan Ascent microplate luminometer (Labsystems; Helsinki, Finland). For some experiments, ATP release was assessed using high-performance liquid chromatography (HPLC). For that purpose, etheno-derivatives of adenine compounds in cell culture supernatants were generated as described elsewhere (32). Briefly, 150 μl of culture supernatants or of nucleotide standard solution was incubated for 30 min at 72°C in the presence of 1 M chloroacetaldehyde and 25 mM Na2HPO4 in a final reaction volume of 200 μl. Samples were placed on ice, alkalinized with 50 μl of 0.5 M NH4HCO3, and analyzed as described previously using a Waters HPLC system (Milford, MA) equipped with a fluorescence detector (15, 32).
Mitogen-activated protein kinase activation.
To assess activation of the p42 and p44 extracellular signal-regulated kinase (ERK) members of mitogen-activated protein kinases (MAPK), HEK-293 cells were grown to confluence as described above and incubated overnight in serum-free culture medium before the experiment. The cells were then stimulated as indicated, kept at 37°C on a vibration isolation table, and placed on ice. Supernatants were discarded and cells were lysed with 150 μl/well mammalian protein extraction reagent containing a proteinase and phosphatase inhibitor cocktail. Cells were disrupted by ultrasonication, cell lysates were centrifuged, and 50-μl aliquots of the supernatants were boiled for 5 min with 50 μl SDS sample buffer containing 50 μM dithiothreitol. Proteins were separated by polyacrylamide gel electrophoresis using 12% Tris-glycine gels (Invitrogen) and transferred onto Immobilon polyvinylidene difluoride membranes (Millipore; Bedford, MA). Membranes were subjected to immunoblotting with anti-phospho-ERK antibodies (Cell Signaling; Danvers, MA) that recognize the phosphorylated and thus activated forms of p42 and p44 ERK MAPKs. Then secondary horseradish-conjugated antibodies were added and bands were detected with ECL reagent (General Electric Healthcare, Little Chalfont, UK). The blots were stripped and reprobed with antibodies that recognize total ERK (Santa Cruz Biotechnology; Santa Cruz, CA). Western blots were analyzed by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD), and results were normalized on the basis of total ERK band intensities.
Intracellular cAMP concentrations.
HEK-293 cells cultured as described above were stimulated for 1 min at 37°C on a vibration isolation table as described below, and concentrations of cAMP in the cytosol were assessed using a commercial cAMP assay kit (cAMP-Screen) from Applied Biosystems (Bedford, MA).
Smooth muscle contraction assay.
The use of laboratory animals was in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. Male C57BL/6J wild-type (WT) mice (20–25g, 8–15 wk) were obtained from Charles River (Wilmington, MA). Homozygous P2Y2 knockout (P2Y2 KO) mice with a C57BL/6J genetic background were a generous gift of Drs. Beverly H. Koller (University of North Carolina, Chapel Hill, NC) and Volker Vallon (University of California San Diego, La Jolla, CA). Mice were anesthetized using isoflurane vapor (TW Medical; Lago Vista, TX) and euthanized with an overdose of the anesthetic drug. The thoracic aorta was excised and placed in KH buffer saturated with a gas mixture composed of 95% O2 and 5% CO2. The aorta specimens were dissected to remove surrounding fatty and connective tissue and were cut transversely into rings of 2- to 3-mm lengths. These rings were mounted on two wires connected to a force displacement transducer of an F30 Harvard Apparatus tissue bath (Harvard Apparatus; Holliston, MA). In some experiments, the endothelial cell layer was removed by rubbing the luminal side of the aorta using a 5-0 silk suture. Aorta rings, bathed in KH buffer, were then subjected to an initial load tension of 0.8 g and allowed to equilibrate for 45–60 min with changes of the bathing fluid before experiments. Contraction and relaxation was measured isometrically and recorded using a MLS060 AD Instrument. Data are expressed as means ± SD and represented as the percentage of contraction relative to the maximum contraction of each aorta ring in response to KCl (110 mM). To test for the presence of intact endothelium, the response of tissue specimens to acetylcholine was tested as previously described (13). Aorta rings were considered to possess intact endothelium when relaxation in response to acetylcholine (10 μM) was >50% of precontraction values induced by phenylephrine (0.1 μM), while preparations showing <10% of such values were considered devoid of endothelium (13).
ATP release from isolated aorta rings.
Whole thoracic aorta specimens were harvested as described above, cut open lengthwise, suspended in 2 ml of tissue bath solution consisting of KH buffer saturated with 95% O2 and 5% CO2, pH 7.4 at 37°C, and allowed to equilibrate for 1 h. Samples of the bath solution were taken before stimulation of tissue specimens with 1 μM phenylephrine, and additional samples were collected at different time points after stimulation. After centrifugation, samples were boiled for 5 min and ATP concentrations were determined using the luminometric method described above.
Statistical analysis.
All values are expressed as means ± SD. Statistical analyses were performed with the two-tailed paired Student's t-test, and differences were considered statistically significant at P < 0.05.
RESULTS
Adrenergic receptor expression in HEK-293 cells.
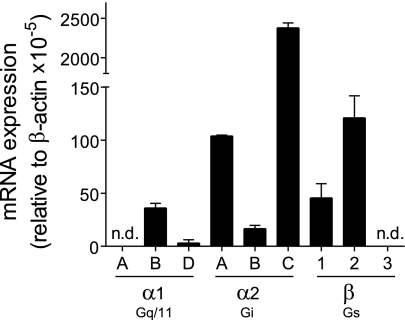
RT-PCR analysis revealed that HEK-293 cells express mRNA of all ADR subtypes with the exception of α1A- and β3-receptors (Fig. 1). The ADR subtypes with highest relative mRNA abundance were α2C, α2A, and β2, and we found mRNA encoding two of the three α1-receptor subtypes. Some previous reports suggest that HEK-293 cells may not express endogenous α1-receptors (34, 57). The mRNA pattern shown in Fig. 1 suggests that the HEK-293 cell lines used in these studies and in ours could differ with regard to their ADR subtype expression patterns. The presence of α1-receptors in our cell line was further confirmed by Ca2+ mobilization experiments, which showed that stimulation with the α1-receptor-specific agonist phenylephrine induces strong Ca2+ signaling (data not shown).
Fig. 1.
Expression of adrenergic receptor (ADR) subtypes in human embryonic kidney (HEK)-293 cells. Expression of adrenergic receptors in HEK-293 cells was determined using real-time RT-PCR analysis with commercially available primer sets (Qiagen). Expression levels below the detection limit of the RT-PCR analysis are designated with “not detectable” (n.d.). Data were normalized using expression of the housekeeping gene β-actin. Values are expressed as means ± SD of triplicate determinations.
α1-Receptor stimulation induces ATP release through pannexin-1 hemichannels.
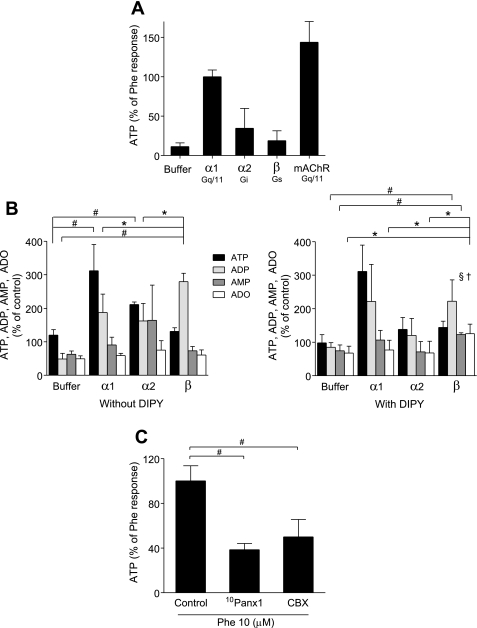
Resting HEK-293 cells supernatants had an average ATP concentration of 0.02 μM. We used phenylephrine (10 μM), UK14,304 (1 μM), and isoproterenol (1 μM) to stimulate the α1-, α2-, and β-receptor subtypes, respectively. Stimulation of α1-receptors triggered robust ATP release, with average extracellular ATP concentrations reaching 0.2 μM, which corresponds to a release of 0.16 pmol ATP per cell. Stimulation of α2-receptors triggered weaker ATP release, while stimulation of β-receptors did not cause significant extracellular ATP accumulation (Fig. 2A). These findings suggest that the Gq-coupled α1-receptors induce stronger ATP release than members of the other two ADR receptor families. Interestingly, the unrelated Gq-coupled muscarinic acetylcholine receptors (mAChR) that are endogenously expressed in HEK-293 cells (17) elicited similarly strong ATP release in response to stimulation with acetylcholine (10 μM).
Fig. 2.
ATP release from HEK-293 cells in response to ADR stimulation is mediated by pannexin-1 hemichannels. A: ATP released into the supernatant was determined with an ATP bioluminescence assay kit. HEK-293 cells were stimulated with the ADR agonists phenylephrine (Phe; 10 μM) for α1-receptors, UK14,304 (1 μM) for α2-receptors, and isoproterenol (1 μM) for β-receptors, or with an equivalent volume of DMEM as a vehicle control (buffer). As a control, we included muscarinic acetylcholine receptors (mAChR) that were stimulated with acetylcholine (10 μM). ATP concentrations in the supernatants were determined 3 min after stimulation. The data are shown as percentage of phenylephrine response and are expressed as means ± SD of triplicate determinations. B: ATP, ADP, AMP, and adenosine (ADO) accumulation in the supernatant after ADR stimulation in the presence or absence of dipyridamole (DIPY, 10 μM) was determined using HPLC analysis. HEK-293 cells were preincubated with DIPY for 10 min and stimulated with the ADR agonists or with HBSS as vehicle control (buffer) as described above, and ATP, ADP, AMP, and ADO concentrations in the supernatants were determined after 3 min. The data are shown as percentage of unstimulated controls and are expressed as means ± SD. Statistical analysis was done with Student's t-test; n = 4; #P < 0.01; *P < 0.05. Differences between responses in the absence versus presence of DIPY are marked with the following symbols: †P < 0.05, §P < 0.01. C: effect of pannexin-1 hemichannel inhibitors on ATP release in response to α1-receptor stimulation. HEK-293 cells were preincubated with 10Panx1 (100 μM) or carbenoxolone (CBX; 20 μM) for 10 min and stimulated with 10 μM phenylephrine, and ATP concentrations in the supernatants were determined after 3 min using the bioluminescence assay. The data are shown as percentage of phenylephrine response and are expressed as means ± SD. Statistical analysis was done with Student's t-test; #P < 0.01; n = 4.
HPLC analysis showed that ADR receptor stimulation caused extracellular accumulation of hydrolytic breakdown products of ATP, primarily of ADP and AMP (Fig. 2B). Specifically, we found that β-receptor stimulation resulted in a significant increase of extracellular ADP concentrations, while ATP, AMP, and adenosine concentrations did not change. To evaluate the possibility of rapid cellular re-uptake of adenosine, we performed the experiments described above in the presence of nucleoside transport inhibitor dipyridamole (DIPY; 10 μM). In the presence of DIPY, β-receptor stimulation resulted in a significant increase of extracellular AMP and adenosine concentrations (Fig. 2B). Taken together, these results suggest that stimulation of Gq/11 (α1) or Gi/o (α2)-coupled ADR result in the accumulation of ATP, while stimulation of the Gs-coupled β-receptors induces accumulation of extracellular ADP that is partially converted to adenosine. These findings indicate that the different ADR subtypes result in distinct nucleotides release patterns or kinetics of ATP hydrolysis by ecto-nucleotidases.
We investigated the mechanism whereby α1-receptor stimulation induces ATP release. Inhibition of pannexin-1 hemichannels with the mimetic blocking peptide 10Panx1 (100 μM) or inhibition of gap junction channels with carbenoxolone (20 μM) significantly reduced α1-receptor-induced ATP release from HEK-293 cells (Fig. 2C). Taken together, these results suggest that pannexin-1 hemichannels play a central role in ATP release from HEK-293 cells.
α1-Receptor stimulation induces MAPK activation.
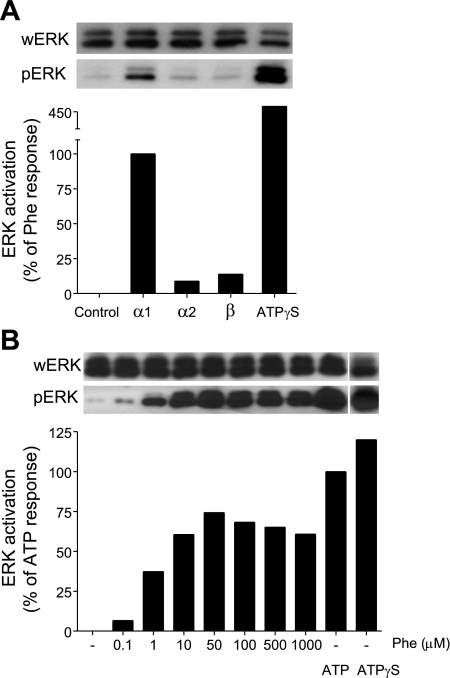
MAPKs are important intracellular signaling molecules that convey signals triggered by cell surface receptors to appropriate functional cell responses. To investigate whether purinergic signaling is involved in ADR-induced MAPK activation, we stimulated HEK-293 cells with the selective ADR receptor agonists described above and assessed p42 and p44 ERK MAPK activation using immunoblotting with antibodies that recognize the phosphorylated (and therefore activated) forms of these MAPKs. We found that stimulation of α1-receptors induced robust p42 and p44 ERK phosphorylation, while stimulation of α2- or β-receptors causes weak, if any, phosphorylation of p42 ERK (Fig. 3A). α1-Receptor stimulation induced dose-dependent and rapid ERK activation that reached a maximum between 30 s and 1 min after stimulation with 50 μM phenylephrine (Fig. 3B). Addition of exogenous ATP or of the nonhydrolyzable analog ATPγS induced ERK phosphorylation, suggesting that α1-receptor-induced ATP release may cause subsequent ERK activation in HEK-293 cells.
Fig. 3.
α1-Receptor stimulation induces ERK activation in HEK-293 cells. A: HEK-293 cells were stimulated with different ADR agonists: phenylephrine (10 μM) for α1-receptors, UK14,304 (1 μM) for α2-receptors, and isoproterenol (1 μM) for β-receptors. Controls were treated with ATPγS (20 μM). After 1 min, the cells were lysed and activation of ERK was determined by immunoblotting of the phosphorylated form of ERK (pERK). Total ERK levels in each sample were determined with antibodies that recognize whole ERK (wERK). Intensities of bands were estimated and normalized with NIH ImageJ software, and data are expressed as percentage of phenylephrine response. The data shown are representative of at least two different experiments with similar results. B: HEK-293 cells were stimulated with different concentrations of the α1-receptor agonist phenylephrine. Controls were stimulated with ATP (20 μM) and ATPγS (20 μM). ERK activation was assessed after 1 min as described above. The data are expressed as percentage of the response to ATP.
Purinergic receptor expression in HEK-293 cells.
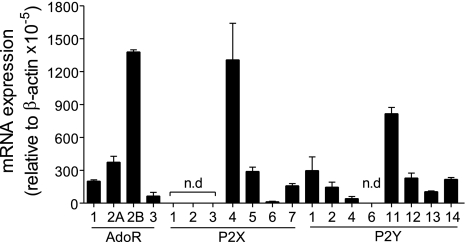
The data above suggest that, similar to chemotaxis receptors in neutrophils, adrenergic receptors in HEK-293 cells may trigger ATP release that promotes cell activation by stimulating purinergic receptors. To define the expression pattern of purinergic receptors in HEK-293 cells, we performed real-time RT-PCR analysis as described above. We detected mRNA of all known adenosine receptors (P1) and of most P2 receptor subtypes with the exception of P2X1, P2X2, P2X3, and P2Y6 (Fig. 4). Among the different purinergic receptor subtypes, mRNA encoding A2b, P2X4, and P2Y11 was most abundant in HEK-293 cells.
Fig. 4.
Purinergic receptor profile of HEK-293 cells. Real-time RT-PCR analysis was used to assess purinergic receptor mRNA expression in HEK-293 cells using the primer sets and experimental approach described previously (11). Expression levels below the detection limit of the RT-PCR assay are marked with “n.d.” (not detectable). Data were normalized using β-actin expression, and values are shown as means ± SD of triplicate determinations. AdoR, adenosine receptor.
P2 receptors are involved in α1-receptor-induced ERK activation.
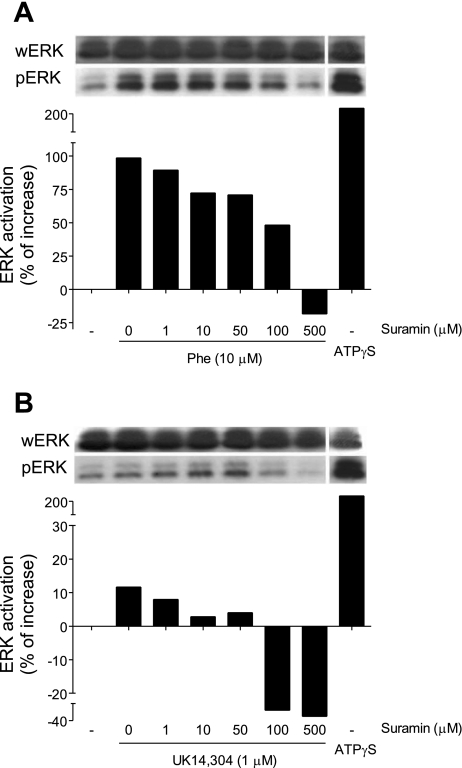
To test whether purinergic feedback mechanisms are involved in α-receptor-induced ERK activation, HEK-293 cells were pretreated with different concentrations of the P2 receptor antagonist suramin 10 min before stimulation of α1- or α2-receptors with phenylephrine (10 μM) or UK14,304 (1 μM). Suramin caused dose-dependent inhibition of ERK in response to α1-receptor stimulation and diminished basal ERK activation levels in cells stimulated with α2-agonist (Fig. 5, A and B). These findings indicate that ERK activation in response to α1 stimulation involves P2 receptors. The fact that suramin inhibited ERK activation to levels below baseline may indicate inhibition of mechanically induced cell stimulation.
Fig. 5.
P2 receptors are involved in α1-receptor-induced ERK activation. HEK-293 cells were treated for 10 min with the indicated concentrations of the P2 receptor antagonist suramin. Then the cells were stimulated with the α1-receptor agonist phenylephrine (10 μM; A) or with the α2-receptor agonist UK14,304 (1 μM; B). As positive controls, we included cells that were exposed to ATPγS (20 μM). After stimulation for 1 min, ERK activation was determined using immunoblotting, and results were analyzed as described in Fig. 3. Data are representative of at least two different experiments with similar results, and values are shown as percent increase over baseline control levels.
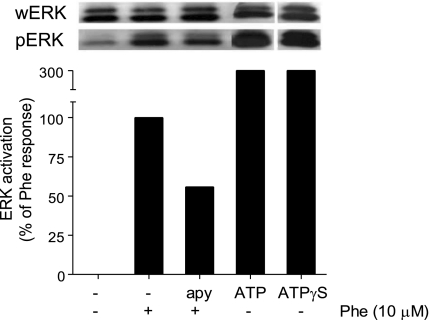
To further study the involvement of ATP release in cell activation by α1-receptors, we treated HEK-293 cells with apyrase (20 U/ml), an enzyme that hydrolyses extracellular ATP, and assessed MAPK activation in response to α1 stimulation. Apyrase treatment decreased α1-induced ERK activation by ∼50% (Fig. 6), which suggests that ATP release and autocrine activation of purinergic receptors is involved in α1-induced cell stimulation.
Fig. 6.
ERK activation in response to α1-receptor stimulation requires ATP. HEK-293 cells were treated with apyrase (apy; 20 U/ml), a scavenger of extracellular ATP. After 10 min, the cells were stimulated with the α1-receptor agonist phenylephrine (10 μM), and ERK activation was determined as described above. Cells stimulated with ATP (20 μM) and ATPγS (20 μM) were included as positive controls.
HEK-293 cells hydrolyze extracellular ATP.
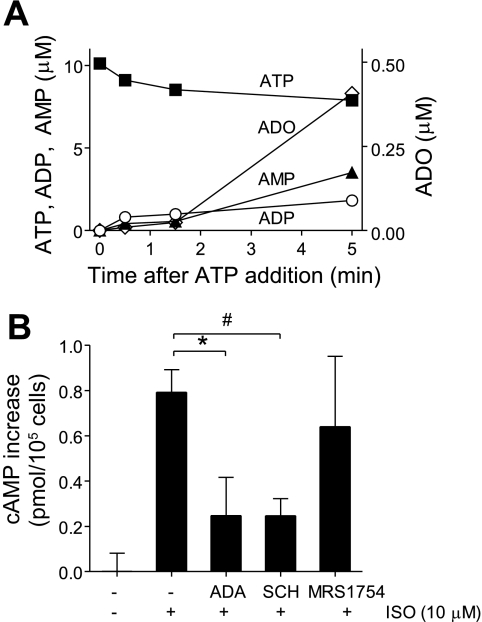
In neutrophils, we found that extracellular adenosine and A3 receptors control specific aspects of cell migration in chemotactic gradient fields (15). Neutrophils hydrolyze released ATP to adenosine that is required for A3 receptor activation. Because of these findings, we studied whether HEK-293 cells are also capable of generating adenosine through breakdown of released ATP. We added exogenous ATP (10 μM) to HEK-293 cells, incubated the cells at 37°C for different periods, and analyzed changes in extracellular concentrations of ATP and its hydrolytic products. We found that HEK-293 cells hydrolyzed ∼25% of the added ATP within 5 min, resulting in the accumulation of extracellular ADP, AMP, and adenosine (Fig. 7A). Adenosine concentrations reached levels of ∼0.4 μM within 5 min, suggesting that HEK-293 cells are indeed capable of converting release ATP to adenosine and that feedback through P1-receptors could be involved in ADR-induced purinergic signaling responses of HEK-293.
Fig. 7.
β-Receptor-induced HEK-293 cell responses involve adenosine receptor stimulation. A: HEK-293 cells were incubated with exogenously added ATP (10 μM) for the indicated times, and hydrolysis of ATP was determined by assaying the concentrations of ATP, ADP, AMP, and ADO in the supernatants using HPLC. B: HEK-293 cells were pretreated with adenosine deaminase (ADA; 20 U/ml), an enzyme that scavenges extracellular adenosine, or with the A2 adenosine receptor (A2) antagonists SCH58261 (50 nM; SCH) or MRS1754 (10 nM) that inhibit A2a or A2b, respectively. After 10 min, the cells were stimulated with the β-receptor agonist isoproterenol (10 μM; ISO), and increases in intracellular cAMP levels were determined after 1 min. Data are shown as increase in cAMP concentration over baseline levels of unstimulated controls, and results are expressed as means ± SD. Statistical analysis was done with Student's t-test; #P < 0.01; *P < 0.05; n = 4.
Adenosine receptors mediate β-receptor-induced signaling responses.
Numerous GPCRs regulate downstream cell responses by modulating intracellular concentrations of the second messenger cAMP. The β- and A2 adenosine receptors are both Gs-coupled receptors, which stimulate adenylyl cyclases that elevate intracellular cAMP concentrations, resulting in the suppression of cell functions through cAMP/PKA signaling pathways (18). The data presented above indicate that stimulation of β-receptors can result in the accumulation of extracellular ADP and adenosine and that HEK-293 cells are able to hydrolyze extracellular ATP to adenosine. These results suggest that adenosine and feedback through adenosine receptors could be involved in downstream signaling events triggered by β-receptors. We found that stimulation of HEK-293 cells with the β-receptor agonist isoproterenol (10 μM) increases intracellular cAMP concentrations by an average of 0.79 ± 0.10 pmol/105 cells (Fig. 7B). Removal of extracellular adenosine by addition of adenosine deaminase (20 U/ml) decreased β-receptor-induced cAMP production by ∼65%, indicating that adenosine is indeed involved in cAMP accumulation in response to β-receptor stimulation. HEK-293 cells express both A2a and A2b receptors that are Gs-coupled GPCRs and are known to trigger intracellular cAMP accumulation (52). Thus, we investigated the potential roles of these adenosine receptor subtypes in β-receptor-induced cAMP production. Pretreating HEK-293 cells with the A2a antagonists SCH58261 (50 nM) for 10 min before β-receptor stimulation blocked cAMP production by ∼69%, while inhibition of A2b with the selective antagonist MRS1754 (10 nM) did not significantly reduce β-receptor-induced cAMP production (Fig. 7B). These results suggest that A2a-type adenosine receptors are involved in the accumulation of intracellular cAMP in response to β-receptor stimulation.
α1-Receptor stimulation of aorta tissue causes ATP release.
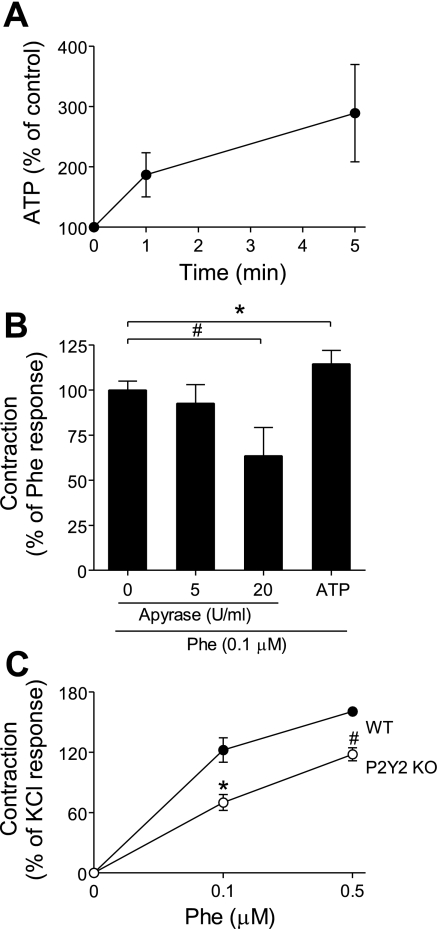
Our results with HEK-293 cells suggest that ADR stimulation involves ATP release and autocrine activation of purinergic receptors that control downstream signaling events such as MAPK phosphorylation. Adrenergic receptors play an important physiological role in the regulation of blood pressure by modulating vascular tone and smooth muscle contraction (16). Thus, we used aorta tissue ring experiments to evaluate whether purinergic signaling is involved in adrenergic responses under physiological conditions. α1 stimulation of aorta rings from WT mice with 1 μM phenylephrine induced strong tissue contraction that was accompanied by the release of ATP into the tissue bath solution, where ATP concentrations reached levels of ∼100 nM within 5 min (Fig. 8A). These data suggest that ATP release may be involved in the control of vascular smooth muscle contraction in response to adrenergic stimulation.
Fig. 8.
ATP and purinergic signaling contribute to adrenergic responses of mouse aorta specimens. A: mouse thoracic aorta sections were stimulated with the α1-receptor agonist phenylephrine (1 μM), and ATP released into the tissue bath solution was determined at the indicated time points using an ATP bioluminescence assay kit. Data are expressed as percentage of baseline readings of unstimulated control tissue. Values are shown as means ± SD of triplicate determinations. B: mouse aorta rings were isolated as described above, and the endothelial layer was removed. The aorta rings were mounted on an apparatus that allows assessment of isometric contraction force and were treated with the indicated concentrations of the ATP scavenger apyrase for 10 min. Then, the aorta rings were stimulated with the α1-receptor agonist phenylephrine (0.1 μM) and contraction was recorded. The response to extracellular ATP was determined by adding exogenous ATP (10 μM). The data shown are means ± SD and are expressed as percentage of phenylephrine response. Statistical analysis was done with Student's t-test; #P < 0.01; *P < 0.05; n = 3. C: mouse aorta rings from wild-type mice (WT, n = 4) or from P2Y2 knockout mice (P2Y2 KO, n = 4) were prepared as described above, stimulated with the indicated concentrations of phenylephrine and contraction was recorded. The data shown are means ± SD of maximum contraction responses, and results are expressed as percentage of contraction in response to KCl (110 mM). Statistical analysis was done with Student's t-test; #P < 0.01; *P < 0.05.
Purinergic signaling is involved in aorta ring contraction.
Mouse vascular smooth muscle cells express several P2 receptor subtypes including P2X1, P2X2, P2X4, P2Y1, P2Y6, as well as P2Y2 (3, 28, 31). Extracellular ATP has been shown to induce contraction of smooth muscle cells (39). To determine whether contraction of aorta rings in response to adrenergic stimulation requires purinergic receptor activation, we investigated the effect of apyrase on tissue contraction in response to 0.1 μM phenylephrine. Pretreatment with apyrase significantly reduced contraction in response to α1-receptor stimulation (Fig. 8B). Subsequent removal of apyrase from the tissue bath solution followed by the addition of exogenous ATP (10 μM) restored tissue contraction. Taken together, these findings suggest that ATP release and purinergic signaling are required for α1-receptor-induced contractile responses of vascular tissues.
While the role of P2X1-receptors in vasoconstriction is established (4), P2Y receptors may also be involved (14) and we focused in this study on P2Y2 receptors, because our previous work with neutrophils has shown that this P2 receptor subtype is an integral part of the purinergic signaling system that controls neutrophil responses (15). Using aorta rings from P2Y2 KO and WT mice, we found that tissue contraction in response to α1-receptor stimulation was significantly lower in the absence of P2Y2 receptors (Fig. 8C). These findings indicate that P2Y2 receptors are at least in part involved in α1-receptor-induced vascular smooth muscle contraction.
DISCUSSION
Endogenous catecholamines regulate many physiological processes by stimulating G protein-coupled adrenergic receptors (49). The different adrenergic receptor subtypes preferentially associate with specific Gα-subunits that dissociate from the β- and γ-subunits of the heterotrimeric G proteins to initiate specific intracellular signaling processes. The α1-receptor family receptors couple to Gq/11, while the α2- and β-receptor subtypes couple to Gi and Gs, respectively (24). We have previously shown that stimulation of chemotaxis receptors induces the release of cellular ATP from neutrophils and that purinergic feedback mechanisms control various aspects of chemotaxis (15). Chemotaxis receptors also belong to the GPCR receptors; for example, formyl peptide receptors (FPR) and interleukin-8 receptors (e.g., CXCR1) couple to Gi, while other chemotaxis receptors such as RANTES receptors (CCR5) have been reported to couple to Gq (40, 54, 56). Here we could show that stimulation of α1-receptors causes ATP release, indicating that purinergic signaling is not limited to neutrophil chemotaxis but that ATP release is a more general mechanism associated with GPCR receptor activation.
Cellular ATP release in response to receptor stimulation, cellular stress such as hypoxia, acidosis, and mechanical stimulation has been observed with many cell types (42, 44, 61). While stress-associated ATP release serves as a danger signal that controls apoptosis and the homing of phagocytes to injured cells (22, 35, 36, 59), receptor-induced ATP release from normal, healthy cells has been identified as an important inside-out signaling mechanism that plays a central role in autocrine cell activation and intercellular communication. For example, ATP release in response to FPR stimulation of neutrophils coordinates complex aspects of chemotaxis, and ATP release from stimulated T cells is a critical part of T cell activation (15, 51, 62).
Recent studies demonstrate that connexin and pannexin hemichannels facilitate ATP release in response to cell stimulation (19, 20, 51). We found here that pannexin-1 hemichannels play a particularly important role in ATP release from HEK-293 cells in response to α1-receptor stimulation (Fig. 2C). Our findings further indicate that different ADR subtypes differ from α1-receptors with regard to the ATP concentrations they induce in the extracellular space (Fig. 2B). These differences may be due to differences in the release of ATP or its hydrolytic products through pannexin-1, due to the release of ATP or its products by distinct transport mechanisms, or due to differential extracellular processing of released ATP by ecto-nucleotidases.
Interestingly, we found that α1- and α2-receptors induce ATP release, albeit to different extents. The Gq/11-coupled α1-receptors but not the Gi/o-coupled α2-receptors seem to require P2 receptors for MAPK activation. In contrast to the α-receptors, the Gs-coupled β-receptors do not seem to require P2 receptors but rather adenosine and A2a receptors (Fig. 9). Thus, the three adrenergic receptor subfamilies appear to associate with different types of purinergic receptors to form functional receptor pairs that elicit appropriate cell responses to catecholamine stimulation. Increasing evidence supports the notion that GPCRs form homo- and heteromers that modulate receptor specificity and functional responses. Specifically, A2a receptors have been shown to form multimeric complexes with dopamine, GABA, glutamate, and cannabinoid receptors (1, 11, 12, 21, 23). Receptor oligomerization affects ligand binding properties and the types of signaling pathways that are triggered by the different GPCRs (45). The ADR/purinergic receptor systems we found in this study may act through such oligomerization or through more loosely associated receptor pairs that link to different G proteins and could explain GPCR promiscuity, where activation of a particular signaling pathway by one GPCR member can modulate intracellular downstream signaling of another GPCR (55). This phenomenon indicates promiscuous coupling of a specific GPCR with different classes of heterotrimeric G proteins (26).
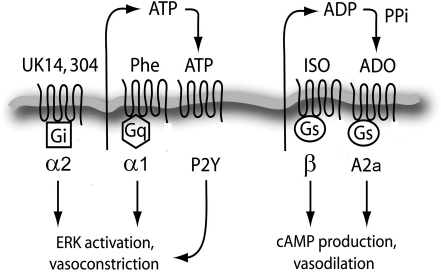
Fig. 9.
Schematic depiction of interactions between adrenergic and purinergic receptors. The stimulation of Gq/11-coupled α1-receptors with phenylephrine induces strong ATP release, MAPK activation, and vasoconstriction via P2 purinergic receptors. Stimulation of Gs-coupled β-receptors with isoproterenol induces extracellular ADP/adenosine accumulation and A2a activation, which result in cAMP signaling.
In addition to ADR stimulation by catecholamines, direct stimulation of P1 and P2 receptors—for example by ATP that is released from neurons or endothelial cells—can directly regulate vascular tone and other important physiological processes (7). ATP released from sympathetic nerves in the adventitia has been proposed to stimulate P2X1, P2X2, P2X4, P2Y2, and P2Y6 of smooth muscle cells, resulting in vasoconstriction (46). Adenosine, on the other hand, causes vasodilatation through A2 receptors in vascular smooth muscle cells (7). Endothelial cells release ATP and UTP, for example, in response to mechanical stress associated with blood flow changes, which results in the stimulation of P2Y2 receptors among other P2 receptors and nitric oxide production that induces vasodilatation (9). Taken together with our findings, these observations suggest that adrenergic and purinergic receptors interact through complex paracrine and autocrine signaling networks that control smooth muscle contraction and vascular tone. In support of this notion, several reports have shown that postjunctional α-receptor stimulation can lead to the release of adenine nucleosides from the endothelium and from smooth muscle cells (25, 53, 60). Sedaa et al. (53) have shown that transmural mechanical stimulation induces ATP release from blood vessels, which suggests that ATP release in response to phenylephrine stimulation has likely two separate components, a direct release in response to α-receptor activation as suggested by our in vitro studies with HEK-293 cells and indirect ATP release in response to mechanical stress during vasoconstriction. Administrating exogenous ATP affects α1/2-receptor-induced vasoconstriction in the human forearm (30), which further supports our notion that purinergic and adrenergic receptors are intricately linked.
Previous studies using P2Y2 KO mice have identified important roles for P2Y2 in neutrophil chemotaxis (15), K+ secretion in the colon (38), Ca2+ signaling in the lung (27), and regulation of blood pressure and renal Na+ reabsorption (48). In addition, P2Y2 receptors have been identified recently as a critical sensor that allows monocyte and macrophages to find and eliminate apoptotic cells (22). Our data with aorta tissue specimens from P2Y2 KO mice show that P2Y2 receptors also play a role in the contraction of vascular smooth muscles in response to adrenergic stimulation, which supports the results of our in vitro experiment that indicated that downstream signaling responses of adrenergic receptors depend on purinergic receptor stimulation.
In summary, we conclude that adrenergic receptors require purinergic signaling, which regulate functional cell responses through positive and negative purinergic feedback mechanisms. Adrenergic receptors are main targets of therapeutic strategies aimed at treating a wide range of diseases such as asthma, heart failure, hypertension, arrhythmia, and shock (2, 29, 41, 58). Our findings suggest that such therapeutic strategies could be improved by modulating GPCR signaling with pharmacological agents that target purinergic receptors.
GRANTS
This study was supported by National Institutes of Health Grants GM-51477, GM-60475, AI-072287, and AI-080582; by Congressionally Directed Medical Research Program (CDMRP) Grant PR043034 (to W. G. Junger); and by a Shock Society/Novo Nordisk research grant for early career investigators (to Y. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Dr. Marco Hefti for carefully reviewing the manuscript.
Present address of A. Li: Department of General Surgery, Harbor-UCLA Medical Center, Torrance, CA 90509.
REFERENCES
- 1.Agnati LF, Ferre S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev 55: 509–550, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bakris G. An in-depth analysis of vasodilation in the management of hypertension: focus on adrenergic blockade. J Cardiovasc Pharmacol 53: 379–387, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Beny JL. Characterization of purine receptors in mouse thoracic aorta. J Cardiovasc Pharmacol 44: 171–177, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci 19: 99–107, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res 26: 959–969, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Brodde OE. Physiology and pharmacology of cardiovascular catecholamine receptors: implications for treatment of chronic heart failure. Am Heart J 120: 1565–1572, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep 60: 12–20, 2008 [PubMed] [Google Scholar]
- 8.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194: 335–342, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G, Cocks T, Kasakov L, Wong HK. Direct evidence for ATP release from non-adrenergic, non-cholinergic (“purinergic”) nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol 49: 145–149, 1978 [DOI] [PubMed] [Google Scholar]
- 11.Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Muller C, Woods AS, Hope BT, Ciruela F, Casado V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferre S. Striatal adenosine A2A and cannabinoid CB1-receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 32: 2249–2259, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Casado V, Cortes A, Mallol J, Perez-Capote K, Ferre S, Lluis C, Franco R, Canela EI. GPCR homomers and heteromers: a better choice as targets for drug development than GPCR monomers? Pharmacol Ther 124: 248–257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan SL, Fiscus RR. Vasorelaxant response to isoprenaline, nitric oxide donor, calcitonin gene-related peptide and vasoactive intestinal peptide in aortic rings of adult C57BL/6J mice. Eur J Pharmacol 431: 229–236, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem 270: 26152–26158, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Civantos Calzada B, Aleixandre de Artinano A. Alpha-adrenoceptor subtypes. Pharmacol Res 44: 195–208, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Conklin BR, Chabre O, Wong YH, Federman AD, Bourne HR. Recombinant Gq alpha. Mutational activation and coupling to receptors and phospholipase C. J Biol Chem 267: 31–34, 1992 [PubMed] [Google Scholar]
- 18.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J 375: 517–529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays 31: 953–974, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life 58: 409–419, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Duran-Prado M, Malagon MM, Gracia-Navarro F, Castano JP. Dimerization of G protein-coupled receptors: new avenues for somatostatin receptor signalling, control and functioning. Mol Cell Endocrinol 286: 63–68, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrada C, Ferre S, Casado V, Cortes A, Justinova Z, Barnes C, Canela EI, Goldberg SR, Leurs R, Lluis C, Franco R. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology 55: 190–197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flordellis C, Paris H, Karabinis A, Lymperopoulos A. Pharmacogenomics of adrenoceptors. Pharmacogenomics 5: 803–817, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto M, Shinozuka K, Bjur RA, Westfall DP, Hattori K, Masumura S. The effects of age on the release of adenine nucleosides and nucleotides from rat caudal artery. J Physiol 489: 841–848, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther 99: 25–44, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Homolya L, Watt WC, Lazarowski ER, Koller BH, Boucher RC. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y(2) receptor (−/−) mice. J Biol Chem 274: 26454–26460, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orleans-Juste P, Marceau F, Thorin E, Sevigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res 85: 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellum JA, Pinsky MR. Use of vasopressor agents in critically ill patients. Curr Opin Crit Care 8: 236–241, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J 336: 513–523, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279: 36855–36864, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Y, Yang Y, Cui Y, Yazawa H, Gong W, Qiu C, Wang JM. Receptors for chemotactic formyl peptides as pharmacological targets. Int Immunopharmacol 2: 1–13, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Lei B, Zhang Y, Han C. Sustained norepinephrine stimulation induces different regulation of expression in three alpha1-adrenoceptor subtypes. Life Sci 69: 301–308, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J Gastroenterol Hepatol 22, Suppl 1: S31–S37, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal 4: 769–781, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115: 455–465, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol 564: 269–279, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyagi Y, Kobayashi S, Nishimura J, Fukui M, Kanaide H. Dual regulation of cerebrovascular tone by UTP: P2U receptor-mediated contraction and endothelium-dependent relaxation. Br J Pharmacol 118: 847–856, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller A, Strange PG. CCL3, acting via the chemokine receptor CCR5, leads to independent activation of Janus kinase 2 (JAK2) and Gi proteins. FEBS Lett 570: 126–132, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Papiris SA, Manali ED, Kolilekas L, Triantafillidou C, Tsangaris I. Acute severe asthma: new approaches to assessment and treatment. Drugs 69: 2363–2391, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol 16: 237–270, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Pirotton S, Communi D, Motte S, Janssens R, Boeynaems JM. Endothelial P2-purinoceptors: subtypes and signal transduction. J Auton Pharmacol 16: 353–356, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prezeau L, Rives ML, Comps-Agrar L, Maurel D, Kniazeff J, Pin JP. Functional crosstalk between GPCRs: with or without oligomerization. Curr Opin Pharmacol 10: 6–13, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Ralevic V. Purines as neurotransmitters and neuromodulators in blood vessels. Curr Vasc Pharmacol 7: 3–14, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 48.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 459: 356–363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubino A, Burnstock G. Evidence for a P2-purinoceptor mediating vasoconstriction by UTP, ATP and related nucleotides in the isolated pulmonary vascular bed of the rat. Br J Pharmacol 118: 1415–1420, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1: ra6, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 15: 813–827, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Sedaa KO, Bjur RA, Shinozuka K, Westfall DP. Nerve and drug-induced release of adenine nucleosides and nucleotides from rabbit aorta. J Pharmacol Exp Ther 252: 1060–1067, 1990 [PubMed] [Google Scholar]
- 54.Seifert R, Wenzel-Seifert K. The human formyl peptide receptor as model system for constitutively active G-protein-coupled receptors. Life Sci 73: 2263–2280, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci 19: 87–93, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol 86: 529–543, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Theroux TL, Esbenshade TA, Peavy RD, Minneman KP. Coupling efficiencies of human alpha 1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. Mol Pharmacol 50: 1376–1387, 1996 [PubMed] [Google Scholar]
- 58.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54: 1747–1762, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med 8: 207–220, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Westfall DP, Sedaa K, Bjur RA. Release of endogenous ATP from rat caudal artery. Blood Vessels 24: 125–127, 1987 [DOI] [PubMed] [Google Scholar]
- 61.Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl− transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol 586: 2779–2798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 23: 1685–1693, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]