Abstract
The role of S100A1 in skeletal muscle is just beginning to be elucidated. We have previously shown that skeletal muscle fibers from S100A1 knockout (KO) mice exhibit decreased action potential (AP)-evoked Ca2+ transients, and that S100A1 binds competitively with calmodulin to a canonical S100 binding sequence within the calmodulin-binding domain of the skeletal muscle ryanodine receptor. Using voltage clamped fibers, we found that Ca2+ release was suppressed at all test membrane potentials in S100A1−/− fibers. Here we examine the role of S100A1 during physiological AP-induced muscle activity, using an integrative approach spanning AP propagation to muscle force production. With the voltage-sensitive indicator di-8-aminonaphthylethenylpyridinium, we first demonstrate that the AP waveform is not altered in flexor digitorum brevis muscle fibers isolated from S100A1 KO mice. We then use a model for myoplasmic Ca2+ binding and transport processes to calculate sarcoplasmic reticulum Ca2+ release flux initiated by APs and demonstrate decreased release flux and greater inactivation of flux in KO fibers. Using in vivo stimulation of tibialis anterior muscles in anesthetized mice, we show that the maximal isometric force response to twitch and tetanic stimulation is decreased in S100A1−/− muscles. KO muscles also fatigue more rapidly upon repetitive stimulation than those of wild-type counterparts. We additionally show that fiber diameter, type, and expression of key excitation-contraction coupling proteins are unchanged in S100A1 KO muscle. We conclude that the absence of S100A1 suppresses physiological AP-induced Ca2+ release flux, resulting in impaired contractile activation and force production in skeletal muscle.
Keywords: S100, excitation-contraction coupling, calcium signaling, muscle
in skeletal and cardiac muscle, action potential (AP) depolarization triggers Ca2+ release from the sarcoplasmic reticulum (SR), which in turn enables actomyosin interaction and contractile force generation in a process termed excitation-contraction (EC) coupling. The small Ca2+-binding protein S100A1 positively modulates EC coupling in both skeletal and cardiac muscle (34, 39, 40, 48). In skeletal muscle, S100A1 localizes to sarcolemmal invaginations known as transverse tubules (t-tubules) and the adjacent junctional faces of the SR (jSR) (7, 14, 40). This region is termed the triad junction and houses the Ca2+ release machinery of the muscle fiber (11, 16). S100A1 binds to the Ca2+ release channel of the jSR ryanodine receptor type-1 (RyR1) and enhances RyR1-mediated Ca2+ release (17, 39, 40, 48). We have recently demonstrated that S100A1 competes with calmodulin (CaM) for the previously well-characterized CaM binding domain (CaMBD) on RyR1 (40, 55), a site documented to sensitize RyR1 to activation (43, 49). Furthermore, single flexor digitorum brevis (FDB) muscle fibers isolated from transgenic mice lacking S100A1 [S100A1 knockout (KO)] demonstrate depressed global Ca2+ transients upon stimulation by a single AP (41) or voltage clamp depolarization (39).
The delayed rising phase and reduced amplitude of the Ca2+ transient seen in S100A1 KO fibers (40) could be due to alterations in RyR1 activation kinetics, which have been demonstrated in single channel experiments (48). Alternatively, changes in fiber excitation preceding RyR1 activation could account for these differences. Specifically, a latent or depressed AP would also explain the depressed Ca2+ transients in S100A1 KO fibers. Here, therefore, we first optically recorded the propagated AP with a voltage-sensitive dye (di-8-aminonaphthylethenylpyridinium, di-8-ANEPPS) in intact wild type (WT) and KO FDB fibers to evaluate any possible contribution of an altered AP to the depressed Ca2+ transients of KO fibers. Next, we utilized a myoplasmic Ca2+ removal model (32) to characterize the effects of S100A1 on SR Ca2+ release, as calculated from global Ca2+ transients evoked by a single AP or train of APs. Finally, we evaluated whether or not the endogenous effect of S100A1 on single fiber Ca2+ release translates to downstream effects on muscle force production in vivo. To this end, we recorded tension generated by the tibialis anterior (TA) muscle of anesthetized S100A1 KO and WT animals in response to various stimulation paradigms. We find that, as predicted by deficiencies in AP-evoked Ca2+ release, TA muscles from S100A1 KO animals produce less force and fatigue more rapidly compared with their WT counterparts. Importantly, fiber size, fiber type, and expression of key EC coupling proteins were not changed in S100A1−/− muscle. These findings support an in vivo role for the modulation of skeletal muscle function by S100A1.
MATERIALS AND METHODS
Animal care.
All animals were housed in a pathogen-free facility at the University of Maryland, Baltimore. The animals were handled according to authorized procedures of the Institutional Animal Care and Use Committee, University of Maryland, Baltimore (Baltimore, MD). Mice were anesthetized using regulated delivery of isofluorane gas. After the these studies were completed, anesthetized mice were euthanized by cervical dislocation.
FDB fiber preparation.
Fibers were prepared using enzymatic dissociation of FDB muscles of 6- to 7-wk-old C57 × 129 WT and S100A1 KO mice and were cultured as previously described (30). S100A1−/− and WT control animals were obtained from Dr. Danna Zimmer, Texas A&M University. The generation and genotyping of these animals have been previously reported (40).
AP recordings.
Twenty-four hour cultured FDB fibers were loaded with 5 μM di-8-ANEPPS in the incubator for 30 min, followed by three washes in Ringer solution. Fiber cultures were mounted on a Zeiss LSM 5 LIVE high-speed confocal system and stimulated with dual platinum field electrodes. Fiber fluorescence was excited with a 488-nm diode laser, and fluorescence emission above 505 nm was sampled following repeated line scans through the interior of fibers (100 μs/line). The line scan was oriented perpendicular to the long axis of the muscle fiber at a depth of ∼15–20 μm into the belly of the fiber. Significant measures were taken to ensure that resulting signals were propagated APs and not artifacts imposed by stimulation (see results). Signals were converted to −ΔF/F0 values, and four trials were averaged to increase the signal-to-noise ratio. APs were triggered using the same 1-ms electrical stimulus as in Ca2+ release assays. All single fiber and whole muscle recordings were performed at room temperature, 22°C.
Fluo-4AM Ca2+ transient recordings.
AP-evoked Ca2+ transients were monitored using the fluorescent indicator Fluo-4AM as previously described (40).
Calculation of SR Ca2+ release flux.
To determine AP-induced Ca2+ release flux, we applied a protocol consisting of a single stimulus and five consecutive tetani (200 ms; 100 Hz) separated by intervals of 200 ms (Fig. 4) providing six long relaxation intervals that allowed characterization of Ca2+ removal in the absence of release (32, 33, 47). To calculate free [Ca2+] using digitally filtered (45) fluo-4 fluorescence ratio traces [R = (F − F0)/F0], dissociation constant KD,fluo and rate constant koff,fluo of the indicator were assumed to be 1 μM (18) and 90 s−1 (46), respectively. Rmax (fluorescence ratio of the Ca2+-saturated indicator) was determined from ionomycin-permeabilized fibers and Rmin calculated as −Rmax [Ca2+]0/KD,fluo where [Ca2+]0 is the concentration of resting free Ca2+. Because [Ca2+]0, measured in indo-1-loaded fibers, was not significantly different in WT and KO fibers (40), we used the mean value 55.7 nM for the analysis. A Ca2+ binding and transport model was used to simulate the signal decay in the relaxation intervals. The simulations started ca. 20 ms after the pulse end. Binding to troponin and parvalbumin (fast Ca2+-specific T-sites and slow Ca2+-Mg2+ P sites) and to ATP was calculated as described by Baylor and Hollingworth (9). ATP-bound Ca2+ and Ca2+ transport rate were assumed to be proportional to free [Ca2+] [scaling factor F =3.6 (Ref. 9) and rate constant kuptake, respectively]. After an adjustment for our experimental temperature (20°C), the rate constants used were as follows: T-sites: kon,T,Ca = 115 μM−1s−1, koff,T,Ca = 1 50 s−1; P-sites: kon,P,Ca = 54.0 μM−1s−1, koff,P,Ca = 0.65 s−1, kon,P,Mg = 0.043 μM−1s−1, koff,P,Mg = 3.9 s−1, kuptake = 1,000 s−1. The total concentrations [T]tot and [P]tot were 0.240 and 1.5 mM, respectively. kon,P,Mg and kuptake were altered by iteration to minimize the least squares deviation between calculated and measured fluorescence ratio in the relaxation phases. Free [Ca2+] and the Ca2+ occupancies of all model compartments [Fluo-4, T-sites, P-sites, ATP (F-sites) and transport] were summed, and the release flux was calculated as the time derivative of the sum.
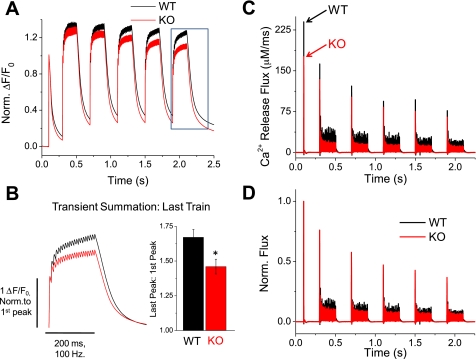
Fig. 4.
Ca2+ release flux is suppressed in S100A1−/− fibers. A: average Fluo-4 fluorescence transients in WT and KO fibers elicited by a high-frequency repetitive stimulation protocol (5 repeats of 200 ms, 100-Hz trains following a single AP), designed to partially saturate cytosolic Ca2+ buffers for calculation of Ca2+ release flux. Traces normalized to initial transient peak to highlight differences in transient summation during the train. B: left, boxed traces from Fig. 4A, time expanded and normalized to amplitude of the initial peak to examine transient summation of last train. Right, quantification of transient facilitation between WT and KO fibers (P < 0.01, WT: n = 7, KO: n = 7). C: average Ca2+ release flux of WT and KO fibers calculated from Fluo-4 fluorescence transients, as described in materials and methods and results. S100A1−/− fibers show a decreased Ca2+ release flux for an initial stimulus that becomes further suppressed compared with WT fibers during the train. D: traces from C normalized to peak amplitude of release flux from the initial single stimulus during the train. S100A1 KO fibers show increased fractional inactivation during the train compared with WT counterparts.
Extracellular application of 4-chloro-m-cresol and measurements of single fiber Ca2+ release.
4-Chloro-m-cresol (4-CmC; Aldrich, Milwaukee, WI) was diluted in DMSO and locally applied to FDB fibers using a focal perfusion system. For experiments evaluating 4-CmC effects on Ca2+ release (see supplementary Fig. 1 at the AJP-Cell Physiology website), FDB fibers were loaded with Fluo-4FF, a lower Ca2+ affinity indicator dye (Kd = 9.7 μM) than with Fluo-4 to prevent saturation of the indicator dye with larger Ca2+ release events. In experiments with 1 mM 4-CmC to elicit maximal release, fibers were pretreated with 50 μM N-benzyl-p-toluene sulfonamide (BTS) to minimize fiber contraction and movement artifacts, as well as 5 μM cyclopiazonic acid, to inhibit SR Ca2+-ATPase (SERCA)1 function and prevent SR Ca2+ reuptake.
Contractile function.
As a measure of whole muscle force production, we recorded tension generated by the TA muscle in vivo, as previously described (31). Briefly, the distal TA tendon of anesthetized animals was surgically released and attached to a load cell. A 26-gauge needle was then inserted through the proximal tibia to stabilize the leg. The load cell was adjusted via a micromanipulator to stretch the muscle to a set resting length (11 mm), which was measured with digital calipers. TA contraction was then triggered via subcutaneous stimulation of the peroneal nerve, and the resulting force generated was sampled at 1 kHz and analyzed with Polyview acquisition software. We utilized several stimulation protocols, which are detailed in results, to evaluate force generation in S100A1 KO and WT animals. After contractile function experiments, animals were euthanized and the TAs were isolated and weighed. As muscle length was fixed in all experiments, and muscle density was assumed to be a constant 1.06 mg/mm3, physiological cross-sectional area (PCSA = mass/density × length) of the TA was solely a function of muscle mass. Force (F) was therefore normalized to TA mass to calculate specific force P0 = F/PCSA.
Muscle sectioning, measurements of fiber diameter, and fiber typing.
Immediately after the functional studies, TA muscles were dissected out and snap frozen in liquid nitrogen. Sections of frozen muscles (10 μm thick) were cut on a cryotome and collected onto glass slides (Superfrost Plus; VWR, West Chester, PA) and labeled for immunofluorescence. Sections were labeled with rabbit antibodies against dystrophin (1:10, Dys-2, Lab Vision, Fremont, CA) and with mouse antibodies to the slow-twitch isoform of myosin heavy chain (MHC) (1:500, M4276, Sigma, St. Louis, MO). Tissue sections were washed three times with 1% BSA-PBS for 10 min before incubation with species-specific secondary antibodies coupled to Alexa dye 568 (dystrophin) or 488 (MHC) (1:100, Invitrogen, Carlsbad, CA). All fibers in a minimum of five fields from multiple sections of each TA muscle from 3 WT and 4 KO animals were blindly evaluated for minimum diameter of each fiber in the field and for the proportion of MHC-slow positive fibers. Minimum fiber diameter was measured using ImageJ software (NIH, Bethesda, MD; http://rsb.info.nih.gov/ij/).
Western blot and lysate preparation.
Muscle homogenates were analyzed for RyR1, SERCA1, Parvalbumin, and GAPDH protein content as described previously (38). Briefly, TA muscles from WT and S100A1 KO mice were dissected, homogenized, and supplemented with protease inhibitor cocktail (Roche Diagnostics). The homogenates were subjected to centrifugation at 10,000 RPM for 10 min at 4°C. The supernatant was extracted and stored. Protein concentrations were measured using a Nanodrop-1000 spectrophotometer (Thermo Scientific). Samples for electrophoresis were not boiled but were solubilized at 70°C for 10 min. Protein sample (10, 20, or 30 μg) was fractionated by either 4–12% bis-Tris SDS-PAGE or 3–8% Tris-acetate SDS-PAGE gels and transferred to PVDF membrane, at 20 V overnight at 4°C using electrophoretic transfer under wet conditions. Blots were then processed and probed for RyR1 (1:1,000, mouse monoclonal), SERCA1 (1 in 1,000, mouse monoclonal), Parvalbumin (1 in 1,000, goat polyclonal), and GAPDH (1 in 1,000, mouse monoclonal) diluted in 5% (wt/vol) milk in phosphate-buffered saline with 0.1% (vol/vol) Tween. Anti-RyR was purchased from Affinity BioReagents, Golden, CO; anti-SERCA1, anti-Parvalbumin, and anti-GAPDH were purchased from Sigma (St. Louis, MO). Blots were incubated with the appropriate horseradish peroxidase (HRP)-labeled secondary antibodies (KPL, Gaithersburg, MD). Films were developed following the exposure to chemiluminescent substrates to detect HRP on immunoblots. Either SuperSignal West Pico or the more sensitive SuperSignal West Femto (Pierce, Rockford, IL) ImageJ was used to quantify the intensity of bands following background subtraction.
Statistical analysis.
All statistical analyses were performed using OriginPro8.0. All data are presented as means ± SE unless otherwise noted. Normality of data sets was ensured for each statistical calculation. All significance tests used Student's t-test, with significance set at P < 0.05.
RESULTS
The AP is unchanged between WT and S100A1 KO muscle fibers.
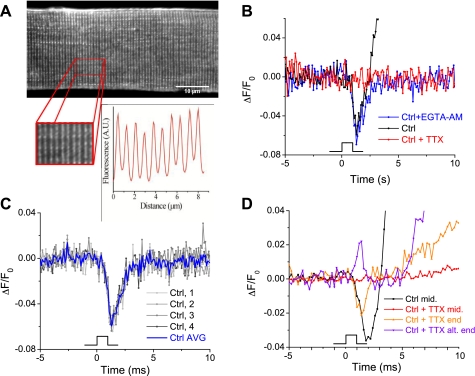
In skeletal muscle, the depolarizing AP triggers the movement of voltage sensors in the dihydropyridine receptor (DHPR, an L-type Ca2+ channel), which in turn activates Ca2+ release from the mechanically linked RyR1 (41, 44). Therefore, either alterations in voltage sensor activation or AP waveform could explain the depressed Ca2+ transients in fibers lacking S100A1. We have recently demonstrated that the initial RyR1-activating component of voltage sensor charge movement (termed “Qβ”) is not changed in S100A1-deficient fibers, ruling out voltage sensor activation as a putative culprit for suppressed release (38, 39). To evaluate any role of the propagated AP, here we measured AP properties using the potentiometric, lipophilic dye di-8-ANEPPS. This dye effectively stains the sarcolemmal and t-tubule membrane systems of skeletal muscle (Fig. 1A), and its fluorescence emission decreases upon depolarization. It has been used previously to measure the AP in cultured FDB fibers and demonstrates rapid kinetics that essentially mimic those of electrical recordings of the AP (13). No differences in di-8-ANEPPS staining pattern or intensity were detected between WT and KO fibers (data not shown). Before a comparison between fibers from WT and S100A1 KO mice, we first took measures to ensure that all di-8-ANEPPS signals represented propagated APs and not artifacts resulting from electrical stimulation or from fiber contraction. In Fig. 1B, a control fiber (black trace) is stimulated at time 0 and a rapid decrease in fluorescence, attributed to the depolarizing phase of the AP, is evident. During and after repolarization, the fluorescence markedly overshoots the baseline value due to a large and reproducible movement artifact contaminating these relatively small fluorescence signals. To ensure this signal resulted from an AP, we added 0.001 mM TTX to the bath and imaged the same fiber again after 60 s. As can be seen in the red trace of Fig. 1B, TTX completely eliminated any fluorescence change. In an effort to eliminate fiber movement, we coloaded another group of fibers with 50 μM EGTA-AM simultaneously with the potentiometric dye to buffer released Ca2+ and prevent contraction during electrical stimulation. We found that this eliminated any movement artifact without affecting the AP time to peak or peak fluorescence (blue trace, quantified below). With the use of this coloading method, the AP signals were highly reproducible from pulse to pulse (Fig. 1C). Typically four pulses were averaged to increase signal to noise, as demonstrated in Fig. 1C. N-benzyl-p-toluene sulfonamide, a contraction inhibitor commonly used in skeletal muscle, was not utilized to minimize movement artifacts, because it has been previously demonstrated to alter AP properties in mammalian muscle fibers (54).
Fig. 1.
Optical recordings of the propagated action potential in flexor digitorum brevis (FDB) fibers. A: FDB fiber stained with di-8-aminonaphthylethenylpyridinium (di-8-ANEPPS) demonstrates lipophilic staining of the sarcolemma and T-system. Inset shows the doublet staining pattern with matched fluorescent intensity profile characteristic of the T-tubules. B: ΔF/F0 recordings of di-8-ANEPPS-stained fibers excited with a 1-ms field stimulus to generate an action potential (AP). Under control conditions, fibers demonstrated a large movement artifact during and after repolarization (black trace). This was abrogated by loading fibers with 50 μM EGTA-AM to buffer released calcium and prevent contraction (blue trace). Treatment of fibers with 100 nM TTX eliminated any fluorescence change (red trace). C: with the use of EGTA-AM/di-8-ANEPPS coloading protocol, AP recordings were highly reproducible and 4 APs were averaged for each trial to improve signal to noise. D: electrical stimulus artifacts in FDB fibers. Electrical signals elicited in the longitudinal middle of the FDB fiber always showed a downward deflection regardless of stimulus polarity (black trace) and were completely abrogated by TTX (red trace). However, even in the presence of TTX, electrical artifacts were seen at the ends of many fibers (orange and purple trace). These local signals alternated directionality with alternating stimulus polarity and promoted local depolarization, which triggered Ca2+ release and local contraction [evidenced by the smaller movement artifacts following stimulation compared with control conditions, which elicited a homogenous fiber contraction (black trace)].
Electrical artifacts caused by local, direct fiber de- or hyperpolarization from field stimulation would be evident by a remaining di-8-ANEPPS signal in the presence of TTX. Such direct fiber polarization signals should alternate in directionality of the di-8-ANEPPS signal when alternating the polarity of field stimulation or show prolongation of the signal with increasing stimulus duration (hence not “all or none” behavior). When imaging the middle of the muscle fiber in regard to its longitudinal axis, we found no such evidence of electrical artifacts. However, when imaging either ends of the FDB fibers, we were able to identify electrical artifacts from field stimulation in TTX-treated fibers. This is depicted in Fig. 1D. The black trace represents the propagated AP recorded in the middle of the fiber evoked by stimulation at time 0. Upon treatment with TTX, the optical signal in the middle of the fiber is eliminated, as expected (red trace). However, upon imaging the ends of the fiber, brief stimulation artifacts that alternated with the polarity of stimulation were present following treatment with TTX (purple and orange traces). These electrical signals increased in duration upon prolonged stimulation (data not shown). The local depolarizations appeared to be independent of fiber orientation with regard to the field electrodes and were never found in the middle of the muscle fiber. Hence, APs were always recorded from the middle of the fiber and always stimulated using alternating polarity to check for unidirectionality of the AP signal. These APs presumably were propagated from the end of the fiber where the 1-ms pulse produced a local depolarization to trigger an AP (see discussion).
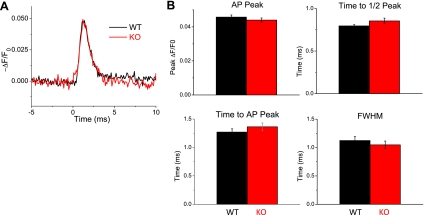
Figure 2A shows average AP traces optically recorded from 9 KO and 10 WT fibers. Visually, the propagated AP appears very similar between groups. These fibers were treated with 50 μM EGTA-AM before stimulation to prevent movement artifacts. An additional group of fibers (WT: n = 20, KO: n = 17) was tested without EGTA-AM, and AP properties were found to be virtually identical between EGTA-treated and nontreated groups. Therefore, data between groups were pooled and analyzed. Figure 2B shows pooled data of AP properties between WT and KO fibers. No significant differences were found in the AP peak (WT ΔF/F0 = 0.052 ± 0.006, KO = 0.05 ± 0.005), time to peak (WT = 1.27 ± 0.26 ms, KO = 1.36 ± 0.27 ms), time to half-peak (WT = 0.795 ± 0.06 ms, KO = 0.85 ± 0.14 ms), or full-width half-maximum (WT = 1.12 ± 0.32 ms, KO = 1.05 ± 0.27 ms). These findings suggest that the AP is unchanged in the absence of S100A1 and therefore cannot account for alterations in SR Ca2+ release.
Fig. 2.
The AP is unchanged in S100A1−/− fibers. A: average recordings of the AP in the t-system, reported as −ΔF/F0 di-8-ANEPPS signal, show similar AP waveforms in wild-type (WT) and knockout (KO) fibers. B: properties of APs in WT and S100A1−/− FDB fibers. No significant change in AP peak amplitude, time to half-peak, time to peak, or full-width half-maximum (FWHM) was found between groups (P > 0.05, WT: n = 20, KO: n = 17).
AP-initiated Ca2+ release flux is suppressed in S100A1-deficient fibers.
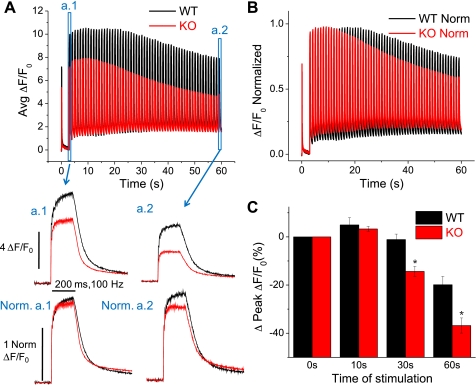
Our previous reports evaluated Ca2+ transients in S100A1−/− fibers elicited by a single AP (40) or step-like depolarizations (39). Here we sought to build on this work by evaluating Ca2+ transients elicited by prolonged, high-frequency stimulation-induced AP activity, a more physiological firing pattern of motor units in small mammals (19). Figure 3A (top) demonstrates the average fluo-4 fluorescence signals (ΔF/F0) of fibers stimulated by a single AP and then followed by 100-Hz trains of stimuli lasting 200 ms and repeated every 1 s for 60 s. The average fluorescence records in response to trains of stimuli at the beginning and end of this stimulation paradigm are presented in Fig. 3, a.1 and a.2, respectively, in a time-expanded version. The 100-Hz train produced a continuous increase in the amplitude of the Ca2+-dependent fluorescence signal throughout the train in WT fibers. By normalizing the traces in Fig. 3, a.1 and 3a.2, to the first response in their respective train, the relative differences in Ca2+ transient summation during the train can be appreciated (Norm a.1 and Norm a.2). Summation during the first train of the stimulation paradigm appears roughly similar between WT and KO fibers (Norm. a.1), whereas summation during the last train following prolonged stimulation is reduced in KO fibers (Norm. a.2) In addition to the transient summation during the individual tetani, both WT and KO fibers showed an initial increase in the peak amplitude of the tetanic Ca2+ transients that reached a maximum after about 10 s of the stimulation protocol (Fig. 3A, top). When normalized to the amplitude of the initial train-induced fluorescence signals, the percent increase in tetanic amplitude at 10 s was not significantly different between WT and KO fibers (Fig. 3C) (WT% initial ΔF/F0 = 5.0 ± 3.0%, n = 5; KO = 3.3 ± 1.1%, n = 9; P = 0.53). This initial facilitation of tetanic Ca2+ transient amplitude is consistent with previous single fiber analyses of repetitive stimulation (5) and is generally attributed to elevated inorganic phosphate initially stimulating release (4, 8). WT fibers then sustained maximal tetanic amplitude for a longer duration than KO fibers, after which both WT and KO fibers eventually showed a consistent decline in train amplitude (Fig. 3B). By evaluating the normalized traces displayed in Fig. 3B, it is clear that the amplitude of the tetanic Ca2+ transient declines more rapidly in fibers lacking S100A1. After 30 s and at the end (t = 60 s) of this high-frequency stimulation paradigm, KO fibers displayed a significantly greater percent drop in tetanic transient amplitude compared with WT fibers (Fig. 3C) (t = 30 s: WT% initial ΔF/F0 = −1.1 ± 2.3%; KO = −14.3 ± 2.1%; P < 0.01; t = 60 s: WT% initial ΔF/F0 = −19.9 ± 3.4%; KO = −36.8 ± 3.1%; P < 0.01). The larger drop in normalized Ca2+ transient amplitude suggests a greater relative decline of SR Ca2+ release during repetitive stimulation of fibers lacking S100A1.
Fig. 3.
Prolonged high-frequency stimulation exacerbates the suppression of Ca2+ transients in S100A1−/− fibers. A: average responses of WT and KO fibers to high-frequency stimulation paradigm consisting of a single pulse, followed by 200-ms, 100-Hz trains repeated every 1s for 60 s. a.1 shows a time-expanded view of the initial train, and a.2 shows an expanded view of the final train, where now Ca2+ transient amplitude is suppressed ∼50% in KO fibers compared with controls. Below a.1 and a.2 are their respective records normalized to the amplitude of the initial response in the tetanus to highlight differences in Ca2+ transient summation during the train between WT and KO fibers. B: traces in A normalized to the amplitude of the initial response highlight the relatively greater decrease of tetanic Ca2+ transient amplitude during prolonged high-frequency stimulation of S100A1 KO fibers. C: quantification of the change in peak amplitude of the tetanic Ca2+ transient seen in B. At 30 and 60 s, KO fibers demonstrated significantly greater relative decrease of tetanic Ca2+ transient amplitude compared with WT counterparts (P < 0.01; WT: n = 5, KO: n = 9).
To assess the kinetics of SR Ca2+ release, we next utilized a Ca2+ removal model (32) to calculate the time course of SR Ca2+ release flux underlying the AP-evoked Fluo-4 Ca2+ transients in WT and KO fibers. In essence, the properties of the reuptake and binding that lead to the decrease in free calcium concentration following cessation of release are empirically characterized from the decay time course of the fluorescent Ca2+ transient. The rate of SR Ca2+ release is then calculated as the derivative of free, bound, and transported calcium. To characterize removal, we utilized a prolonged, high-frequency, repetitive stimulation protocol (a single AP followed by 5 repeats of 200 ms, 100-Hz trains with 200-ms recovery between trains, Fig. 4A) to promote the saturation of cytosolic Ca2+ buffers. In accordance with all previous protocols, S100A1−/− fibers demonstrate suppressed Ca2+ transients in response to both twitch and tetanic stimulation [data not shown, pooled twitch and tetanic amplitudes from initial twitch and train in Figs. 3 and 4 stimulation paradigms: WT 1AP (twitch) ΔF/F0 = 6.95 ± 0.45, n = 12; KO = 5.19 ± 0.29, n = 16; P < 0.01; WT 20AP (tetani) ΔF/F0 = 9.66 ± 0.69; KO = 6.77 ± 0.46, P < 0.01]. Figure 4A demonstrates average WT and KO responses to this high-frequency stimulation protocol normalized to the amplitude of the initial Ca2+ transient. From these records it is evident that transient summation during the train appears roughly similar between WT and KO fibers for the first train but rapidly becomes impaired within seconds of high-frequency stimulation. Figure 4B zooms in on the last train in the series of five trains in Fig. 4A (box) and shows the average WT and KO responses normalized to the initial peak of the last train. Summation was then quantified as the ratio of the amplitude of the last peak in the train to the first peak. There was significantly reduced summation during the train of stimuli in fibers lacking S100A1 (WT summation = 1.67 ± 0.06, n = 7; KO = 1.46 ± 0.05, n = 7; P < 0.01).
By fitting the decaying phase of the transient at the end of each train and utilizing the same fixed and free parameters for uptake systems (see materials and methods), release flux was then calculated from the average fluorescence time courses of WT and KO fibers. The average SR Ca2+ release flux time courses from 7 WT and 7 KO fibers are presented in Fig. 4C. Peak initial flux from a single AP was reduced by 26% in KO fibers compared with WT counterparts. Both groups demonstrated partial inactivation of release flux that essentially reached a steady state during the trains of stimuli, as well as some cumulative suppression of release over the time course of the stimulation protocol. Flux was suppressed by ∼50% at the end of each train in KO fibers compared with controls (Fig. 4C). As evidenced by the normalized flux time courses in Fig. 4D, KO fibers demonstrated greater fractional inactivation of release flux during the trains of stimuli. However, following the 200 ms of recovery between trains, KO fibers demonstrated a similar relative recovery before the next train.
To determine whether the amount of releasable SR Ca2+ was limiting Ca2+ release in KO fibers, we utilized 4-CmC to potentiate release channel activity. 4-CmC directly activates the isolated SR Ca2+ release channel (21), and a low dose of 4-CmC has been shown to potentiate voltage-gated SR Ca2+ release without significantly increasing resting [Ca2+] (39, 51). Here therefore, we evaluated Ca2+ transients (measured here with Fluo4-FF, please see materials and methods) during maximal tetanic stimulation (400 ms, 100 Hz) of WT and KO fibers before and after 60 s local application of 150 μM 4-CmC. After 4-CmC application, both groups showed a marked potentiation of the tetanic Ca2+ transient, and the peak amplitude of the Ca2+ transient in KO fibers now approximated that of WT controls (supplementary Fig. 1A). A higher dose of 4-CmC (1 mM) has been shown to directly elicit a massive release of SR Ca2+ and has been used as an indicator of maximum SR-releasable Ca2+ (23). 4-CmC (1 mM) elicited a similar Fluo-4 FF signal in WT and KO fibers (supplementary Fig. 1B). This result suggests that the amount of releasable SR Ca2+ is not the primary factor limiting Ca2+ release in S100A1 KO fibers and is consistent with a direct role of S100A1 on RyR1 channel function.
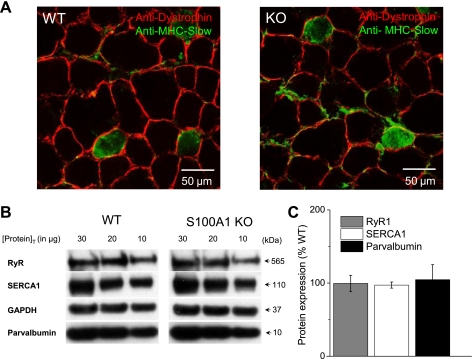
S100A1−/− and WT muscle demonstrate similar muscle morphology and expression of key proteins involved in EC coupling.
Previous studies using adenoviral delivery of S100A1 (40) and patch pipette dialysis of S100A1 protein (38) into KO fibers to rescue functional phenotypes suggests a specific effect of S100A1 on SR Ca2+ release. However, compensatory changes in muscle morphology, muscle fiber type, or protein expression could all potentially contribute to alterations in EC coupling and muscle function seen in S100A1 KO muscle. Therefore, we next sought to evaluate key properties of muscle morphology and protein expression in S100A1 KO and WT muscle. Frozen TA muscle from functional studies detailed below were sectioned and stained with anti-dystrophin and anti-MHC-slow antibodies to allow quantification of both muscle fiber diameter and the percentage of MHC-slow positive fibers (Fig. 5A). Fiber diameter was not significantly different between WT and KO muscle fibers (WT = 40.8 ± 1.0 μm, n = 99 fibers, N = 3 muscles; KO = 39.4 ± 0.8 μm, n = 109 fibers, N = 4 muscles, Table 1). Likewise, the proportion of MHC-slow positive fibers was similar between groups (WT = 10.3 ± 2.1% slow fibers, N = 3 muscles, KO = 11.1 ± 2.1% slow fibers, N = 4 muscles, Table 1). We have previously demonstrated that fiber capacitance is also unchanged in S100A1 KO muscle fibers (38). These results suggest that alterations in fiber size, capacitance, and the proportion of slow-type fibers cannot explain the functional deficiencies in S100A1 KO muscle.
Fig. 5.
Muscle morphology and expression of key excitation-contraction (EC) coupling proteins in S100A1 KO and WT muscle. A: representative tibialis anterior (TA) sections from WT (left) and KO (right) muscle stained with anti-dystrophin (red) and anti-MHC-Slow (green) antibodies. Fiber diameter and the proportion of slow-type fibers were unchanged between WT and KO muscle (Table 1). B: representative Western blots of key proteins in EC coupling. TA homogenates containing 30, 20, and 10 μg protein (to prevent the measurement of saturated signals) were probed for RyR1, SERCA1, and Parvalbumin, with GAPDH used as a loading control. C: protein expression in S100A1 KO muscle, relative to the percent expression in WT muscle. Values expressed as means ± SD (data from three replicates for each protein concentration, each from three different WT and KO TA samples). The expression of key players in EC coupling were unchanged in S100A1-deficient muscle.
Table 1.
WT and S100A1 KO Muscle morphological properties and protein expression
| Muscle Fiber Diameter, μm | MHC-Slow Fibers, % + | RyR1 Expression, %WT | SERCA Expression, %WT | Parvalbumin Expression, %WT | |
|---|---|---|---|---|---|
| WT | 40.8 ± 1.0 | 10.3 ± 2.1 | |||
| KO | 39.4 ± 0.8 | 11.1 ± 2.1 | 99.5 ± 11.1* | 97.2 ± 4.1* | 104.6 ± 20.4* |
Values are means ± SE.
Values are means ± SD. WT, wild type; KO, knockout; MHC, myosin heavy chain; RyR, ryanodine receptor type-1; SERCA, sarcoplasmic reticulum Ca2+ ATPase.
We next checked for compensatory changes in the expression of key EC coupling proteins in WT and S100A1 KO muscle. Homogenates from frozen TA muscles were probed with anti-RyR1, anti-SERCA1, and anti-parvalbumin antibodies. Protein samples of 10, 20, and 30 μg were probed to ensure that we obtained signals that were linear and not saturated to validate comparisons between groups (Fig. 5B). RyR1, SERCA1, and parvalbumin expression were all unchanged in S100A1 KO muscle (Fig. 5C, Table 1). We have previously demonstrated that DHPR expression also is not altered in S100A1 KO muscle (38). Combined, these results suggest that compensatory changes in several key proteins involved in the EC coupling pathway do not account for alterations in SR Ca2+ release.
S100A1 KO animals produce less force than WT counterparts.
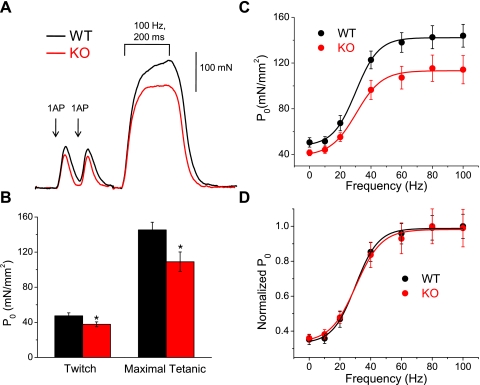
To address the physiological relevance of S100A1 in skeletal muscle function, we next determined whether the endogenous modulation of Ca2+ release by S100A1 at a single fiber level translates to whole muscle function in vivo. As a measure of function, we recorded the maximal isometric contractile force produced by the TA muscles of anesthetized S100A1 KO and WT mice. Figure 6A shows representative force recordings from a WT and KO animal. The first two peaks are the twitch force from a single AP, with the third, sustained peak the maximum tetanic force resulting from a train of stimuli at 100 Hz for 200 ms. We used 100 Hz stimulation to elicit peak tetanic force, because stimulation at a greater frequency leads to a submaximal fused tetanus. There appears to be a decrease in the amount of force generated upon twitch and tetanic stimulation in muscle lacking S100A1, results typical of the animals tested.
Fig. 6.
S100A1−/− muscle produces less contractile force in vivo. A: representative force recordings from WT and KO animals. The initial two peaks are from single stimuli, whereas the third larger force transient was elicited by a 200-ms, 100-Hz train of stimuli that generated maximal tetanic force. B: pooled data from 9 WT and 10 KO animals. Twitch force was significantly reduced ∼23% in KO animals (P < 0.05), while maximal tetanic forcewas reduced ∼25% (P < 0.01). C: specific force (P0) plotted vs. the frequency of stimulation for 8 WT and 9 KO animals. Continuous line is the best fit of a sigmoidal function (Eq. 1 in text) to each data set. D: normalized force vs. frequency curves shows that the frequency dependency of force generation is similar in WT and KO animals.
Figure 6B shows the pooled data of specific force (P0) recorded from twitch and tetanic stimulation in 9 WT and 10 KO animals. KO animals elicited decreased force in response to a single AP (WT Twitch P0 = 47.3 ± 3.5 mN/mm2, KO = 37.7 ± 2.8 mN/mm2; P < 0.05), as well as in response to a 200-ms, 100-Hz train of APs to elicit maximal tetanic force (WT Tetanic P0 = 145.3 ± 8.7 mN/mm2, KO = 109 ± 11.1 mN/mm2; P < 0.01). A summary of the biometrics and force data from these experiments is listed in Table 2. There were no differences in body weight or muscle weight of these animals.
Table 2.
Descriptive statistics for contractile force measurements
| N | Sex | Body Weight, g | TA Weight, mg | Twitch Force, mN | Tetanic Force, mN | Twitch P0, mN/mm2 | Tetanic P0, mN/mm2 | Fatigue, % | Fatigue Tau, s | |
|---|---|---|---|---|---|---|---|---|---|---|
| WT | 9 | 4M, 5F | 20.04 ± 0.96 | 41.31 ± 3.09 | 168 ± 13 | 515 ± 31 | 47.3 ± 3.5 | 145.3 ± 8.7 | 65.5 ± 6.2 | 107.1 ± 11.6 |
| KO | 10 | 8F, 2M | 19.4 ± 1.23 | 41.47 ± 2.77 | 138 ± 10* | 388 ± 40* | 37.7 ± 2.8* | 109 ± 11.1* | 75.9 ± 3.4 | 50.1 ± 13.3* |
Values are means ± SE. M, male; F, female; TA, tibialis anterior; P0, specific force.
P < 0.05.
Next, we evaluated the in vivo specific force versus frequency (P0 vs. F) relationship in these animals by eliciting TA contraction with AP trains of 10, 20, 40, 60, 80, and 100 Hz. As noted above, the peak of the tetanic transient decreased consistently with increased frequency after 100 Hz in both WT and KO animals, as monitored up to 350 Hz, and is not included in this analysis. Figure 6C shows the average P0 versus F relationship from 8 WT and 9 KO animals. There data were fit by a sigmoidal function:
| (1) |
where P0min gives the minimum specific force, P0max gives the maximum specific force, Fhalf defines the frequency where P0 = 0.5 of P0max, and 1/k is a measure of the steepness of the P0 vs. F relationship. The WT fit demonstrated parameters of P0min = 47.5 mN/mm2, P0max = 142.8 mN/mm2, Fhalf = 30.1 Hz, and k = 7.4 Hz. The KO fit demonstrated parameters of P0min = 38.0 mN/mm2, P0max = 113.0 mN/mm2, Fhalf = 30.4 Hz, and k = 8.3 Hz. While specific force was significantly decreased at 0, 40, 60, 80, and 100 Hz stimulation in animals lacking S100A1, there was no difference in the frequency dependence of the development of force, as illustrated by the normalized P0 vs. F fits in Fig. 6D and quantified by the very similar Fhalf and k values of the sigmoidal fits to WT and KO P0 vs. F relationships.
S100A1 KO muscle fatigues more rapidly than WT counterparts.
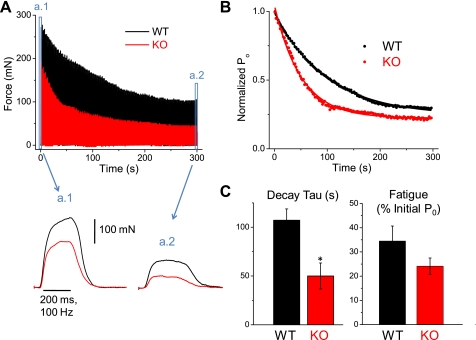
Given the more rapid decline in Ca2+ transient amplitude (Fig. 3, B and C) and greater fractional inactivation (Fig. 4B) during repetitive stimulation of isolated muscle fibers lacking S100A1, we next sought to evaluate the effects of S100A1 on the maintenance of maximal force during repetitive stimulation in vivo. The fatiguing paradigm consisted of 200-ms, 100-Hz trains to elicit maximal force, spaced every 2 s, for 300 s. Force was recorded continuously for each of the 150 tetani over the time of stimulation. Figure 7A (bottom) illustrates representative force recordings from WT and KO animals during the first tetanus at time = 0 (a.1) and during the last tetanus after 300 s of the fatiguing paradigm (a.2). Force was significantly reduced in both groups at the end of this protocol, with WT and KO animals exhibiting a ∼65 and 75% loss of force, respectively (Table 2). Figure 7A, top, illustrates the individual fatigue time course of these same two animals. Between each tetanus, force returned back to baseline. By simple visual inspection the KO appears to show a more rapid decline in maximal contractile force, particularly over the first 100 s of stimulation. Individual fatigue records were normalized to the initial force generated by each animal, and the data points representing the peak of each tetanus were fit to an exponential decay function to quantify the rate of fatigue between WT and KO animals (Fig. 7B). The decay in force over time of stimulation was described by a single exponential:
| (2) |
where P0 is the specific force, A is the amplitude, t is the time (s), τ is the decay time constant, and P0(0) is the steady-state offset. Figure 7B shows the average decay from normalized fatigue records of WT and KO animals. Each WT and KO fatigue record was fit and the decay time constants averaged as an indicator of the rate of fatigue. As demonstrated by the box plot in Fig. 7C, animals lacking S100A1 demonstrated a significantly faster rate of decline in peak force compared with WT counterparts (WT τ = 107.1 ± 11.6 ms, n = 7 animals; KO τ = 50.1 ± 13.3 ms, n = 8 animals; P = 0.007). The final level of this exponential fit represents the specific force following the fatiguing paradigm relative to initial force, which was not found to be significantly different between groups (WT = 0.345 ± 0.062, KO = 0.241 ± 0.034, P > 0.05). These results suggest that muscle lacking S100A1 fatigues more rapidly than WT counterparts.
Fig. 7.
S100A1−/− muscle fatigues more rapidly that WT muscle. A: top, decline in maximum tetanic force during fatiguing stimulation in representative WT (black) and S100A1−/− (red) TA muscles. Muscles were stimulated with 200-ms, 100-Hz trains every 2 s for 300 s. The KO muscle displays a greater initial rate of force loss. The peaks of each of the 150 force transients recorded throughout the fatiguing paradigm were fit to a single exponential decay. Bottom, tetanic force transients from WT and KO muscle before (a.1; t = 0 s) and after (a.2; t = 300 s) fatiguing paradigm. B: average decline in force from the individual decay fits of 7 WT and 8 KO animals, normalized to the specific force at the beginning of the fatiguing paradigm. The decay τ of these exponential fits was used as an indicator of the rate of fatigue. C: pooled data of the rate of force decline in WT and KO animals. KO animals demonstrated a significantly increased rate of fatigue (WT τ = 107.1 ± 11.6 ms, n = 7 animals; KO τ = 50.1 ± 13.3 ms, n = 8 animals; P = 0.007). The final level of fatigue was not found to be significantly different between groups (WT = 0.345 ± 0.062, KO = 0.241 ± 0.034, P > 0.05).
DISCUSSION
Here we demonstrate, for the first time, an in vivo role for S100A1 in the modulation of skeletal muscle contractile force and fatigue. We show that muscles lacking S100A1 exhibit decreased twitch and tetanic force production and fatigue more rapidly than WT counterparts (Figs. 6 and 7). Suppressed force was anticipated based on our finding that KO fibers exhibit decreased SR Ca2+ release flux in response to AP stimulation (Fig. 4), and more rapid fatigue was suggested by the greater decline in Ca2+ transient amplitude with prolonged stimulation of KO fibers (Figs. 3 and 4). We provide evidence that alterations in release are likely due to S100A1 modulation of RyR1 and not a consequence of upstream regulation of the propagated AP (Fig. 2) or voltage sensor of EC coupling (39). We also provide evidence that functional differences are not a result of compensatory changes in muscle morphology, fiber type, or expression of key EC coupling proteins (Fig. 5). These endogenous effects of S100A1 are consistent with previous findings that exogenous S100A1 enhances RyR1-mediated Ca2+ release on a single channel level (48) and in skinned fibers (35). Furthermore, our findings and others' suggest that these effects are independent of alterations in SERCA1 function (17, 35, 39, 40) or the releasable SR Ca2+ content (supplementary Fig. 1) (35). The accumulating data therefore strongly supports endogenous S100A1 directly modulating RyR1 Ca2+ release.
Electrical properties of WT and S100A1-deficient muscle.
In our laboratory, we recently found that S100A1 modulates the AP in sympathetic ganglion neurons; however, this seems to be dependent on S100A1 augmentation of CaV1 channel currents (20). The AP in skeletal muscle propagates along the sarcolemma and into the T-system, with the depolarizing phase mediated by the opening of voltage-dependent Na+ channels, and repolarization due in part to the inactivation of Na+ channels and the opening of voltage-dependent K+ and Cl− channels, with minimal influence of voltage-dependent Ca2+ channels (22, 24, 53). The afterdepolarization of skeletal muscle is composed of two phases, an early one considered to be caused by spreading of the spike into the T-system and a late one due to an accumulation of potassium ions in the T-system, which increases with repetitive APs (6, 24, 26). While no reports of S100A1 regulation of Na+ channels or sarcolemmal morphology have been documented to our knowledge, a recent report does show prolonged ventricular repolarization (due to suppressed K+ currents) in S100A1-deficient mice (1). If this were recapitulated in skeletal muscle, it would most likely lead to prolonged Ca2+ release in KO fibers, in disagreement with our previous findings (40). Therefore, we hypothesized AP properties would be similar in WT and KO fibers. Nevertheless, a latent or depressed AP would explain the depressed Ca2+ transients in KO fibers, and therefore as an appropriate control, we assayed the propagated AP in the T-system of S100A1−/− and WT FDB fibers. We found no differences in AP properties between groups, and therefore conclude that a depressed AP cannot explain the blunted Ca2+ release in the absence of S100A1.
Our use of membrane potential-sensitive dyes did uncover some noteworthy electrical properties of FDB fibers in culture. In the presence of TTX (used to block muscle fiber APs), stimulation artifacts were detected at the ends, but not in the middle, of fibers stained with di-8-ANEPPS (Fig. 1D). The increased sensitivity to local depolarization may be due to current entry or exit across the fiber membrane at the ends of the fiber, with longitudinal current flow in the fiber interior over most of the fiber length. Of note, these local depolarizations also elicited substantial Ca2+ transients and contraction that locally appeared roughly similar to those evoked by an AP, and therefore measures should be taken to ensure the homogenous, all-or-none nature of Ca2+ transients when using this muscle fiber preparation.
Force production in S100A1−/− animals.
The deficits in contractile force recorded here appear solely attributable to a decrease in the specific force of fast twitch skeletal muscle from S100A1−/− animals. S100A1 KO animals exhibit alterations in cardiac function (1, 34) and central nervous system behavior (2), and therefore any evaluation of skeletal muscle performance must attempt to bypass these influences. This complicates the interpretation of standard murine assays of muscular performance used in the field, such as treadmill/wheel running or grip strength assays. For this reason we directly stimulated the dorsiflexors of the anesthetized animal and recorded contractile force to evaluate in vivo effects specific to skeletal muscle.
There are several intermediate steps linking Ca2+ release with the development of tension that could be influenced by the lack of S100A1. Importantly, Most et al. (35) found that S100A1 did not alter maximal Ca2+-dependent tension development and had no effect on the pCa-tension relationship in skinned murine EDL and soleus fibers. Our findings are in agreement with this report, as the similar force-frequency relationship between WT and KO animals (Fig. 6D) argues against a significant in vivo role for the modulation of myofilament sensitivity by S100A1 in our murine model. One technical limitation of this study is the inability to record Ca2+ and force development simultaneously in S100A1 KO muscle. Future studies should aim to measure both parameters simultaneously under physiological conditions to optimally address the relationship between suppressed Ca2+ release and contractile force.
Fatigue in S100A1−/− muscle.
The increased rate of fatigue in KO muscles (Fig. 7) suggests that deficiencies in SR Ca2+ cycling may become exacerbated upon repetitive stimulation of the muscle (Figs. 3 and 4). Interestingly, these deficiencies in Ca2+ cycling do not appear attributable to the impaired ability of the SERCA1 to pump Ca2+ back into the SR. In cardiac muscle, S100A1 enhances SERCA2a function, resulting in more efficient Ca2+ cycling, specifically upon stressing the heart (25, 34). However, enhanced SERCA2a function is mediated through phospholamban (25), which is not highly expressed in fast twitch skeletal muscle. In agreement with this proposed mechanism, S100A1 was shown to have no effect on SR Ca2+ load and SERCA1 pump function in skinned fast-twitch EDL muscle and SR vesicles (35) or terminal cisternae preparations (17). Our studies potentiating Ca2+ release with 4-CmC are consistent with this finding and suggest that the releasable SR Ca2+ store is not a limiting factor in the suppression of Ca2+ release in S100A1 KO fibers (supplementary Fig. 1). Additionally, KO FDB fibers exhibit no changes in the decaying phase of electrically evoked Ca2+ transients compared with WT fibers (39, 40), an indicator of normal SERCA1 function. This, combined with our present findings (Figs. 3B and 4B), suggests impaired RyR-mediated Ca2+ release, which is exacerbated upon repetitive challenging of the Ca2+ release machinery, may be responsible for the increased rate of fatigue in S100A1-deficient muscle.
It is difficult to directly compare the decline in Ca2+ transient amplitude from isolated single fibers (Fig. 3) to the fatigue of contractile force in whole muscles from living animals (Fig. 7). First, the isolated fiber is an overly simplified model for fatigue, as it lacks the complexity of neurovascular compensation for fatigue inherent to the living animal. Second, impaired Ca2+ cycling is not the only determinant of fatigue, even in the absence of neurovascular flow. However, declining SR Ca2+ release is a major, if not predominant, contributor to the decline in force associated with repetitive activity. This is demonstrated by the fact that pharmacologically enhancing release to prefatigue levels with RyR1-agonists overcomes the majority of force decline associated with fatigue (3, 52). The decline in release has been attributed to a number of mechanisms which both directly and indirectly affect RyR1 (3). Depletion of cellular ATP/elevation of Mg2+, acidosis, AP failure, inadequate voltage-sensor activation, elevated inorganic phosphate (Pi), and the production of reactive oxygen species (ROS) may all contribute to impaired release concomitant with fatigue. Additionally, defective store-operated calcium (SOC) entry has been linked to exaggerated muscle fatigue during prolonged stimulation (36). However, acidosis and AP failure have been shown to contribute minimally to the decline in AP-evoked release in intact fibers (15, 27, 37), and no overt evidence of S100A1 modulation of SOC entry, ROS, or Pi production exists to our knowledge. Therefore, while each of these possibilities must still be addressed as potential mechanisms of fatigue modulated by S100A1, a predominant role appears unlikely.
Increased depletion of cellular ATP presents one attractive contributor to the more rapid rate of fatigue in S100A1−/− muscle, based on the findings of Boerries et al. (12), who showed that S100A1 interacts with the mitochondrial F1-ATPase to increase ATP content in cardiomyocytes (12). ATP must be bound to a cytoplasmic regulatory site on RyR1 to activate the channel normally, with a half-maximal stimulation (EC50) of ∼0.5 mM ATP (29). Therefore, decreased ATP content in KO fibers, which, when depleted by fatigue, more rapidly undershoots the EC50 of RyR1 activation, could contribute to the declining release concurrent with repetitive activity in muscle lacking S100A1. Furthermore, as most ATP is bound to Mg2+ at rest, the free Mg2+ content has been shown to rise from ∼1 to 2 mM or more when ATP is depleted (50). Mg2+ strongly inhibits RyR1 (10, 28), and the S100A1's enhancement of RyR1 activity is proposed to be sensitive to Mg2+ (35). Decreased [ATP] or increased [Mg2+] could independently or additively contribute to the accelerated decline in Ca2+ release with repetitive stimulation of KO fibers.
An alternative, provocative hypothesis to account for the impaired release with repetitive stimulation of KO fibers stems from the competition between S100A1 and CaM for endogenous regulation of RyR1. S100A1 and CaM compete for the same binding site (calmodulin binding domain, CaMBD) on RyR1 at physiological Ca2+ concentrations (40, 55). S100A1 binds and increases RyR1 Po at nanomolar Ca2+ concentrations (48). Apo-CaM also binds to the CaMBD at nanomolar Ca2+ and is a weak activator of RyR1, while at micromolar [Ca2+]i, as occurs in the stimulated muscle fiber, Ca-CaM binds more tightly to the channel and acts as a strong inhibitor of RyR1 (42, 49). Mutation of a single amino acid in the CaMBD of RyR1 (L3625D) reduces CaM binding and regulation of recombinant RyR1 (56), and this same mutation abrogates S100A1 binding (D. J. Weber, personal communication). Experiments with a genetic mouse strain expressing the RyR1-L3625D mutation suggest that both S100A1 activation of RyR1 and Ca-CaM inhibition of both the isolated and in situ Ca2+ release channel are markedly impaired (G. Meissner, M. F. Schneider, unpublished observations).
These findings suggest that endogenously, as cytosolic Ca2+ increases with repetitive stimulation, Ca-CaM binds the CaMBD of RyR1 and inhibits release, perhaps through displacement of S100A1 bound to this same site. This putative role of Ca-CaM is consistent with what is seen here in S100A1 KO fibers, where, as its endogenous competitor has been removed, Ca-CaM is free to bind and regulate RyR1. In the normalized records of Fig. 3 and 4, it is evident that following prolonged stimulation and an elevation of cytosolic Ca2+, fibers lacking S100A1 show reduced summation of the Ca2+ transient during the train of APs (Fig. 3, a.2 and Fig. 4B) and greater transient rundown (Fig. 3, B and C), consistent with an increased inhibitory role of Ca-CaM. In further support of this role, Fig. 4D shows that S100A1 KO fibers demonstrate more pronounced inactivation of Ca2+ release flux during a train of stimuli. These data support the hypothesis that in the absence of S100A1, Ca-CaM plays a larger role in the inactivation of Ca2+ release during prolonged stimulation, which may contribute to the rapid fatigue of S100A1-deficient muscle (Fig. 7). Important additional experiments must be performed to focus on the precise mechanism by which S100A1 and CaM may regulate muscular fatigue.
In summary, our results support the hypothesis that S100A1 is an important endogenous regulator of skeletal muscle performance in vivo. Our data suggest that ablation of S100A1 leads to impaired SR Ca2+ release, which further deteriorates upon repetitive challenging of the Ca2+ release machinery. Accumulating evidence suggests suppressed release is attributable to a direct effect of S100A1 on RyR1, independent of AP, voltage sensor, or SERCA1 dysfunction. Impaired Ca2+ handling is consistent with the decreased contractile force and rapid fatigue of S100A1-deficient muscle.
GRANTS
This work was supported by grants from the National Institutes of Health AR055099 (to M. F. Schneider), K01AR053235 (to R. M. Lovering), MDA 4278 (to R. M. Lovering), and Deutsche Forschungsgemeinschaft ME 713/18 (to W. Melzer), as well as TAMU start-up funds (D. B. Zimmer). B. L. Prosser was supported by the Interdisciplinary Training Program in Muscle Biology T32-AR007592.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Ackermann GE, Domenighetti AA, Deten A, Bonath I, Marenholz I, Pedrazzini T, Erne P, Heizmann CW. S100A1 deficiency results in prolonged ventricular repolarization in response to sympathetic activation. Gen Physiol Biophys 27: 127–142, 2008 [PubMed] [Google Scholar]
- 2.Ackermann GE, Marenholz I, Wolfer DP, Chan WY, Schafer B, Erne P, Heizmann CW. S100A1-deficient male mice exhibit increased exploratory activity and reduced anxiety-related responses. Biochim Biophys Acta 1763: 1307–1319, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol 104: 296–305, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol 415: 433–458, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almers W. Potassium concentration changes in the transverse tubules of vertebrate skeletal muscle. Fed Proc 39: 1527–1532, 1980 [PubMed] [Google Scholar]
- 7.Arcuri C, Giambanco I, Bianchi R, Donato R. Annexin V, annexin VI, S100A1 and S100B in developing and adult avian skeletal muscles. Neuroscience 109: 371–388, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Balog EM, Fruen BR, Kane PK, Louis CF. Mechanisms of Pi regulation of the skeletal muscle SR Ca2+ release channel. Am J Physiol Cell Physiol 278: C601–C611, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol 551: 125–138, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blazev R, Lamb GD. Low [ATP] and elevated [Mg2+] reduce depolarization-induced Ca2+ release in rat skinned skeletal muscle fibres. J Physiol 520: 203–215, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107: 2587–2600, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boerries M, Most P, Gledhill JR, Walker JE, Katus HA, Koch WJ, Aebi U, Schoenenberger CA. Ca2+-dependent interaction of S100A1 with F1-ATPase leads to an increased ATP content in cardiomyocytes. Mol Cell Biol 27: 4365–4373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiFranco M, Capote J, Vergara JL. Optical imaging and functional characterization of the transverse tubular system of mammalian muscle fibers using the potentiometric indicator di-8-ANEPPS. J Membr Biol 208: 141–153, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta 1450: 191–231, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Dutka TL, Lamb GD. Transverse tubular system depolarization reduces tetanic force in rat skeletal muscle fibers by impairing action potential repriming. Am J Physiol Cell Physiol 292: C2112–C2121, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Fahrenbach WH. Sarcoplasmic reticulum: ultrastructure of the triadic junction. Science 147: 1308–1309, 1965 [DOI] [PubMed] [Google Scholar]
- 17.Fano G, Marsili V, Angelella P, Aisa MC, Giambanco I, Donato R. S-100a0 protein stimulates Ca2+-induced Ca2+ release from isolated sarcoplasmic reticulum vesicles. FEBS Lett 255: 381–384, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J 65: 865–881, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature 314: 164–166, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Ochoa EO, Prosser BL, Wright NT, Contreras M, Weber DJ, Schneider MF. Augmentation of Cav1 channel current and action potential duration after uptake of S100A1 in sympathetic ganglion neurons. Am J Physiol Cell Physiol 297: C955–C970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrmann-Frank A, Richter M, Sarkozi S, Mohr U, Lehmann-Horn F. 4-Chloro-m-cresol, a potent and specific activator of the skeletal muscle ryanodine receptor. Biochim Biophys Acta 1289: 31–40, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Huxley AF, Taylor RE. Local activation of striated muscle fibres. J Physiol 144: 426–441, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez-Moreno R, Wang ZM, Gerring RC, Delbono O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J 94: 3178–3188, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurkat-Rott K, Fauler M, Lehmann-Horn F. Ion channels and ion transporters of the transverse tubular system of skeletal muscle. J Muscle Res Cell Motil 27: 275–290, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kiewitz R, Acklin C, Schafer BW, Maco B, Uhrik B, Wuytack F, Erne P, Heizmann CW. Ca2+ -dependent interaction of S100A1 with the sarcoplasmic reticulum Ca2+-ATPase2a and phospholamban in the human heart. Biochem Biophys Res Commun 306: 550–557, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kirsch GE, Nichols RA, Nakajima S. Delayed rectification in the transverse tubules: origin of the late after-potential in frog skeletal muscle. J Gen Physiol 70: 1–21, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation- contraction coupling in skeletal muscle fibres of the rat. J Physiol 478: 331–339, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laver DR, Baynes TM, Dulhunty AF. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J Membr Biol 156: 213–229, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Laver DR, Lenz GK, Lamb GD. Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine. J Physiol 537: 763–778, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Carroll SL, Klein MG, Schneider MF. Calcium transients and calcium homeostasis in adult mouse fast-twitch skeletal muscle fibers in culture. Am J Physiol Cell Physiol 272: C1919–C1927, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol 286: C230–C238, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melzer W, Rios E, Schneider MF. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys J 51: 849–863, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melzer W, Rios E, Schneider MF. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J Physiol 372: 261–292, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Most P, Remppis A, Pleger ST, Katus HA, Koch WJ. S100A1: a novel inotropic regulator of cardiac performance. Transition from molecular physiology to pathophysiological relevance. Am J Physiol Regul Integr Comp Physiol 293: R568–R577, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Most P, Remppis A, Weber C, Bernotat J, Ehlermann P, Pleger ST, Kirsch W, Weber M, Uttenweiler D, Smith GL, Katus HA, Fink RH. The C terminus (amino acids 75–94) and the linker region (amino acids 42–54) of the Ca2+-binding protein S100A1 differentially enhance sarcoplasmic Ca2+ release in murine skinned skeletal muscle fibers. J Biol Chem 278: 26356–26364, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol 4: 379–383, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Posterino GS, Lamb GD. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J Physiol 551: 219–237, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prosser BL, Hernandez-Ochoa EO, Zimmer DB, Schneider MF. The Qgamma component of intra-membrane charge movement is present in mammalian muscle fibres, but suppressed in the absence of S100A1. J Physiol 587: 4523–4541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prosser BL, Hernandez-Ochoa EO, Zimmer DB, Schneider MF. Simultaneous recording of intramembrane charge movement components and calcium release in wild-type and S100A1-/- muscle fibres. J Physiol 587: 4543–4559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prosser BL, Wright NT, Hernandez-Ochoa EO, Varney KM, Liu Y, Olojo RO, Zimmer DB, Weber DJ, Schneider MF. S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem 283: 5046–5057, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 325: 717–720, 1987 [DOI] [PubMed] [Google Scholar]
- 42.Rodney GG. Calmodulin in adult mammalian skeletal muscle: localization and effect on sarcoplasmic reticulum Ca2+ release. Am J Physiol Cell Physiol 294: C1288–C1297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodney GG, Schneider MF. Calmodulin modulates initiation but not termination of spontaneous Ca2+ sparks in frog skeletal muscle. Biophys J 85: 921–932, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider MF, Chandler WK. Voltage-dependent charge movement in skeletal muscle: a possible step in excitation-contraction coupling. Nature 242: 244–247, 1973 [DOI] [PubMed] [Google Scholar]
- 45.Schuhmeier RP, Dietze B, Ursu D, Lehmann-Horn F, Melzer W. Voltage-activated calcium signals in myotubes loaded with high concentrations of EGTA. Biophys J 84: 1065–1078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirokova N, Garcia J, Pizarro G, Rios E. Ca 2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J Gen Physiol 107: 1–18, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmer J, Muller T, Melzer W. Numerical methods to determine calcium release flux from calcium transients in muscle cells. Biophys J 74: 1694–1707, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treves S, Scutari E, Robert M, Groh S, Ottolia M, Prestipino G, Ronjat M, Zorzato F. Interaction of S100A1 with the Ca2+ release channel (ryanodine receptor) of skeletal muscle. Biochemistry 36: 11496–11503, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Tripathy A, Xu L, Mann G, Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca 2+ release channel (ryanodine receptor). Biophys J 69: 106–119, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westerblad H, Allen DG. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 453: 413–434, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westerblad H, Andrade FH, Islam MS. Effects of ryanodine receptor agonist 4-chloro-m-cresol on myoplasmic free Ca2+ concentration and force of contraction in mouse skeletal muscle. Cell Calcium 24: 105–115, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol 75: 382–388, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Wolters H, Wallinga W, Ypey DL, Boom HB. Ionic currents during action potentials in mammalian skeletal muscle fibers analyzed with loose patch clamp. Am J Physiol Cell Physiol 267: C1699–C1706, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Woods CE, Novo D, DiFranco M, Capote J, Vergara JL. Propagation in the transverse tubular system and voltage dependence of calcium release in normal and mdx mouse muscle fibres. J Physiol 568: 867–880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright NT, Prosser BL, Varney KM, Zimmer DB, Schneider MF, Weber DJ. S100A1 and calmodulin compete for the same binding site on ryanodine receptor. J Biol Chem 283: 26676–26683, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi N, Xin C, Meissner G. Identification of apocalmodulin and Ca2+-calmodulin regulatory domain in skeletal muscle Ca2+ release channel, ryanodine receptor. J Biol Chem 276: 22579–22585, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.