Abstract
The proton-coupled folate transporter (PCFT-SLC46A1) is required for intestinal folate absorption and is mutated in the autosomal recessive disorder, hereditary folate malabsorption (HFM). This report characterizes properties and requirements of the R376 residue in PCFT function, including a R376Q mutant associated with HFM. Gln, Cys, and Ala substitutions resulted in markedly impaired transport of 5-formyltetrahydrofolate (5-FTHF) and 5-methyltetrahydrofolate (5-MTHF) due to an increase in Km and decrease in Vmax in HeLa R1–11 transfectants lacking endogenous folate transport function. In contrast, although the influx Km for pemetrexed was increased, transport was fully preserved at saturating concentrations and enhanced for the like-charged R376K- and R376H-PCFT. Pemetrexed and 5-FTHF influx mediated by R376Q-PCFT was markedly decreased at pH 5.5 compared with wild-type PCFT. However, while pemetrexed transport was substantially preserved at low pH (4.5–5.0), 5-FTHF transport remained very low. Electrophysiological studies in Xenopus oocytes demonstrated that 1) the R376Q mutant, like wild-type PCFT, transports protons in the absence of folate substrate, and in this respect has channel-like properties; and 2) the influx Km mediated by R376Q-PCFT is increased for 5-MTHF, 5-FTHF, and pemetrexed. The data suggest that mutation of the R376 residue to Gln impairs proton binding which, in turn, modulates the folate-binding pocket and depresses the rate of conformational alteration of the carrier, a change that appears to be, in part, substrate dependent.
Keywords: reduced folate carrier, folate receptors, heme carrier protein-1, folate deficiency, folate transport, intestinal folate absorption, choroid plexus
this laboratory recently cloned the proton-coupled folate transporter (PCFT) and established its critical role in intestinal folate absorption with the identification of loss-of-function mutations in this gene in six families with hereditary folate malabsorption (HFM) (18, 32). Since then, another four cases of HFM with mutations in the PCFT gene have been reported (1, 2, 12, 16, 17). HFM is characterized by both impaired intestinal folate absorption and impaired folate transport into the central nervous system (7, 14). The latter is due to a transport defect at the blood:choroid plexus:cerebrospinal fluid (CSF) barrier (31, 33).
Transport mediated by PCFT is electrogenic (18, 19, 22) and is accompanied by proton cotransport and cellular acidification (23). There is a characteristic pH profile in which influx is optimal at a pH of 5.5 and decreases to a low level at physiological pH. Under the latter condition, when a pH gradient is absent, transport is sustained at least in part by the voltage gradient across the cell membrane (18, 19, 22). This decline in transport as the pH is increased is due to both a rise in the influx Km and a fall in influx Vmax that is substrate dependent and substantial for methotrexate (MTX) and folic acid. This change is much more modest for pemetrexed, a new-generation antifolate recently approved for the treatment of lung cancer and mesothelioma (5, 34). The identification of PCFT residues that play key roles in folate and proton binding, and proton coupling, is emerging based on site-directed mutagenesis (23, 24). Mutations that are the basis for HFM also have the potential for providing valuable insights into residues that are critical to function. Recent studies have established a PCFT secondary structure consisting of 12 transmembrane domains, with both NH2 and COOH termini located within the cytoplasm, along with a disulfide bond linking cysteine residues in the first and fourth extracellular loops (19, 25, 35).
Recently, we studied a patient with HFM whose disorder was due to substitution of a Gln for an Arg at position 376 within the predicted 10th transmembrane domain of PCFT. This mutation allowed sufficient residual activity to permit a detailed characterization of the functional defect. This was the same PCFT residue that was mutated in a patient with HFM reported earlier from this laboratory (32). However, in that case, the substituted amino acid was Trp and the functional loss was too profound to allow characterization. This paper addresses the role of this residue, and the impact of the R376Q mutation along with other mutations at this site, on PCFT transport properties reflected in radio-tracer folate fluxes in HeLa cells and electrophysiological measurements in Xenopus oocytes.
MATERIALS AND METHODS
Chemicals.
[3H]pemetrexed, [3′,5′,7,9-3H], folic acid, [3′,5′,7,9-3H], (6S)5-methyltetrahydrofolate (5-MTHF), [3′,5′,7,9-3H](6S)5-formyltetrahydrofolate (5-FTHF), and [3′,5′,7-3H] methotrexate (MTX) were obtained from Moravek Biochemicals (Brea, CA). Nonlabeled MTX and folic acid were purchased from Sigma-Aldrich (St. Louis, MO). Radiolabeled substrates were purified and/or the purity confirmed by liquid chromatography (29). Unlabeled pemetrexed was obtained from Eli Lilly and purified by liquid chromatography. Unlabeled 5-MTHF and 5-FTHF were obtained from Schircks Laboratories (Jona, Switzerland).
Cell lines and culture conditions.
The HeLa R1–11 cell line was utilized as the transfection recipient for analysis of the functional properties of wild-type PCFT and the various mutant PCFT constructs. This cell line lacks genomic reduced folate carrier (RFC) and does not constitutively express PCFT, due to methylation of the promoter (6, 30, 34). These cells were maintained in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Identification of the PCFT mutation in a patient with HFM.
This study was approved by the Albert Einstein College of Medicine's Clinical Committee of Investigation (CCI no. 2006-279). Informed consent was obtained from the patient and family members in accordance with the Declaration of Helsinki. Peripheral blood samples were collected, and genomic DNA was extracted using the Gentra Systems purification kit (Minneapolis, MN). The primers and conditions for polymerase chain reaction were reported previously (18). PCFT genomic fragments that contain exons and flanking introns were purified on 1% agarose gels and sequenced on an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA) at the Albert Einstein Cancer Center Genomics Shared Resource.
Generation of expression vectors for PCFT mutants by site-directed mutagenesis.
An expression vector that expresses COOH-terminal-hemagglutinin (HA)-tagged wild-type PCFT was reported previously (25). DNA constructs containing various substitutions at the R376 residue (R376Q, R376W, R376C, R376E, R376A, R376H, or R376K) were generated with the QuickChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). In each instance, the entire PCFT coding region was sequenced to verify the presence of the desired mutation and absence of polymerase-generated errors.
Transient transfection.
HeLa R1–11 cells were seeded in 20 ml Low Background glass scintillation vials (Research Products International, Prospect, IL). When cells reached ∼60% confluence, transient transfection of wild-type PCFT, mock (vector alone), or one of the PCFT mutant constructs (0.8 μg DNA/vial) was performed using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, 3 μl/vial) according to the manufacturer's protocol.
Transport assay.
Forty-eight hours following transient transfection, cells were washed twice with HBS buffer (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM glucose, pH 7.4). Cells were then incubated in a 37°C water bath in this buffer for 20 min following which the buffer was aspirated and transport was initiated by addition of 0.5 ml prewarmed buffer, adjusted to the desired pH, which contained the tritiated folate or antifolate. HBS buffer was utilized for pH ≥ 7, whereas MBS buffer (20 mM MES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM glucose) was used for pH < 7. Uptake was halted after 1 min by addition of 5 ml ice-cold HBS buffer, and vials were kept on ice. Adherent cells in the vials were washed three times with 5 ml ice-cold HBS buffer and digested by the addition of 0.5 ml of 0.2 M NaOH and incubation at 65°C for 45 min. Radioactivity in 0.4 ml of this solution was measured on a liquid scintillation spectrometer, and the protein level in 10–20 μl was determined by the BCA protein assay (Pierce, Rockford, IL). Influx is expressed as pmol tritiated substrate·mg protein−1·min−1.
Electrophysiological analysis.
Xenopus oocytes were utilized to assess currents associated with folate transport. Wild-type or R376Q-PCFT cRNA (50 nl of 0.5 μg/μl, i.e., 25 ng) or water (50 nl) was injected into stage V/VI oocytes, and electrophysiological measurements were made 3–5 days later. These procedures and the electrical configuration were the same as described previously (4, 23). As with the divalent metal transporter, DMT1, oocytes were voltage clamped to −90 mV to maximize folate-induced currents (8, 13). Oocyte solutions were pH adjusted using Tris (pH 7.5) or MES (pH 5.5). During these experiments, oocytes were continuously perfused with solutions (with and without folates as indicated) at 5 ml/min. For studies of current as a function of substrate concentration (kinetics), oocytes were exposed to pH 5.5 solution followed by a pause to allow the current to stabilize. The oocyte was then exposed to the test folate substrate at pH 5.5 for 2–3 min. Following this, the oocytes were again perfused with pH 5.5 buffer alone for 2–3 min before exposure to the next concentration of substrate. The current measurement obtained with the substrate was subtracted from the baseline current measured at pH 5.5 without substrate. Kinetic parameters (Km) and maximum current (Imax) were calculated on the basis of the Michaelis-Menten equation.
Assessment of PCFT expression levels in the crude membrane fraction and on the cell surface.
Wild-type PCFT and all mutants were HA-tagged at the C-terminus. PCFT expression in the crude membrane fraction was assessed by Western blot using an anti-HA antibody (see below). PCFT biotinylation and the pull-down assay were described previously (24). Briefly, HeLa R1–11 cells were transiently transfected with equal amounts of DNA, with empty vector (mock), wild-type PCFT, or its substituted mutant constructs in six-well plates. Two days later, cells were incubated with EZ-Link Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockford, IL, 1 mg/ml) in PBS (pH 8.0) at room temperature for 30 min. The cells, on ice, were treated with 0.7 ml hypotonic buffer [0.5 mM Na2HPO4 and 0.1 mM EDTA (pH 7.0)] containing protease inhibitor (Roche, Indianapolis, IN) and were then scraped from the plates. The crude membrane fraction was pelleted by centrifugation at 4°C and resuspended in 0.4 ml ice-cold lysis buffer [50 mM Tris base, 150 mM NaCl, 1% NP-40, and 0.5% sodium deoxycholate (pH 7.4)] containing protease inhibitors. After centrifugation, 25 μl of the supernatant was stored for Western blot analysis of the crude membrane fraction, and the remaining supernatant was mixed on a rotator with 50 μl of streptavidin-agarose beads in suspension (Thermo Scientific) overnight at 4°C. The streptavidin-agarose beads were washed four times, each for 20 min, with 0.5 ml lysis buffer before the proteins were eluted from the beads by heating for 5 min at 95°C in 2× SDS-PAGE loading buffer containing dithiothreitol (DTT).
For Western blot analysis, samples were resolved on 12.5% SDS-PAGE. Proteins eluted off the beads (see previous step) were loaded directly on gels while the crude membranes were mixed (1:1) with DTT-containing 2× SDS-PAGE sample loading buffer at room temperature before loading on gels. Proteins were transferred to Amersham Hybond membranes (GE Healthcare, Piscataway, NJ) which were first blocked with 10% dry milk in TBST (20 mM Tris, 135 mM NaCl, and 1% Tween 20, pH 7.6) overnight and then probed for 2 h with a rabbit anti-HA antibody (no. H6908, Sigma) at a dilution of 1:5,000 in TBST containing 0.1% dry milk. The membranes were probed again with anti-rabbit IgG-horseradish peroxidase conjugate (Cell Signaling Technology) in TBST at a 1:5,000 dilution and agitated on a shaker. The blots were developed with Amersham ECL Plus reagent (GE Healthcare) and exposed to autoradiography film (Denville Scientific, Metuchen, NJ). For actin loading control, the blot was first probed with rabbit β-actin antibody (Cell Signaling Technology), then incubated in stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, and 62 mM Tris-Cl, pH 6.7) and reprobed with the anti-HA antibody.
Statistical analyses.
Influx kinetic parameters (Km and Vmax), determined with tritiated substrates, were obtained from a nonlinear regression of influx as a function of extracellular folate/antifolate concentration according to the Michaelis-Menten equation. Values indicated are means ± SE from at least three independent experiments. Statistical comparisons were performed by a one-tailed unpaired Student's t-test, one-way ANOVA, Tukey's pairwise comparisons, or two-way ANOVA and the Bonferroni test. The significant difference level was set at P ≤ 0.05. All statistical analyses utilized GraphPad PRISM (version 3.0 for Windows) and Minitab software (version 11.2 for Windows).
RESULTS
The impact of amino acid substitutions at the Arg376 residue on PCFT expression and accessibility at the cell surface.
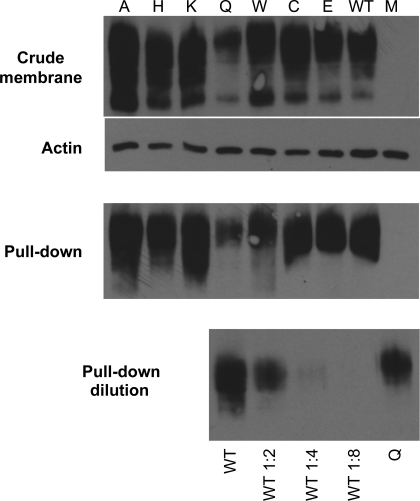
As described in the Supplemental Material (available online at the Journal website), a subject with HFM was identified with a homozygous mutation (position C.1127 G>A, based on NM_080669) located on exon 3 of the pcft gene resulting in a Gln for Arg substitution at the 376th amino acid residue located within the 10th transmembrane domain. To assess the impact of the R376Q mutation and the role of this residue, PCFT constructs were made that encompassed this and a variety of other substitutions with amino acids of different charge, polarity, and size. These included the R376W mutant reported earlier in another subject with HFM (32). First, the stability of these proteins and their trafficking to the cell surface was assessed. Wild-type and mutated PCFT constructs containing an HA epitope at the COOH terminus were transfected in HeLa R1–11 cells that lack genomic RFC and PCFT expression. Figure 1 illustrates one of five independent analyses assessing expression of the various mutant proteins in crude membrane preparations and on the plasma membrane. It can be seen that all mutants except R376Q were expressed at levels comparable to PCFT in crude membrane preparations (Fig. 1, top, upper bands). Expression of the R376Q mutant was lower than that of the wild-type PCFT and other mutants. This pattern was also seen in the Western blot analysis performed after biotinylation of PCFT followed by streptavidin pull-down (Fig. 1, middle). To more accurately compare expression of the wild-type and the R376Q mutant on the plasma membrane, the wild-type PCFT pull-down sample was diluted 1:2, 1:4, and 1:8 before Western blot analysis (Fig. 1, bottom). It can be seen that even at the 1:2 dilution (or 50%), the wild-type PCFT band was of lesser intensity than that of the mutant. Hence, the decrease in expression of the R3767Q PCFT mutant at the plasma membrane is small.
Fig. 1.
Proton-coupled folate transporter (PCFT) protein expression and trafficking to the cell membrane. Top: Western blot of crude membrane preparations of wild-type (WT) PCFT and a variety of mutants with substitutions at the R376 residue, along with actin-loading controls. Middle: Western blot analysis performed after biotinylation of PCFT accessible at the cell surface followed by streptavidin pull-down. The data in both top and middle panels are representative of five Western blots. There was some variability in R376Q PCFT levels in the crude membrane preparations and biotinylation at the cell surface among the experiments. In several cases, expression was comparable to that of wild-type PCFT. Bottom: Western blot following dilution of the wild-type PCFT pull-down sample to more accurately quantify, by comparison, levels of the mutant R376Q PCFT. Ten microliters of the biotinylated wild-type PCFT protein fraction (shown in middle panel) was added to 10, 30, and 70 μl (1:2, 1:4, and 1:8 dilution, respectively) of 2× SDS-loading buffer, and an equal volume of the samples was analyzed by Western blot analysis.
Functional analysis of PCFT R376 mutants; impact of transport substrate.
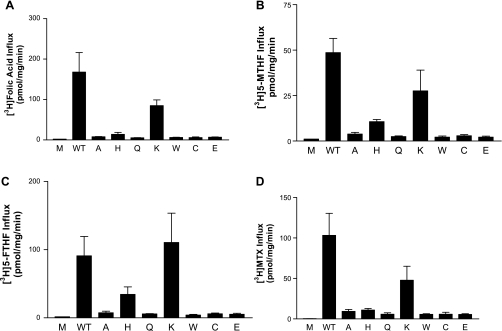
Figure 2 illustrates the functional capacities of the various PCFT mutants using four different tritiated transport substrates at a concentration of 0.5 μM: folic acid (Fig. 2A), 5-MTHF (Fig. 2B), (6S)5-FTHF (Fig. 2C), and MTX (Fig. 2D). It can be seen that under these conditions, little or no function was detected for both mutants (R376W, R376Q) identified in the two patients with HFM. This was also the case for the R376A, R376C, and R376E PCFT mutants. On the other hand, there was substantial preservation of function for the R376K mutant, and to a lesser and variable extent, the R376H mutant. Hence, preservation of charge preserved substantial function.
Fig. 2.
Influx of tritiated folate compounds mediated by PCFT mutants with substitutions at the R376 residue. HeLa cells were transfected with a variety of constructs, and influx was assessed over 1 min at 37°C, pH 5.5, and a substrate concentration of 0.5 μM. A: [3H]folic acid. B: [3H]5-methyltetrahydrofolate ([3H]5-MTHF). C: [3H]5-formyltetrahydrofolate ([3H]5-FTHF). D: [3H]methotrexate (MTX). M and WT, mock- and wild-type-transfected cells, respectively. The data are means ± SE from 3 independent experiments.
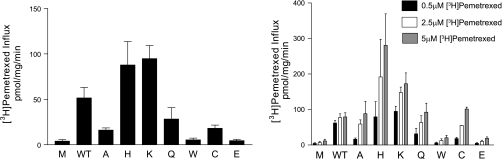
A different pattern emerged when pemetrexed was the transport substrate. Pemetrexed is a new-generation antifolate that has the highest affinity for PCFT among all the folates and antifolates that have been studied (5, 34). As indicated in Fig. 3, left, when extracellular substrate was 0.5 μM, transport activity in cells transfected with the R376H or R376K mutants was greater than that of wild-type PCFT. Furthermore, residual transport activity was present for several of the other mutants (R376A, R376Q, R376C). This was much more apparent when the pemetrexed concentration was increased from 0.5 to 2.5 and 5.0 μM (Fig. 3, right). There was only a small further increase in influx mediated by the wild-type PCFT. On the other hand, transport mediated by the R376A, R376Q, and R376C PCFT mutants was comparable to, and transport mediated by R376H and R376K exceeded that of, wild-type PCFT at a pemetrexed concentration of 5 μM. The only two mutants for which transport function could not be demonstrated at all were R376W, associated with HFM, and the oppositely charged, R376E. Hence, while this carrier prefers a positive charge at the 376 residue, the transporter can operate quite satisfactorily with a nonpolar or polar residue at this site. The increased activities detected at higher substrate concentrations were consistent with a higher Km for these PCFT mutants relative to wild-type PCFT. The data also indicated the important role the transport substrate can play in determining the activity, or activity potential, of a mutated carrier.
Fig. 3.
[3H]pemetrexed influx mediated by PCFT mutants in HeLa R1–11 cells. Left: influx of 0.5 μM pemetrexed. Right: influx compared at pemetrexed concentrations of 0.5, 2.5, and 5 μM. Conditions were the same as in Fig. 2. The data are means ± SE from 3 independent experiments.
Influx kinetics as assessed in HeLa R1–11 cell transfectants.
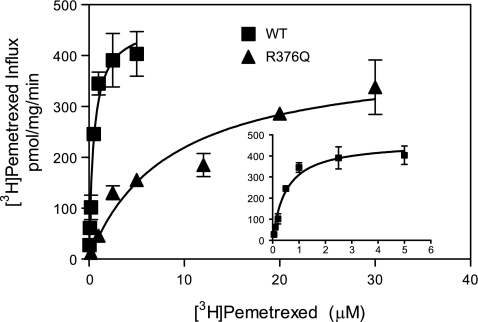
Figure 4 illustrates the influx kinetics for [3H]pemetrexed in HeLa R1–11 cells transiently transfected with wild-type or the R376Q PCFT constructs. It can be seen that there was a substantial increase in the pemetrexed influx Km (P < 0.01) for the R376Q mutant (4.8 μM) compared with wild type-PCFT (0.34 μM). However, there was no significant difference in the influx Vmax (P > 0.79). Because of the technical difficulties in obtaining accurate influx kinetic measurements when carrier affinities are very low, Ki values for 5-FTHF and 5-MTHF were determined on the basis of the inhibition of [3H]pemetrexed influx (Fig. 5). The Ki for 5-FTHF increased from 0.56 to 22.2 μM, while the Ki for 5-MTHF increased from 1.24 to 21.0 μM for wild-type versus the R376Q-PCFT mutant, respectively, on the basis of the measured pemetrexed influx Km indicated above. These kinetic constants are summarized in Table 1. There was no significant difference in the magnitude of the increase in the influx Km for pemetrexed and the increase in influx Ki for the reduced folates for the R376Q-PCFT mutant compared with wild-type PCFT.
Fig. 4.
Influx kinetics mediated by wild-type PCFT and the R376Q mutant as assessed with [3H]pemetrexed in HeLa R1–11 cell transfectants. Inset: [3H]pemetrexed influx kinetics measured over a low concentration range (0.05–6.0 μM) in cells transfected with wild-type PCFT. Influx was assessed over 1 min at 37°C and pH 5.5. The data are representative of 3 independent experiments.
Fig. 5.
Determination of 5-FTHF and 5-MTHF influx Ki values. Influx of [3H]pemetrexed was assessed at concentrations of 0.4 μM or 2.0 μM in HeLa R1–11 cells transfected with either wild-type or R376Q PCFT, respectively. Concentrations of 5-FTHF and 5-MTHF were chosen that produced 40–60% inhibition of [3H]pemetrexed influx. Ki values were determined on the basis of the measured pemetrexed influx Km and the known concentrations of substrate and inhibitors according to the Michaelis-Menten equation. The data are means ± SE from 3 independent experiments.
Table 1.
Comparison of influx kinetic parameters for the wild-type PCFT and the R376Q mutant as assessed with [3H] pemetrexed in Hela R1-11 cells
| Influx Km or Ki, μM |
Influx Vmax, pmol·mg protein−1·min−1 |
|||
|---|---|---|---|---|
| Substrate/Inhibitor | Wild-Type | R376Q | Wild-Type | R376Q |
| Pemetrexed* | 0.34 ± 0.15 | 4.8 ± 1.8 | 203 ± 133 | 174 ± 115 |
| 5-FTHF† | 0.56 ± 0.07 | 22.2 ± 2.6 | ||
| 5-MTHF† | 1.24 ± 0.13 | 21.0 ± 0.6 | ||
Values are means ± SE.
PCFT, proton-coupled folate transporter; 5-FTHF, 5-formyltetrahydrofolate; 5-MTHF, 5-methyltetrahyrofolate.
Influx Km;
Influx Ki based on inhibition of [3H]pemetrexed influx.
Electrophysiological properties.
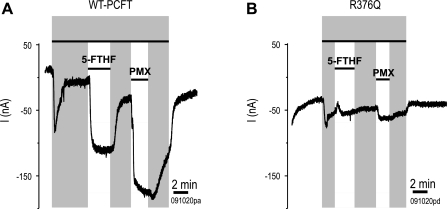
PCFT-mediated transport is electrogenic reflecting the net positive charge of the cotransported folate and proton(s). Figure 6A illustrates currents generated in oocytes injected with wild-type PCFT and clamped at −90 mV. When the pH of the perfusing buffer was decreased from 7.5 to 5.5, current was detected. Hence, protons are transported via the carrier in the presence of a proton gradient in the absence of folate substrate. This channel-like phenomenon is referred to as “slippage” and was observed previously for an H247A PCFT mutant (23). Current was markedly increased when a saturating concentration (50 μM) of either 5-FTHF or pemetrexed was added. When the oocytes were again perfused with folate-free buffer at this pH, some current was detected, again reflecting slippage, returning toward baseline when the medium pH was returned to 7.5. Likewise for the R376-PCFT mutant, slippage was detected upon exposure to pH 5.5 buffer; however, the magnitude of current was markedly decreased upon exposure to 5-FTHF or pemetrexed in comparison to wild-type PCFT (Fig. 6B).
Fig. 6.
Pemetrexed and 5-FTHF induced currents and proton-slippage. Wild-type PCFT (A) and R376Q-PCFT (B) were expressed in Xenopus oocytes clamped to −90 mV, and the elicited currents were recorded. The solution pH was initially 7.5, then switched to pH 5.5 (gray shading) to assess for folate substrate-independent currents. In this low-pH solution, oocytes were then exposed to 50 μM 5-FTHF or 50 μM pemetrexed (PMX), white bars, for 2–3 min following which the sequence was reversed. The data are representative of 4–5 experiments from at least 3 separate donor Xenopus.
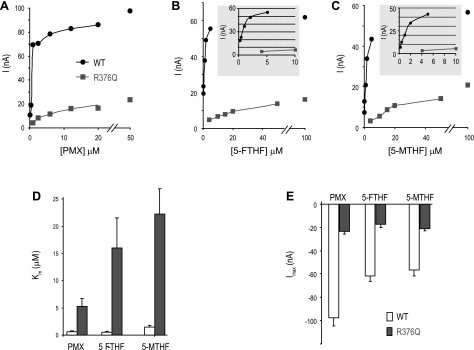
Influx kinetics for pemetrexed (Fig. 7A), 5-FTHF (Fig. 7B), and 5-MTHF (Fig. 7C) in oocytes clamped at −90 mV were also assessed (Fig. 7 and Table 2). It can be seen that the influx Km values of the R376Q-PCFT mutant for pemetrexed, 5-FTHF, and 5-MTHF were all increased compared with wild type-PCFT. There was no significant difference (P > 0.19) in the magnitude of the increases in Km among the three PCFT substrates (Fig. 7D). Figure 7E indicates that there were substantial decreases in the influx Imax for the three substrates in comparison to wild-type PCFT. The magnitude of the change for pemetrexed was greater than for the two reduced folates (P < 0.01). This decrease in Imax for pemetrexed mediated by R376Q-PCFT was surprising since a decrease in Vmax was not observed in HeLa cells that express this construct.
Fig. 7.
Transport kinetics assessed electrophysiologically in Xenopus oocytes. A–C: current (I) as a function of concentration for pemetrexed (PMX; A), 5-FTHF (B), and 5-MTHF (C) measured with a holding potential of 90 mV. The final points are the calculated maximum current (Imax; Vmax). Wild-type PCFT data are indicated by round symbols; data for the R376Q-PCFT mutant are indicated by the square symbols. The ordinate scales are expanded in the insets to B and C to clarify the initial rates for the wild-type PCFT. D: comparison of the influx Km values. E: influx Imax values among the PCFT folate substrates. The data are representative of 4–5 experiments from at least 3 separate donor Xenopus. Wild-type PCFT is indicated by open bars; the R376Q PCFT mutant is indicated by the filled bars.
Table 2.
Comparison of influx kinetic parameters for the wild-type PCFT and the R376Q mutant as assessed electrophysiologically in Xenopus oocytes
| Uptake Km, μM |
Uptake Imax, nAmp |
|||
|---|---|---|---|---|
| Substrate | Wild-Type | R376Q | Wild-Type | R376Q |
| Pemetrexed | 0.72 ± 0.33 | 5.1 ± 1.5 | 113 ± 17 | 30.7 ± 6.4 |
| 5-FTHF | 0.60 ± 0.1 | 23 ± 10 | 62.3 ± 6.2 | 19.6 ± 3.5 |
| 5-MTHF | 1.6 ± 0.25 | 28.3 ± 5.2 | 57.3 ± 4.5 | 20.7 ± 1.8 |
Values are means ± SE.
Imax, maximum current.
The impact of pH on influx mediated by the R376Q mutant.
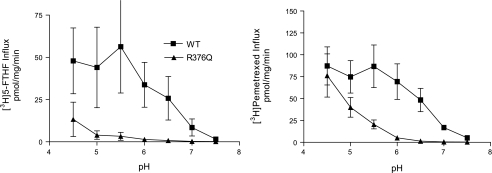
Figure 8, right, illustrates the pH profile for pemetrexed obtained from an analysis of influx as a function of pH. Transport activity mediated by wild-type PCFT at neutral pH was detected along with a rapid rise in influx to a maximum at pH 5.5. There was no further influx increase when the pH was reduced to 5.0 and 4.5. Pemetrexed influx in cells transfected with R376Q-PCFT was negligible at pH 6.0, increased as the pH was decreased, and was comparable to that of wild-type PCFT at pH 4.5. The pH profile for 5-FTHF influx mediated by wild-type PCFT was similar to that observed for pemetrexed (Fig. 8, left). On the other hand, 5-FTHF influx mediated by the R376Q-PCFT mutant was quite different, showing only a small increase at pH 5.0, and while 5-FTHF influx mediated by R376Q-PCFT increased further at pH 4.5, it remained only ∼25% that of wild-type PCFT. Hence, a reduction in pH could rescue the mutated carrier function when pemetrexed was the transport cosubstrate but not when 5-FTHF was the cosubstrate.
Fig. 8.
The pH dependence of transport mediated by wild-type and R376Q PCFT. Left: pH profile for [3H]5-FTHF. Right: pH profile for [3H]pemetrexed. The concentration of both substrates was 0.5 μM. Uptake was assessed over 1 min at 37°C. The data are means ± SE from 3 independent experiments.
DISCUSSION
The current study represents the first analysis of the functional properties of a specific PCFT mutant identified in a subject with HFM. The R376W-PCFT mutation associated with HFM reported earlier lacked function (32); this was more rigorously confirmed in the current report. The data indicate that a positive charge is favored at this residue; indeed, substitution of R376 with the like-charged His or Lys resulted in enhanced pemetrexed transport. On the other hand, despite the salutary impact of the positive charge, it is not essential for function. Substitution with an Ala (nonpolar) or Gln (polar) residue preserved pemetrexed transport activity.
The kinetic basis for alterations in PCFT function appear to be related to the properties of the substituted residues as well as the transport substrate. Hence, while the Ala, Gln, and Cys substitutions resulted in marked loss of function when MTX, folic acid, and the reduced folates were substrates, activity could be detected with pemetrexed as substrate. In fact, transport equal to that mediated by wild-type PCFT could be achieved at saturating concentrations of pemetrexed in HeLa R1–11 cells that express these PCFT constructs (Fig. 3). This was further quantified for the R376Q PCFT mutant by a kinetic analysis of [3H]pemetrexed influx in which Km was substantially increased without a significant change in Vmax. In contrast, irrespective of the pemetrexed concentration, transport could not be detected for the Trp or the oppositely charge Glu mutant PCFTs.
The pH dependence of the R376Q PCFT mutant was markedly changed. As observed previously, there was appreciable transport of 5-FTHF mediated by wild-type PCFT at pH 6.0, comparable to the pH present at the microenvironment of the proximal jejunum where folates are absorbed (15). This was also the case for pemetrexed and, for both substrates, transport mediated by wild-type PCFT was maximum at pH 5.5. However, for the R376Q mutant, 5-FTHF transport at low pH remained trivial while pemetrexed transport was restored to the level of wild-type PCFT. One possible explanation for these observations is that the R376 residue plays an important role in proton binding to the carrier which is the initial event in formation of the tertiary complex. This, in turn, would allosterically alter conformation of the binding pocket resulting in enhanced association with its folate substrates. Mutation of this residue would diminish proton binding, resulting in a decrease in the affinity for folates at the binding pocket. According to this paradigm, when the proton concentration is increased, proton binding to the carrier increases which, in turn, restores a conformation favorable for pemetrexed, but to a lesser extent for 5-FTHF, binding. It is also possible that the enhanced transport of pemetrexed at low pH might be related to protonation of pyrrolopyrimidine moiety that favors binding and translocation. A marked alteration in pH sensitivity was also observed for the H281A PCFT mutant, reported previously, due to a marked increase in influx Km without a change in Vmax; decreasing the pH substantially reversed the Km increase (23). A change in the pH profile was also detected with the E185A PCFT mutation, a residue required for proton coupling (24). Hence, it would appear that a number of residues influence PCFT proton binding which, in turn, modulates folate substrate binding.
Electrophysiological studies confirmed a large, comparable, decrease in the affinity of the R376Q mutant carrier for pemetrexed and the reduced folates. In addition, the influx Imax for 5-MTHF and 5-FTHF mediated by the R376Q mutant was markedly decreased in comparison to the wild-type PCFT. In contrast to the studies in HeLa cells, the Imax for pemetrexed was also markedly decreased when transport was mediated by R376Q-PCFT. The basis for this difference for pemetrexed is not clear but could be related to the voltage clamp (−90 mV) in the oocyte experiments. The validity and physiological relevance of the measurements made in HeLa cells is supported by several observations. First, transport of [3H]pemetrexed mediated by the R376Q, R376A, and R376C mutants was equal to that mediated by wild-type PCFT at a saturating concentration of 5 μM, consistent with the preservation of the pemetrexed influx Vmax. Second, the changes reported for the kinetic parameters for pemetrexed as the pH is increased are much more modest than that observed for other folates and antifolates, further evidence for the unique properties of this antifolate (18, 34). The electrophysiological studies also reproduced in oocytes that express R376Q PCFT the proton slippage associated with expression of the wild-type transporter, albeit of lesser magnitude, consistent with retention of channel-like properties of the mutant carrier.
From the perspective of intestinal folate absorption, dietary folate levels are so low that the wild-type carrier usually operates in a very unsaturated state. The degree of unsaturation is even greater when the carrier is mutated to a form with a very low affinity for its substrates, as in this case. While high oral folate loads can overcome a decrease in PCFT affinity, low maximum transport rates associated with the R376Q-PCFT mutant limits folate delivery at any load as observed for the physiological folate, 5-MTHF, and for 5-FTHF that was used to treat this patient. The relatively low pemetrexed influx Km mediated by the mutated R376Q, compared with the values for the reduced folates, beyond the preservation of influx Vmax, should also contribute to the preservation of activity of this mutant for this substrate by allowing saturation at much lower substrate concentrations than occurs with the reduced folates.
In HFM, when transport mediated by a mutated PCFT is absent, other routes of intestinal folate transport may be utilized, such as RFC. This allows patients with HFM to achieve normal folate blood levels and delivery to systemic tissues that is generally mediated by RFC that has a neutral pH optimum. However, this will not be sufficient to deliver adequate amounts of folates required for the developing infant brain because of the defect in transport into the central nervous system. About half the patients with HFM have neurological complications, often seizures as in this case (see Supplemental Material) (7, 14). Under normal conditions, the CSF folate level is two- to threefold higher than the blood level. This gradient is most likely generated at the choroid plexus where PCFT (33), folate receptor-α (FRα) (27, 28), and RFC (26) are expressed. CSF folate is virtually absent in untreated patients with HFM. Hence, PCFT is essential for this process after birth and is assumed to be required throughout life. However, it is now clear that FRα is also required for the maintenance of CSF folate but with a different temporal role. Hence, when the receptor is mutated in humans, as recently reported, the syndrome of cerebral folate deficiency manifests several years after birth (3, 21). The observations that both PCFT and FRα are at some point required in this process, the presence of one is inadequate in the absence of the other, is consistent with a tandem-like relationship. For instance, PCFT may be required for export of folates from acidified endosomes within the choroid plexus (33). It is of interest that this patient was reported to have seizures following administration of folic acid; this is a phenomenon observed previously in HFM as well as other seizure disorders (11, 20). While this may be coincidental, it is important to point out that folic acid is not found in nature, and binds essentially irreversibly to folate receptors (10). This may, in fact, impair transport of physiological folates across the choroid plexus. Accordingly, patients with HFM should not be treated with folic acid (14).
GRANTS
This work was supported in part by a grant from the National Cancer Institute, National Institutes of Health, CA082621 (to I. D. Goldman), EY017732 (to M. F. Romero), the American Heart Association, SDG2640146 (M. H. Chang), and the St. Baldrick's Foundation (to K. Mahadeo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
Present address of E. S. Unal: Department of Surgery, University of Istanbul, Istanbul, Turkey.
REFERENCES
- 1.Atabay B, Turker M, Ozer EA, Mahadeo K, Diop-Bove NK, Goldman ID. Mutation of the proton-coupled folate transporter gene (PCFT-SLC46A1) in Turkish siblings with hereditary folate malabsorption. Pediatr Hematol Oncol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borzutzky A, Crompton B, Bergmann AK, Giliani S, Baxi S, Martin M, Neufeld EJ, Notarangelo LD. Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter. Clin Immunol 133: 287–294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cario H, Bode H, Debatin KM, Opladen T, Schwarz K. Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology 73: 2127–2129, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Chang MH, DiPiero J, Sonnichsen FD, Romero MF. Entry to “formula tunnel” revealed by SLC4A4 human mutation and structural model. J Biol Chem 283: 18402–18410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 6: 404–417, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther 8: 2424–2431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine (Baltimore) 81: 51–68, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Jebnoun S, Kacem S, Mokrani CH, Chabchoub A, Khrouf N, Zittoun J. A family study of congenital malabsorption of folate. J Inherit Metab Dis 24: 749–750, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev 56: 1085–1097, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lanzkowsky P, Erlandson ME, Bezan AI. Isolated defect of folic acid absorption associated with mental retardation and cerebral calcification. Blood 34: 452–465, 1969 [PubMed] [Google Scholar]
- 12.Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, Glaser F, Jansen G, Drori S, Assaraf YG. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood 112: 2055–2061, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie B, Ujwal ML, Chang MH, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes. Pflügers Arch 451: 544–558, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Mahadeo KM, Min SH, Diop-Bove N, Kronn D, Goldman ID. Hereditary folate malabsorption. In: GeneReviews [Internet], edited by Pagon RA, Bird TC, Dolan CR, Stephens K. Seattle, WA: University of Washington, 2010 [Google Scholar]
- 15.McEwan GT, Lucas ML, Denvir M, Raj M, McColl KE, Russell RI, Mathan VI. A combined TDDA-PVC pH and reference electrode for use in the upper small intestine. J Med Eng Technol 14: 16–20, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Meyer E, Kurian MA, Pasha S, Trembath RC, Cole T, Maher ER. A novel PCFT gene mutation (p.Cys66LeufsX99) causing hereditary folate malabsorption. Mol Genet Metab 99: 325–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min SH, OH SY, Karp GI, Poncz M, Zhao R, Goldman ID. The clinical course and genetic defect in the PCFT in a 27-year-old woman with hereditary folate malabsorption. J Pediatr 153: 435–437, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol 293: C1669–C1678, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Reynolds EH. Anticonvulsants, folic acid, and epilepsy. Lancet 1: 1376–1378, 1973 [DOI] [PubMed] [Google Scholar]
- 21.Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P, Wevers R, Grosso S, Gartner J. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet 85: 354–363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umapathy NS, Gnana-Prakasam JP, Martin PM, Mysona B, Dun Y, Smith SB, Ganapathy V, Prasad PD. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci 48: 5299–5305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem 284: 17846–17857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol 297: C66–C74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta 1178: 1407–1414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta 1513: 49–54, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res 52: 3396–3401, 1992 [PubMed] [Google Scholar]
- 28.Weitman SD, Weinberg AG, Coney LR, Zurawski VR, Jennings DS, Kamen BA. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res 52: 6708–6711, 1992 [PubMed] [Google Scholar]
- 29.Zhao R, Babani S, Gao F, Liu L, Goldman ID. The mechanism of transport of the multitargeted antifolate, MTA-LY231514, and its cross resistance pattern in cell with impaired transport of methotrexate. Clin Cancer Res 6: 3687–3695, 2000 [PubMed] [Google Scholar]
- 30.Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumor cells: contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res 10: 718–727, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 11:e4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 110: 1147–1152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT - SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem 284: 4267–4274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities compared with the reduced folate carrier. Mol Pharmacol 74: 854–862, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry 49: 2925–2931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.