Abstract
The number of intermediate-conductance, Ca2+-activated K+ channels (KCa3.1) present at the plasma membrane is deterministic in any physiological response. However, the mechanisms by which KCa3.1 channels are removed from the plasma membrane and targeted for degradation are poorly understood. Recently, we demonstrated that KCa3.1 is rapidly internalized from the plasma membrane, having a short half-life in both human embryonic kidney cells (HEK293) and human microvascular endothelial cells (HMEC-1). In this study, we investigate the molecular mechanisms controlling the degradation of KCa3.1 heterologously expressed in HEK and HMEC-1 cells. Using immunofluorescence and electron microscopy, as well as quantitative biochemical analysis, we demonstrate that membrane KCa3.1 is targeted to the lysosomes for degradation. Furthermore, we demonstrate that either overexpressing a dominant negative Rab7 or short interfering RNA-mediated knockdown of Rab7 results in a significant inhibition of channel degradation rate. Coimmunoprecipitation confirmed a close association between Rab7 and KCa3.1. On the basis of these findings, we assessed the role of the ESCRT machinery in the degradation of heterologously expressed KCa3.1, including TSG101 [endosomal sorting complex required for transport (ESCRT)-I] and CHMP4 (ESCRT-III) as well as VPS4, a protein involved in the disassembly of the ESCRT machinery. We demonstrate that TSG101 is closely associated with KCa3.1 via coimmunoprecipitation and that a dominant negative TSG101 inhibits KCa3.1 degradation. In addition, both dominant negative CHMP4 and VPS4 significantly decrease the rate of membrane KCa3.1 degradation, compared with wild-type controls. These results are the first to demonstrate that plasma membrane-associated KCa3.1 is targeted for lysosomal degradation via a Rab7 and ESCRT-dependent pathway.
Keywords: endocytosis, Rab7, lysosomes, endosomal sorting complex required for transport
small- and intermediate-conductance, Ca2+-activated K+ channels (KCa2.3 and KCa3.1) are present in vascular endothelial cells and have been shown to play an important role in the endothelium-derived hyperpolarizing factor (EDHF) response, a powerful regulator of vascular tone (16, 22, 23, 45, 56, 64). EDHF signaling is thought to be initiated by the activation of KCa2.3 and KCa3.1, leading to hyperpolarization of the endothelium, followed by hyperpolarization of the adjacent vascular smooth muscle, resulting in relaxation (24, 25, 31). Recent studies demonstrate that the impaired function or expression of KCa2.3 and/or KCa3.1 abrogate the EDHF response, resulting in blood pressure elevation (6, 16, 35, 56, 64), suggesting that these channels may be novel targets for the development of alternate antihypertensive therapies (23, 65, 70). Numerous activators of KCa3.1 and KCa2.3 have been identified (20, 21, 57, 58, 62), which have subsequently been shown to modulate vascular tone and hence blood pressure, suggesting that this channel may be targeted for therapeutic benefit (9, 16, 35, 68, 70). An alternate approach to modulating the activity of individual channels (Po) present in the membrane is to regulate the number of channels (N), as N is similarly directly proportional to current flow and hence the physiological response of the cell. The number of channels in the membrane (N) is regulated by the balance between the anterograde and retrograde trafficking pathways of the channels. Considerable progress has been made in understanding the mechanisms involved in the assembly/trafficking of KCa2.3 and KCa3.1 toward the plasma membrane (18, 40, 41, 51, 63); however, the mechanisms of endocytosis and downstream sorting of these channels are virtually unknown. Deciphering the molecular machinery that governs the (post)endocytic trafficking of KCa2.3 and KCa3.1 is the first step in advancing our understanding of how the number of channels is regulated at the plasma membrane, and whether this will result in an altered EDHF response. In this respect, new therapeutic strategies that focus on regulation of ion channel density at the cell surface are emerging. For example, molecules which correct the trafficking of mutant forms of CFTR (50) and hERG (60) have recently been described. Additional studies have focused on the pharmacological regulation of channel stability within the membrane and their subsequent fate following endocytosis (55).
Very recently, we demonstrated that plasma membrane KCa2.3 has a long half-life (∼13 h), whereas KCa3.1 is rapidly internalized (within 60–90 min) (28). Furthermore, the long plasma membrane half-life of KCa2.3 could be attributed to a dynamic recycling of the channel back to the cell surface in an RME-1- and Rab35/EPI64C-dependent manner. In contrast, KCa3.1 does not enter this recycling pathway after being internalized and is rapidly degraded (28). The degradation of many cell surface proteins and receptors occurs within the lysosomal lumen and depends on the function of the multivesicular body (MVB) sorting pathway (32, 43, 47). The endosomal sorting complex required for transport (ESCRT)-0, -I, -II, and -III plays a critical role in the targeting of proteins for lysosomal degradation. In the classic model, cargo recognition is mediated by interactions with the ESCRT-0 (Hrs) complex as well as with ESCRT-I (tumor susceptibility gene 101, TSG101). ESCRT-II appears to function downstream or in parallel to ESCRT-I and facilitates the recruitment and assembly of ESCRT-III (composed of charged multivesicular body proteins, e.g., CHMP4). Finally, Vps4, a AAA ATPase, dissociates the ESCRT complex and facilitates the fusion of MVBs with lysosomes, resulting in the degradation of the cargo (17, 48). However, recent studies have revealed that ESCRT-independent routes to lysosomes exist. For example, lysosomal targeting of the activated PAR1 receptor requires sorting nexin 1 (SNX1), but it is independent of ESCRT-0 (Hrs) and ESCRT-I (TSG101) function (33). Also, the δ-opioid G protein-coupled receptor (DOR) does not require TSG101 for agonist-induced lysosomal degradation (36). In other studies, cargo engagement directly with ESCRT-III, via binding to Alix/Bro1 (independent of ESCRT-I or ESCRT-II), has been implicated (26, 67). Moreover, it has been suggested that ESCRT-dependent and ESCRT-independent mechanisms of MVB biogenesis exist in mammalian cells (61). These data emphasize the intricate mechanism of recruitment and function of different ESCRT components for lysosomal targeting of membrane proteins. Thus, we have undertaken studies to elucidate the molecular mechanisms involved in the retrograde trafficking of KCa3.1 from the plasma membrane.
In this study, we demonstrate that, subsequent to endocytosis, KCa3.1 is targeted to the lysosomes for degradation, and that this process follows an ESCRT-dependent pathway, with components of the ESCRT family of proteins acting at early, intermediate, and later stages of channel sorting into lysosomes. Furthermore, we demonstrate a role for the small-molecular-weight guanine nucleotide-binding protein Rab7 as a regulator of the lysosomal degradation of KCa3.1. To our knowledge, the present results are the first to demonstrate that membrane KCa3.1 is targeted for degradation through a MVB pathway.
METHODS
Molecular biology.
Generation of KCa3.1 (also referred to as IK1 or SK4) in pcDNA3.1(+) (Invitrogen, Carlsbad, CA) has been previously described (63). The biotin ligase acceptor peptide (BLAP) sequence (GLNDIFEAQKIEWHE) was inserted into the extracellular loop between transmembrane domains S3 and S4 of KCa3.1 as described (28). The COOH-terminal myc epitope-tagged KCa3.1 was previously described (63). The NH2-terminal, hemagglutinin (HA)-tagged full-length TSG101 (pcGNM2/TSG-F) and COOH-terminal portion of TSG101 (pcGNM2/TSG-3′) expression vectors were generously provided by Dr. E. O. Freed (National Institutes of Health, Bethesda, MD) and Dr. Z. Sun (Stanford University, Palo Alto, CA), respectively. The green fluorescent protein (GFP)- and hemagglutinin (HA)-tagged Rab7 constructs (14) were obtained from Addgene [Addgene plasmid 12605 for the wild type (WT) and Addgene plasmid 12660 for the dominant negative (DN) form]. The human CHMP4B and VPS4B expression vectors were obtained from Open Biosystems. To convert CHMP4B to a DN form, CHMP4B was fluorescently tagged by subcloning it into pECFP-N1 vector (BD Biosciences) using the XhoI and KpnI restriction sites. The ATPase-defective mutant VPS4B(E235Q), which acts as a DN, was generated using the Stratagene QuikChange site-directed mutagenesis strategy (Stratagene, La Jolla, CA). The fidelity of all constructs utilized in this study was confirmed by sequencing (ABI PRISM 377 automated sequencer, University of Pittsburgh, Pittsburgh, PA) and subsequent sequence alignment (NCBI BLAST) using GenBank accession numbers NP_789782.1 for CHMP4B and NP_004860.2 for VPS4B.
Cell culture.
Human embryonic kidney (HEK293) cells were obtained from the American Type Culture Collection (Manassas, VA). The human microvascular endothelial cell line, HMEC-1 (1), was generously provided by Dr. Edwin Ades [Centers for Disease Control, Atlanta, GA (CDC)], Mr. Francisco J. Candal (CDC), and Dr. Thomas Lawley (Emory University, Atlanta, GA). HMEC-1 cells possess morphological, phenotypical, and functional characteristics of human microvascular endothelial cells (1, 5) and are therefore physiologically relevant for our study. The cells were cultured and transfected as previously described (28).
Antibodies.
Polyclonal α-streptavidin antibody (Ab) was obtained from Genscript (Piscataway, NJ). Monoclonal HA (HA.11) and c-myc (clone 9E10) antibodies were obtained from Covance (Richmond, CA). Monoclonal α-tubulin and monoclonal α-Rab7 were obtained from Sigma-Aldrich (St. Louis, MO). Monoclonal anti-lysosome-associated membrane protein 2 (Lamp2) directed against the human epitope (H4B4) (developed by J. Thomas August and James E. K. Hildreth) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development (Bethesda, MD) and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). Rabbit α-VPS4A and α-VPS4B polyclonal antibodies were generously provided by Dr. W. I. Sundquist (University of Utah, Salt Lake City, UT). The monoclonal α-TSG101 Ab was obtained from GeneTex (Irvine, CA).
Biotinylation of KCa3.1 using recombinant biotin ligase.
BLAP-tagged KCa3.1, heterologously expressed in HEK293 or HMEC-1 cells, was enzymatically biotinylated using recombinant biotin ligase (BirA), as described (28). BirA was either purchased from Avidity (Aurora, CO) or expressed from pET21a-BirA (generously provided by Dr. Alice Y. Ting, Massachusetts Institute of Technology, Cambridge, MA) in Escherichia coli according to previously published methods (12). Plasma membrane BLAP-tagged KCa3.1 was then labeled with streptavidin-Alexa 488 or streptavidin-Alexa 555 (Invitrogen), and the cells were either incubated for various periods of time at 37°C, as indicated in the text, or immediately fixed and permeabilized (28). Nuclei were labeled with DAPI (Sigma-Aldrich). Cells were imaged in one of two ways, as indicated in the figure legends. In some cases, cells were subjected to laser confocal microscopy using an Olympus FluoView 1000 system. To ensure maximal X-Y spatial resolution, sections were scanned at 1,024 × 1,024 pixels, with sequential three-color image collection to minimize cross talk between the channels imaged. In other experiments, cells were imaged using a wide-field Olympus IX-81 with motorized stage. Multiple planes were imaged, deconvolved using a point-spread function, and presented as a projection image.
Immunofluorescence.
To assess colocalization of internalized KCa3.1 with lysosomes, BLAP-tagged KCa3.1 was labeled with streptavidin-Alexa 555 as above and the cells were then incubated for 5 h at 37°C, in the presence of the lysosomal protease inhibitors leupeptin (100 μM)/pepstatin (1 μg/ml; L/P) (Sigma-Aldrich). The cells were then fixed/permeabilized as described (41) and the lysosomes labeled with α-Lamp2 antibody, followed by labeling with Alexa 488-conjugated goat anti-mouse IgG antibody. Intracellular HA-tagged Tsg101 was labeled with α-HA antibody, followed by a goat anti-mouse IgG-Alexa 488 (Invitrogen). Imaging was carried out as above.
Immunoprecipitations and immunoblots.
Our immunoprecipitations (IP) and immunoblot (IB) protocols have been previously described (28, 29, 40, 41). Briefly, cells were lysed and equivalent amounts of total protein were precleared with protein G-agarose beads (Invitrogen) and incubated with the indicated antibody. Normal IgG was used as negative control. Immune complexes were precipitated with protein G-agarose beads, and the proteins were resolved by SDS-PAGE followed by IB. To eliminate interference by the heavy and light chains of the immunoprecipitating antibody in the IP, mouse IgG Trueblot ULTRA (eBioscience) was used as a secondary antibody for the detection of immunoprecipitated proteins in the IB.
Determination of degradation rate for plasma membrane localized KCa3.1.
The degradation rate for endocytosed membrane KCa3.1 was determined as described (28). Briefly, the channel was specifically biotinylated using BirA and labeled with streptavidin, as above. Next, cells were incubated for various periods of time at 37°C, as indicated. The cells were then lysed and equivalent amounts of total protein were separated by SDS-PAGE, followed by IB for streptavidin. As streptavidin remains tightly coupled to the channel during SDS-PAGE, this provides a direct correlate to the immunofluorescence (IF) studies detailed above. Bands were quantified by the densitometry function of Quantity One software (Bio-Rad, Hercules, CA). Data were corrected for background and nonspecific labeling (measured on cells treated only with streptavidin, in the absence of biotinylation). The obtained band intensities for the various time points were normalized relative to the intensity at time 0 and are reported. The blots were also probed for tubulin, as a protein-loading control.
Short-interfering RNA treatment.
Smartpool short-interfering RNA (siRNA) constructs against the coding region of either human Rab7, TSG101, or the two isoforms of VPS4 (A and B) were obtained from Dharmacon Research (Chicago, IL). Each siRNA smartpool comprised a mixture of four oligonucleotide duplexes. The siGENOME Non-Targeting siRNA Pool no. 2 (Dharmacon Research), was used as control. HEK293 cells were plated at ∼50% confluence and transfected with 50 nM siRNA duplexes (for Rab7) and up to 100 nM siRNA for TSG101 and VPS4, using DharmaFECT 1, according to the manufacturer's directions. Experiments were carried out 72-h posttransfection.
Transmission electron microscopy of quantum dot-labeled KCa3.1.
For an ultrastructural analysis of channel localization following endocytosis, HEK293 cells stably expressing KCa3.1 were grown on plastic coverslips for 24 h before labeling. Next, the channel was enzymatically biotinylated at the cell surface as described above, then labeled with streptavidin conjugated with QuantumDot-655 (Invitrogen). These QDots can be visualized in electron micrographs as dense geometric structures (19). Following incubation at 37°C for different periods of time, the cells were fixed in 2.5% glutaraldehyde in PBS for 1 h at room temperature. Samples were rinsed three times for 5 min in PBS and postfixed in 1% osmium tetroxide containing 1% potassium ferricyanide for 1 h at room temperature and then rinsed three times in PBS. The cells were dehydrated in ascending grades of ethanol (30%, 50%, 70%, 90%) 10 min each, and three changes of absolute alcohol for 15 min at room temperature. Samples were infiltrated three times with epon (1 h each). Samples were embedded in epon overnight at 37°C and then 48 h at 65°C. Ultrathin sections were mounted on grids and stained in 2% uranyl acetate in methanol and then 1% lead citrate before imaging with a JEOL 1011 (Joel, Tokyo, Japan) electron microscope operated at an accelerating voltage of 80 kV.
Chemicals.
All chemicals were obtained from Sigma-Aldrich, unless otherwise stated.
Statistical analysis.
All data are presented as means ± SE, where n indicates the number of experiments. Statistical analysis was performed using a Student's t-test, to compare the IB band intensities at time 0 for KCa3.1 expressed together with either the WT or DN form of a certain construct. For the normalized values of band intensities, statistical analysis was performed using the nonparametric Kruskal-Wallis test. A value of P < 0.05 was considered statistically significant and is reported.
RESULTS
Membrane KCa3.1 is targeted to the lysosomes for degradation.
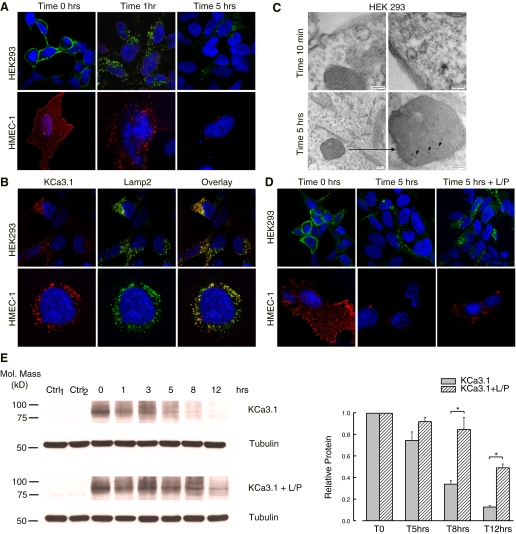
To follow the fate of KCa3.1 channels subsequent to endocytosis, HEK293 and HMEC-1 cells were transfected with BLAP-tagged KCa3.1 (29). This construct will be referred to as KCa3.1 for simplicity throughout the remainder of the manuscript. Previous patch-clamp data demonstrate that this channel maintains the functional characteristics of the WT channel (29). KCa3.1 was enzymatically biotinylated at the cell surface using BirA and labeled with streptavidin-Alexa 488 in HEK cells (Fig. 1A, top) and streptavidin-Alexa 555 in HMEC-1 cells (Fig. 1A, bottom). Cells were incubated at 37°C for the indicated periods of time, and the fate of the endocytosed channel was assessed by confocal microscopy. At time 0 h, the labeled channel is present at the cell surface, as predicted for our labeling protocol. Note that, for HMEC-1 cells, which are only ∼2 μm thick, the images display also the top of the cells, with a discontinuous punctuate appearance of the labeled channels at the cell surface. Importantly, no labeling was observed if we express KCa3.1 with no BLAP tag and carried out the labeling procedure as above. Also, if the BirA-dependent biotinylation step was omitted, streptavidin exposure alone failed to label KCa3.1 (data not shown), confirming the specificity of the labeling observed. After 1-h incubation at 37°C, almost all the channel present at the plasma membrane was endocytosed in both HEK293 and HMEC-1 cells. After 5 h at 37°C, some KCa3.1-containing vesicles are still visible inside the cells, but significant channel has been presumably degraded as assessed by the reduced fluorescence signal. These results show that KCa3.1 is rapidly internalized from the plasma membrane and targeted for degradation, in good agreement with our previous report (29).
Fig. 1.
Membrane Ca2+-activated K+ channel KCa3.1 is targeted to the lysosomes for degradation. A: cells were transfected with biotin ligase acceptor peptide (BLAP)-KCa3.1, and the channel at the cell surface was labeled with streptavidin-Alexa 488 in human embryonic kidney (HEK) cells (top) and streptavidin-Alexa 555 in human microvascular endothelial cells (HMEC-1) (bottom). The fate of the channel inside the cells was addressed by immunofluorescence (IF) upon incubation at 37°C for the indicated periods of time. At time 0, the channel is localized at the plasma membrane. After 1 h at 37°C, nearly all the channel is endocytosed in both HEK and HMEC-1 cells. Following 5 h at 37°C, the endocytosed KCa3.1 is extensively degraded in both cell types, as evidenced by the reduced fluorescence signal. Nuclei were labeled with DAPI (blue). HEK cells are shown as single confocal sections, while HMEC-1 cells are shown as projection images from multiple z-sections. B: the endocytosed BLAP-KCa3.1 (labeled in red) colocalizes with the lysosomal marker lysosome-associated membrane protein 2 (Lamp2; labeled in green) in both HEK and HMEC-1 cells. A confocal optical section imaged through a representative cell is shown. C: ultrastructural analysis using transmission electron microscopy confirms that membrane KCa3.1 is delivered to the lysosomes, following endocytosis. D: cells were transfected with BLAP-KCa3.1 and the channel at the cell surface was labeled as in A. Cells were incubated for 5 h at 37°C either in the presence or absence of lysosomal protease inhibitors leupeptin (100 μM)/pepstatin (1 μg/ml; L/P). At time 0, the channel is localized at the plasma membrane. At the end of the incubation period, the endocytosed KCa3.1 is extensively degraded in control cells, whereas in cells treated with L/P, this process is inhibited, as shown by the strong fluorescent signal still present. E: degradation rate for plasma membrane localized BLAP-KCa3.1 was evaluated in HEK cells by specifically biotinylating the channel using recombinant biotin ligase (BirA). Subsequently, the channel was labeled with streptavidin and the cells were incubated at 37°C for 0, 1, 3, 5, 8, or 12 h in the presence or absence of L/P. Representative blots are shown at left; 20 μg protein was loaded per lane. The data were quantified by densitometry and are plotted as shown (n = 3; *P < 0.05). T, time; Mol, molecular; Ctrl, control.
As lysosomes are the final destination to terminate the fate of many membrane proteins, we hypothesized that membrane KCa3.1 degradation also occurs in the lysosomes. To confirm this, we performed colocalization studies between the endocytosed KCa3.1 and the lysosomal marker Lamp2. The channel was expressed in HEK293 (Fig. 1B, top) and HMEC-1 cells (Fig. 1B, bottom) and labeled at the cell surface with streptavidin-Alexa 555. Next, the channel was allowed to be endocytosed for 5 h at 37°C, in the presence of the lysosomal protease inhibitors L/P. Cells were fixed and processed for double label indirect immunofluorescence, to detect the lysosomal marker Lamp2 (labeled with Alexa 488, see methods). As is apparent in the overlay panels, there is a high degree of colocalization (yellow) between KCa3.1 and Lamp2 in both HEK and HMEC-1 cells, indicating that internalized KCa3.1 is targeted to the lysosomes.
To obtain a direct structural correlate to our IF results, we determined the localization of KCa3.1 following endocytosis using transmission electron microscopy (TEM). For these studies, HEK293 cells were transfected with KCa3.1 and the channel was labeled at the cell surface with streptavidin conjugated with QuantumDot-655, followed by incubation at 37°C in the presence of L/P for various periods of time. As shown in Fig. 1C, the channel is rapidly internalized from the plasma membrane in to endocytic vesicles (top). After 5 h, the channel is localized in the lysosomes, as shown by the pronounced QDots staining of these organelles (bottom).
We next confirmed the lysosomal targeting and proteolysis of membrane KCa3.1 by evaluating the degradation of the channel in cells treated with L/P. HEK293 and HMEC-1 cells were transfected with KCa3.1, and the channel at the cell surface was labeled with streptavidin-Alexa 488 in HEK cells (Fig. 1D, top) and streptavidin-Alexa 555 in HMEC-1 cells (Fig. 1D, bottom). Cells were incubated at 37°C for 5 h in the presence or absence of L/P, and the fate of the endocytosed channel was assessed by confocal microscopy. At time 0 h, the labeled channel is present at the cell surface. At the end of the incubation period, in cells treated with L/P for 5 h at 37°C, channel degradation is inhibited and KCa3.1 accumulates inside the cells in a vesicular compartment with a perinuclear distribution, as compared with control cells, where the channel is extensively degraded. Taken together, these data confirm the lysosomal nature of the vesicles where the channel accumulates.
To quantify the degradation rate of membrane KCa3.1 in HEK293 cells under normal conditions and conditions where the lysosomal proteolysis is inhibited, we used biotinylation as previously described (29). KCa3.1 stably expressed in HEK cells was biotinylated at the cell surface with BirA, then labeled with streptavidin, in the same way as for the IF measurements. Next, the channel was allowed to be internalized for various periods of time at 37°C with or without L/P treatment. Subsequently, the cells were lysed and the complex KCa3.1/streptavidin still present inside the cells was probed by IB, with antibodies against streptavidin. To control for nonspecific labeling, HEK cells not expressing KCa3.1 were labeled as above (Ctrl1 lane in Fig. 1E) or KCa3.1-expressing cells were labeled with streptavidin in the absence of enzymatic biotinylation (Ctrl2 lane in Fig. 1E). As is apparent, no channel is detected in these controls, confirming the specificity of our labeling protocol. As shown in Fig. 1E, subsequent to endocytosis, membrane KCa3.1 is extensively degraded within 12 h in control cells. That is, only 12 ± 1% (n = 3) of the membrane protein was still detected at the end of the incubation period. In contrast, in L/P-treated cells, channel degradation is significantly inhibited, with 49 ± 4% (n = 3; P < 0.05) of membrane KCa3.1 still present. Taken together, these data indicate that lysosomes are the final destination of the endocytosed membrane KCa3.1 channel.
Rab7 is required for the degradation of KCa3.1 by the lysosomes.
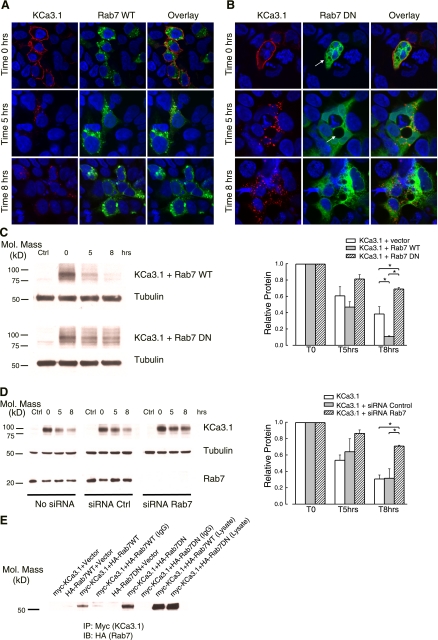
Recent studies demonstrate that Rab7 controls intracellular vesicle traffic to late endosomes/lysosomes and lysosome biogenesis in mammalian cells (7, 59). Thus, we investigated the role of Rab7 as a regulator of the distal stages of the KCa3.1 endocytic pathway. HEK293 cells were cotransfected with KCa3.1 and either the WT or a DN form of GFP-HA-tagged Rab7. The plasma membrane-localized channel was labeled with streptavidin-Alexa 555 and allowed to internalize for either 5 or 8 h at 37°C. In agreement with previous reports (7), WT GFP-Rab7 primarily localized to vesicles with a perinuclear distribution (Fig. 2A, middle). Overexpression of WT Rab7 did not affect the plasma membrane localization of KCa3.1, as shown by the strong labeling of the channel, at time 0 h (Fig. 2A, left). As is also seen for these cells, the endocytosed KCa3.1 is extensively degraded after 8-h incubation at 37°C (Fig. 2A, left). In contrast, overexpression of DN GFP-Rab7 caused a diffuse, widespread signal and the accumulation of large vacuoles (Fig. 2B, arrow, middle), as previously reported (3). At time 0 h, KCa3.1 is also localized to the plasma membrane, similar to WT Rab7-expressing cells. Quantification of band intensities at time 0 obtained from IB (see below; compare lane 2 for WT and DN Rab7 in Fig. 2C) revealed that DN Rab7 does not affect cell surface expression of KCa3.1, as compared with WT (n = 3; P > 0.05). However, DN Rab7 had a strong inhibitory effect on membrane KCa3.1 degradation, as shown by the clear fluorescent signal associated with the channel at the end of 8-h incubation period (Fig. 2B, left).
Fig. 2.
Rab7 is required for the degradation of KCa3.1 by the lysosomes. A and B: HEK cells were doubly transfected with BLAP-KCa3.1 and either wild-type (WT) green fluorescent protein (GFP)-Rab7 (A) or dominant negative (DN) GFP-Rab7 (B). The channel was labeled at the cell surface with streptavidin-Alexa 555 and cells incubated at 37°C for either 5 or 8 h. Transient expression of DN Rab7 results in endosomal accumulation of KCa3.1, compared with the wild-type Rab7. C: to quantify the effect of Rab7 activity on the degradation rate of membrane KCa3.1, HEK cells were transfected with BLAP-KCa3.1 and either GFP-Rab7 construct. Cells were enzymatically biotinylated with BirA, labeled with streptavidin, and then blotted for streptavidin at the indicated periods of time. A representative blot is shown, together with the results obtained from densitometry quantification (n = 3; *P < 0.05). D: HEK cells stably expressing BLAP-KCa3.1 were either mock-transfected, transfected with short interfering RNA (siRNA) control, or Rab7-specific siRNA. At 72-h posttransfection, the channel was streptavidin-labeled at the cell surface and incubated at 37°C for the indicated periods of time. The degradation rate of the channel was assessed by immunoblotting (IB). A representative blot is shown, together with the results obtained from densitometric quantification (n = 3; *P < 0.05). E: coimmunoprecipitation of myc-KCa3.1 with either hemagglutinin (HA)-tagged WT or DN Rab7 was carried out in HEK cells as described in methods. KCa3.1 was immunoprecipitated using either an anti-myc Ab (lanes 1–3, 5, and 6) or an anti-V5 Ab as IgG control (lanes 4 and 7) and subsequently IB for Rab7 using an anti-HA Ab. When only a single construct was transfected (myc or HA), the empty vector was included to maintain the plasmid at the same final concentration. Rab7 was detected by IB in lanes 3 and 6, confirming association between KCa3.1 and Rab7.
To quantify the rate of KCa3.1 degradation in HEK cells expressing either the WT or DN Rab7, we biotinylated, then labeled the channel at the cell surface with streptavidin, as above. The cells were incubated at 37°C for either 5 or 8 h, and the amount of KCa3.1 present was assessed by immunoblotting. As shown in Fig. 2C, right, in the presence of WT Rab7 the channel is extensively degraded, the detected amount of protein at the end of the incubation period being only 11 ± 1% (n = 3) of the initial value. This value is significantly lower than the amount of protein detected after 8 h at 37°C in control cells, not transfected with Rab7 (38 ± 9%, n = 3; P < 0.05), suggesting that, as previously reported (7), overexpression of Rab7 WT might induce a dominant active phenotype, accelerating the rate of channel degradation. In contrast, DN Rab7 dramatically inhibits the rate of degradation. After 8-h incubation at 37°C, the levels of membrane KCa3.1 still present inside the cells measured 69 ± 2% (n = 3; P < 0.05) of the original values.
To confirm a role for Rab7 in the degradation of KCa3.1, we utilized an siRNA approach. As above, the channel was streptavidin-labeled at the cell surface and the degradation rate was measured for untransfected cells, cells transfected with control siRNA, and cells transfected with Rab7 siRNA. As shown in Fig. 2D (bottom IB), 72 h after transfection with Rab7 siRNA duplexes, endogenous Rab7 is not detected by IB, whereas the scrambled control siRNA had no effect on Rab7 levels in HEK cells. Moreover, after 8-h incubation at 37°C, the channel is extensively degraded in untransfected cells, being only 31 ± 5% (n = 3) of the initial membrane protein, as well as in siRNA control-transfected cells (32 ± 11%, n = 3, P > 0.05). In contrast, in Rab7 siRNA-transfected cells, channel degradation is significantly inhibited, with 71 ± 1% (n = 3; P < 0.05) of membrane KCa3.1 still present.
Because Rab proteins are known to associate with cargo proteins (46, 54), we determined whether KCa3.1 is associated with Rab7 by coimmunoprecipitation (co-IP). HEK cells were cotransfected with myc-tagged KCa3.1 and either the WT or the DN form of the GFP-HA-tagged Rab7. As shown in Fig. 2E, we observed an association of myc-KCa3.1 with both WT and DN forms of Rab7. These findings are consistent with Rab7 being a key regulatory molecule in the degradation pathway of KCa3.1.
KCa3.1 is targeted to MVB via the ESCRT pathway—role of TSG101.
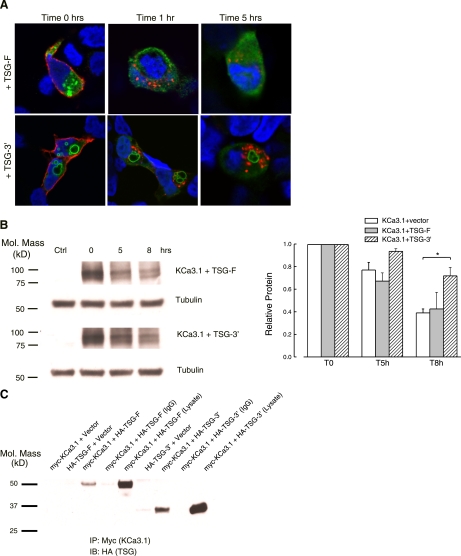
Because KCa3.1 is targeted for lysosomal degradation, we determined the role of the ESCRT family of proteins in this process. Initially, we focused on TSG101, a core component of the ESCRT-I complex (17, 48). We coexpressed KCa3.1 with either WT, full-length TSG101 (TSG-F), or its NH2-terminally truncated form (TSG-3′). Previous studies have shown that overexpression of TSG-3′ disrupts the cellular endosomal sorting machinery (39). Plasma membrane KCa3.1 was labeled at the cell surface with streptavidin-Alexa 555, after which the cells were incubated at 37°C for the indicated periods of time (Fig. 3A). As shown in Fig. 3A, top, TSG-F displayed a highly punctate, putatively endosomal expression pattern. In contrast, transfection of cells with TSG-3′ (Fig. 3A, bottom) resulted in the formation of large vacuolar structures, as reported (30, 39). As shown in Fig. 3A for time 0 h, the channel is expressed at the cell surface in both TSG-F or TSG-3′-transfected cells. Moreover, quantification of band intensities at time 0 obtained from IB (see below, compare lane 2 for TSG-F and TSG-3′ in Fig. 3B) revealed that the truncated form of TSG101 does not affect cell surface expression of KCa3.1, compared with the full-length TSG101 (n = 3; P > 0.05). Within 1-h incubation at 37°C, the channel was almost completely endocytosed in the presence of TSG-F (Fig. 3A, top) as well as TSG-3′ (Fig. 3A, bottom), similar to what we observed for control, nontransfected cells (Fig. 1A). After 5-h incubation at 37°C, TSG-3′-expressing cells show a pronounced staining associated with the channel, indicative of a reduced degradation rate, compared with the control.
Fig. 3.
Effects of overexpressing the full-length and the NH2-terminally truncated form of TSG101 on KCa3.1 degradation. A: subcellular localization of TSG-F and NH2-terminally truncated TSG-3′ in HEK cells cotransfected with BLAP-KCa3.1. The channel was labeled at the cell surface with streptavidin-Alexa 555. After incubation at 37°C for the indicated times, the cells were fixed, permeabilized, and stained for TSG101 using anti-HA Ab and visualized by confocal microscopy. At time 0 h, BLAP-KCa3.1 is localized at the plasma membrane. After 5-h incubation at 37°C, cells expressing the dominant negative form of TSG101 show a suppression of channel degradation, and its accumulation in vesicles. Images are single confocal sections. Nuclei were labeled with DAPI. B: the degradation rate for plasma membrane-localized BLAP-KCa3.1 was evaluated in HEK cells by specifically biotinylating the channel using BirA (see methods). The data were quantified by densitometry and plotted as shown (n = 3; *P < 0.05). C: coimmunoprecipitation of myc-KCa3.1, with either HA-tagged TSG-F or TSG-3′, was carried out in HEK cells as described in methods. KCa3.1 was immunoprecipitated using either an anti-myc Ab (lanes 1–3, 6, and 7) or an anti-V5 Ab as IgG control (lanes 4 and 8) and subsequently IB for TSG101 using an anti-HA Ab. When only a single construct was transfected (myc or HA), the empty vector was included to maintain the plasmid at the same final concentration. TSG101 was detected by IB in lanes 3 and 7, confirming association between KCa3.1 and TSG101. Note the difference in the molecular weight between the full-length and the truncated form.
The effects of full-length and mutant TSG101 constructs on the rate of KCa3.1 degradation were quantified using surface biotinylation as above (Fig. 3B). After 8 h at 37°C, the level of membrane KCa3.1 still detected inside the cells was 43 ± 14% for the TSG-F-expressing cells and 72 ± 7% for the truncated TSG-3′ construct (n = 3; P > 0.05). Although suggesting an inhibitory effect on channel degradation rate in cells expressing TSG101–3′ relative to the WT TSG-F, the results are not significantly different, owing to the variability obtained for the TSG-F construct. This observation is not uncommon, because previous studies have reported that overexpression of the WT TSG101 might slow the degradation rate and hence induce accumulation of EGF receptor in HeLa cells (30). To obtain a better estimate for the effect of truncated TSG101 on the rate of channel endocytosis, we compared TSG-3′ cells with control cells (transfected only with KCa3.1 and the empty vector). In the latter case, the amount of channel still present inside the cells at the end of 8-h incubation was 39 ± 4% (n = 3; P < 0.05). Unfortunately, siRNAs directed against TSG101 (100 nM for 72 h) resulted in only an ∼40% knockdown of TSG101, as assessed by IB, such that the role of TSG101 knockdown on KCa3.1 degradation was not further explored.
Given that TSG101 is known to directly interact with cargo proteins destined for lysosomal degradation (2), we determined whether endocytosed KCa3.1 associates with TSG101. HEK cells were transfected with myc-tagged KCa3.1 and either the full-length or the 3′-truncated form of HA-tagged TSG101. As shown in Fig. 3C, KCa3.1 coimmunoprecipitated with TSG101-F, and also with the truncated form TSG101–3′, indicative of TSG101 and KCa3.1 being minimally in the same endosomal compartment following endocytosis. These results indicate a role for TSG101 in recruiting membrane KCa3.1 into MVB.
KCa3.1 is targeted to MVB via the ESCRT pathway—role of CHMP4B.
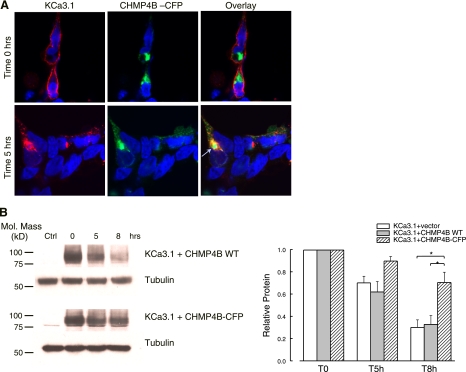
We next determined whether CHMP4B, a member of the ESCRT-III complex (34, 69), is involved in the lysosomal targeting of KCa3.1. It has been previously shown that fluorescently tagged CHMPs behave as dominant negatives, disturbing the regulation of membrane trafficking from endosomes to lysosomes (42). Thus, we cotransfected HEK cells with KCa3.1 and CHMP4B-cyan fluorescent protein (CFP), labeled cell surface KCa3.1 with streptavidin-Alexa 555, and allowed the channel to internalize for 5 h at 37°C. At time 0 h, the channel is localized at the plasma membrane in these cells, as shown in Fig. 4A (top). Moreover, quantification of band intensities at time zero obtained from IB (see below, compare lane 2 for WT and DN CHMP4B in Fig. 4B) showed that overexpression of DN CHMP4B does not affect cell surface expression of KCa3.1, compared with the WT CHMP4B (n = 3; P > 0.05). As shown in Fig. 4A (middle), expression of CHMP4B-CFP induces formation of enlarged endosomal structures, indicative of the dominant negative effect of the fused CFP-CHMP4B. Moreover, endocytosed KCa3.1 accumulates in the CHMP4B-CFP-induced structures, as indicated by the clear colocalization (arrow in the bottom overlay, Fig. 4A).
Fig. 4.
Effect of CHMP4B-cyan fluorescent protein (CFP) overexpression on the distribution and degradation of endocytosed KCa3.1. A: HEK cells were cotransfected with BLAP-KCa3.1 and a CFP-tagged, CHMP4B. KCa3.1 was labeled at the cell surface with streptavidin-Alexa 555 (red), then allowed to be internalized for 5 h at 37°C. In cells overexpressing CHMP4B-CFP, the channel is not degraded, as shown by the strong intracellular signal (left). Moreover, the channel is accumulated in the large CHMP4B-CFP containing intracellular structures (arrow, overlay). Images are single confocal sections. Nuclei were labeled with DAPI. B: the degradation rate for plasma membrane-localized BLAP-KCa3.1 was evaluated in HEK cells by specifically biotinylating the channel using BirA (see methods). The data were quantified by densitometry and plotted as shown (n = 4; *P < 0.05).
The effect of CHMP4B-CFP expression on the degradation rate of membrane KCa3.1 was quantified using surface biotinylation as above. As shown in Fig. 4B, after 8-h incubation at 37°C, CHMP4B-CFP expression significantly inhibited channel degradation (70 ± 9% channel still present inside the cells, n = 4), compared with the CHMP4B WT-expressing cells (33 ± 8%, n = 4; P < 0.05). These data emphasize the importance of a functional CHMP4B for proper sorting of internalized KCa3.1 into the late endosomes.
Lysosomal proteolysis of KCa3.1 is dependent on VPS4 activity.
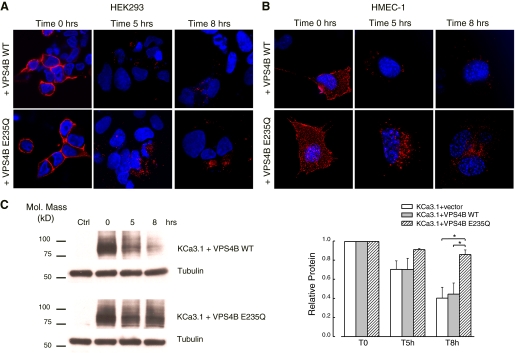
One of the essential steps in MVB biogenesis and function is the recruitment of the AAA ATPase SKD1/VPS4, which drives dissociation of the ESCRT machinery and allows the intraluminal release of vesicles formed by endosomal membrane invagination (52). It has been shown that while VPS4 transiently associates with late endosomes, the ATPase-defective VPS4 mutant (VPS4B E235Q) fails to dissociate from these membranes and severely impairs MVB sorting (27). To test this effect on KCa3.1 degradation, HEK and HMEC-1 cells were doubly transfected with KCa3.1 and either the WT VPS4B or VPS4B E235Q. KCa3.1 was labeled at the cell surface either with streptavidin-Alexa 555 or plain streptavidin, then incubated at 37°C for 5 and 8 h. The rate of membrane KCa3.1 degradation was assessed with both confocal microscopy and IB.
At time 0 h, the labeled channel is expressed at the cell surface as expected, in both HEK cells (Fig. 5A, left) and HMEC-1 cells (Fig. 5B, left). Importantly, as obtained form IB quantification of the band intensities at time 0(see below, compare lane 2 for WT and DN VPS4B in Fig. 5C), expression of VPS4B E235Q does not affect plasma membrane KCa3.1 compared with the WT VPS4B (n = 3; P > 0.05). However, as shown by the large accumulation of KCa3.1-related fluorescent vesicles inside the cells, the protein degradation rate for mutant VPS4B E235Q-expressing cells is dramatically decreased compared with the VPS4B WT-expressing cells, in both HEK (Fig. 5A) and HMEC-1 cells (Fig. 5B). Quantitative immunoblotting (Fig. 5C) showed that after 8-h incubation at 37°C, the channel is not degraded in cells expressing VPS4B E235Q, with 86 ± 5% (n = 3; P < 0.05) of membrane KCa3.1 still present. Wild-type VPS4B has no effect on KCa3.1 degradation, such that, after 8 h, only 44 ± 11%, (n = 3) of the channel remained. These results indicate a role for VPS4 in the correct targeting and lysosomal degradation of KCa3.1. As above for TSG101, siRNAs directed against VPS4A and VPS4B in combination (100 nM for 72 h) resulted in only an ∼60% knockdown of VPS4A/B, as assessed by IB, such that the role of VPS4 knockdown on KCa3.1 degradation was not further explored.
Fig. 5.
Lysosomal proteolysis of KCa3.1 is dependent on Vps4 activity. A: HEK cells were doubly transfected with BLAP-KCa3.1 and either the WT or a DN form (E235Q) of VPS4B. Channel was fluorescently labeled at the cell surface and then incubated at 37°C for the indicated periods of time. As shown by the large accumulation of channel-related vesicles inside the cells, the protein degradation rate for mutant VPS4BE235Q cells is much slower than the one for the wild-type-expressing cells. Images are single confocal sections. B: the degradation rate for plasma membrane-localized BLAP-KCa3.1 was quantified in HEK cells by specifically biotinylating the channel using BirA (see methods). A representative blot is shown. The data were quantified by densitometry and normalized to the starting values for each case (n = 3; *P < 0.05).
DISCUSSION
Endothelial small- and intermediate-conductance Ca2+-activated K+ channels (KCa2.3 and KCa3.1) play a key role in mediating the agonist-induced activation of endothelium-derived hyperpolarizing factor (EDHF) response, one of the main regulators of vascular tone (8, 15, 23–25, 65). For cells to appropriately respond to external agonists, they must maintain the proper protein complement at the cell surface. This can be achieved by both regulating the delivery and removal of the channels to and from the cell surface when necessary. Although there is a clear consensus that KCa2.3 and KCa3.1 together are responsible for initiating the EDHF signaling (16, 22, 35), these channels strikingly differ in their fate subsequent to membrane insertion, as we have recently shown (28). That is, KCa2.3 has a long residency time at the plasma membrane, attributed to a dynamic recycling of the channel back to the cell surface. In contrast, as we present here, KC3.1 is rapidly internalized and targeted for degradation in both HEK and HMEC-1 cells. The striking difference in the way these channels are regulated at the cell surface might be regarded as a “fingerprint” of their physiological role in endothelial cells.
In this study, we characterize, for the first time, the molecular mechanisms responsible for KCa3.1 downstream trafficking and degradation. Using a biotin-ligase acceptor peptide-tagged KCa3.1 recently engineered in our lab, we rapidly biotinylate and streptavidin labeled the channel at the cell surface. This allows us to follow the channels fate subsequent to endocytosis and determine its degradation rate, using a combined imaging and biochemical approach. Our data indicate a degradation pathway in which the channel is delivered to lysosomes. The first evidence supporting this conclusion came from colocalization studies between endocytosed KCa3.1 and the lysosomal marker Lamp-2 in both HEK and endothelial HMEC-1 cells (Fig. 1B) and was confirmed using TEM (Fig. 1C). Additionally, in the presence of lysosomal protease inhibitors L/P, KCa3.1 accumulates inside the cells in a vesicular compartment with a perinuclear distribution, whereas in control cells the protein is mostly degraded over the observed period of time, as indicated by the lack of fluorescence signal associated with the channel (Fig. 1D). Biochemical quantification of the degradation rate of membrane KCa3.1 upon prolonged incubation either in the presence or absence of L/P indicate that the endocytosed channel is degraded within 12 h in control cells, whereas for L/P-treated cells the channel degradation is significantly inhibited (Fig. 1E), conclusively demonstrating that lysosomes are the final destination for the internalized channel.
There is a defined role for the small GTPase Rab7 in regulating the later stages of the endocytic pathway for a number of proteins (10). Moreover, it has been shown that Rab7 acts as a key regulatory protein for proper aggregation and fusion of late endocytic structures in the perinuclear region, and consequently for the biogenesis and maintenance of the lysosomal compartment (7, 66). Herein, we define a role for Rab7 in the degradation of KCa3.1. That is, KCa3.1 immunoprecipitates with both WT and DN Rab7 following transient expression in HEK cells (Fig. 2D), confirming that these proteins associate in the same complex. We also found that Rab7 activity directly correlates with the degradation rate of plasma membrane KCa3.1. Thus, overexpression of the WT-Rab7 accelerates the rate of channel degradation, whereas DN-Rab7 strongly inhibited the degradation rate of membrane KCa3.1 (Fig. 2C). The effect observed with WT-Rab7 could be explained by a dominant active effect on Rab7 function following overexpression as previously reported (7). A role for Rab7 in the degradation of endocytosed KCa3.1 was confirmed by siRNA-dependent knockdown of endogenous Rab7 (Fig. 2D).
Furthermore, we demonstrate that KCa3.1 is routed to lysosomes via the MVB pathway and that the main components of the ESCRT family of proteins are critical in this process. We investigated the roles of Tsg101 (ESCRT-I), CHMP4 (ESCRT-III), and VPS4, which are considered critical proteins for early, intermediate, and late steps of MVB sorting pathway, respectively (17, 48). Previous studies revealed that TSG101 is required for the proper formation of MVBs (47, 49), and that it is also important in selecting which proteins enter the MVB lumen and are delivered to the lysosomes, and which remain on the limiting membrane and escape degradation. However, TSG101 is not universally required for the lysosomal targeting of all membrane proteins (33, 36). We demonstrate that expression of a truncated TSG101 protein significantly inhibits the degradation rate of KCa3.1 (Fig. 3B). Furthermore, we demonstrate a close association between KCa3.1 and TSG101 via co-IP (Fig. 3C), indicative of a role for TSG101 in the targeting KCa3.1 for lysosomal degradation. However, we do not yet know what sorting signal engages KCa3.1 into the MVB pathway. Covalent modification by ubiquitin has been established as a signal for many cargo membrane proteins targeted for lysosomal degradation (48). TSG101 appears to function as the receptor and/or sorting complex that selects ubiquitylated proteins for incorporation into MVB vesicles (4). However, some cargo does not require ubiquitination to enter the ESCRT-I-dependent MVB pathway (13). Whether membrane KCa3.1 requires ubiquitinylation as an MVB sorting determinant remains an open question and further study is needed to address this issue.
While cargo recognition is mediated by the early ESCRT complexes, cargo engagement with ESCRT-III either via ESCRT-I and -II, or via alternative mechanisms, appears to be the next critical step in MVB sorting. ESCRT-III subunits copolymerize at sites of action, delineating and generating vesicles within the lumen of the MVB (3, 53). Recent evidence indicates that ESCRT-III subunits of the CHMP4 family can form circular filaments that can be engaged to deform the membrane to which they are attached (34). For the process to be completed and the cargo delivered into the lumen of lysosomes, the ESCRT complex should be released from the membrane, and this step is controlled by the AAA ATPase protein VPS4 (37). In agreement with these findings, the results of our study demonstrate a role for CHMP4B and VPS4B on the lysosomal degradation of KCa3.1. Upon expression of DN ESCRT-III mutant protein CHMP4B in HEK cells (Fig. 4), as well as an ATP-defective DN mutant of VPS4B in HEK and HMEC-1 cells (Fig. 5), the degradation rate of membrane KCa3.1 was potently inhibited. The observed effect might be attributed to a disturbed endosome-lysosome degradation pathway, based on recent data showing that overexpression of DN forms of these proteins generates deformed and functionally impaired MVBs (27, 42).
In total, we demonstrate that membrane KCa3.1 is targeted for lysosomal degradation, subsequent to endocytosis, and that this process requires components of the ESCRT machinery. Recent studies demonstrate that other potassium channels undergo lysosomal degradation, i.e., the inward-rectifier potassium channel Kir2.1 (38), the ATP-sensitive potassium channel KATP (44), or the HERG channel (11). However, to the best of our knowledge, this is the first study to show the role of ESCRT proteins on lysosomal degradation of a potassium channel. Understanding the molecular mechanisms that govern the downstream sorting and trafficking of KCa3.1 channels in endothelial cells is relevant for cardiovascular pathological conditions. Importantly, our data indicate qualitatively similar mechanisms for channel trafficking and lysosomal degradation in both HEK and HMEC-1 cells. Thus, inhibition of lysosomal proteolysis with L/P induced the accumulation of KCa3.1 in both cell types (Fig. 1D). Also, a functional VPS4B is required for channel degradation in either cell type (Fig. 5, A and B). In line with this observation, we have previously shown that internalized KCa2.3 is recycled back at the plasma membrane via the same molecular machinery (including RME-1, Rab35, and the Rab GAP, EPI64C) in both HEK and HMEC-1 cells (28). This suggests that general mechanisms, governed by similar molecular components, are responsible for the behavior of these channels in both cell types. Hence, our results in HMEC-1 cells, which closely resemble the morphological and functional characteristics of human microvascular endothelia, are relevant for understanding the trafficking of KCa3.1 and KCa2.3 in intact endothelia. However, future studies are required, to confirm these results on endogenously expressed KCa3.1 in native endothelia.
GRANTS
This work was supported by National Institutes of Health Grants HL083060 and HL092157 (to D. C. Devor) and by an American Heart Fellowship (0825542D; to C. M. Balut).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Beth Nickel for technical assistance in TEM. We also acknowledge Dr. Kirk Hamilton for critically reading the manuscript and for many helpful discussions.
REFERENCES
- 1.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99: 683–690, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Auth T, Schluter S, Urschel S, Kussmann P, Sonntag S, Hoher T, Kreuzberg MM, Dobrowolski R, Willecke K. The TSG101 protein binds to connexins and is involved in connexin degradation. Exp Cell Res 315: 1053–1062, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell 3: 271–282, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol 157: 91–101, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouis D, Hospers GA, Meijer C, Molema G, Mulder NH. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4: 91–102, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Kohler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119: 2323–2332, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell 11: 467–480, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, Vanhoutte PM. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol 137: 1346–1354, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceresa BP, Bahr SJ. rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J Biol Chem 281: 1099–1106, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chapman H, Ramstrom C, Korhonen L, Laine M, Wann KT, Lindholm D, Pasternack M, Tornquist K. Downregulation of the HERG (KCNH2) K(+) channel by ceramide: evidence for ubiquitin-mediated lysosomal degradation. J Cell Sci 118: 5325–5334, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chen I, Howarth M, Lin W, Ting AY. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat Methods 2: 99–104, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wang J, Meyers KR, Enns CA. Transferrin-directed internalization and cycling of transferrin receptor 2. Traffic 10: 1488–1501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhury A, Dominguez M, Puri V, Sharma DK, Narita K, Wheatley CL, Marks DL, Pagano RE. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J Clin Invest 109: 1541–1550, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol 31: 641–649, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol 553: 183–189, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies BA, Lee JR, Oestreich AJ, Katzmann DJ. Membrane protein targeting to the MVB/lysosome. Chem Rev 109: 1575–1586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decimo I, Roncarati R, Grasso S, Clemens M, Chiamulera C, Fumagalli G. SK3 trafficking in hippocampal cells: the role of different molecular domains. Biosci Rep 26: 399–412, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Deerinck TJ, Giepmans BN, Smarr BL, Martone ME, Ellisman MH. Light and electron microscopic localization of multiple proteins using quantum dots. Methods Mol Biol 374: 43–53, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Devor DC, Singh AK, Bridges RJ, Frizzell RA. Modulation of Cl− secretion by benzimidazolones. II. Coordinate regulation of apical GCl and basolateral GK. Am J Physiol Lung Cell Mol Physiol 271: L785–L795, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am J Physiol Lung Cell Mol Physiol 271: L775–L784, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, Hoyer J, Kohler R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol 138: 594–601, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 117: 139–155, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann Med 39: 495–516, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Fisher RD, Chung HY, Zhai Q, Robinson H, Sundquist WI, Hill CP. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128: 841–852, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Fujita H, Yamanaka M, Imamura K, Tanaka Y, Nara A, Yoshimori T, Yokota S, Himeno M. A dominant negative form of the AAA ATPase SKD1/VPS4 impairs membrane trafficking out of endosomal/lysosomal compartments: class E vps phenotype in mammalian cells. J Cell Sci 116: 401–414, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Gao Y, Balut CM, Bailey MA, Patino-Lopez G, Shaw S, Devor DC. Recycling of the Ca2+-activated K+ channel, KCa2.3 is dependent upon RME-1, Rab35/EPI64C and an N-terminal domain. J Biol Chem 285: 17938–17953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Chotoo CK, Balut CM, Sun F, Bailey MA, Devor DC. Role of S3 and S4 transmembrane domain charged amino acids in channel biogenesis and gating of KCa2.3 and KCa31. J Biol Chem 283: 9049–9059, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goila-Gaur R, Demirov DG, Orenstein JM, Ono A, Freed EO. Defects in human immunodeficiency virus budding and endosomal sorting induced by TSG101 overexpression. J Virol 77: 6507–6519, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol 141: 881–903, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol 5: 317–323, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell 17: 1228–1238, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanson PI, Roth R, Lin Y, Heuser JE. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol 180: 389–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinton JM, Langton PD. Inhibition of EDHF by two new combinations of K+-channel inhibitors in rat isolated mesenteric arteries. Br J Pharmacol 138: 1031–1035, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hislop JN, Marley A, Von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J Biol Chem 279: 22522–22531, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20: 4–11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansen JA, de Boer TP, Wolswinkel R, van Veen TA, Vos MA, van Rijen HV, van der Heyden MA. Lysosome mediated Kir2.1 breakdown directly influences inward rectifier current density. Biochem Biophys Res Commun 367: 687–692, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Johnson MC, Spidel JL, Ako-Adjei D, Wills JW, Vogt VM. The C-terminal half of TSG101 blocks Rous sarcoma virus budding and sequesters Gag into unique nonendosomal structures. J Virol 79: 3775–3786, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones HM, Hamilton KL, Devor DC. Role of an S4–S5 linker lysine in the trafficking of the Ca(2+)-activated K(+) channels IK1 and SK3. J Biol Chem 280: 37257–37265, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Jones HM, Hamilton KL, Papworth GD, Syme CA, Watkins SC, Bradbury NA, Devor DC. Role of the NH2 terminus in the assembly and trafficking of the intermediate conductance Ca2+-activated K+ channel hIK1. J Biol Chem 279: 15531–15540, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Katoh K, Shibata H, Suzuki H, Nara A, Ishidoh K, Kominami E, Yoshimori T, Maki M. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J Biol Chem 278: 39104–39113, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 3: 893–905, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control K(ATP) channel surface density. J Biol Chem 285: 5963–5973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke 37: 1277–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Parent A, Hamelin E, Germain P, Parent JL. Rab11 regulates the recycling of the beta2-adrenergic receptor through a direct interaction. Biochem J 418: 163–172, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23: 519–547, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458: 445–452, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell 17: 3469–3483, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robert R, Carlile GW, Liao J, Balghi H, Lesimple P, Liu N, Kus B, Rotin D, Wilke M, de Jonge HR, Scholte BJ, Thomas DY, Hanrahan JW. Correction of the Delta phe508 cystic fibrosis transmembrane conductance regulator trafficking defect by the bioavailable compound glafenine. Mol Pharmacol 77: 922–930, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Roncarati R, Decimo I, Fumagalli G. Assembly and trafficking of human small conductance Ca2+-activated K+ channel SK3 are governed by different molecular domains. Mol Cell Neurosci 28: 314–325, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Sachse M, Strous GJ, Klumperman J. ATPase-deficient hVPS4 impairs formation of internal endosomal vesicles and stabilizes bilayered clathrin coats on endosomal vacuoles. J Cell Sci 117: 1699–1708, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Saksena S, Wahlman J, Teis D, Johnson AE, Emr SD. Functional reconstitution of ESCRT-III assembly and disassembly. Cell 136: 97–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci 25: 10930–10940, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schumacher SM, Martens JR. Ion channel trafficking: a new therapeutic horizon for atrial fibrillation. Heart Rhythm 7: 1309–1315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res 99: 537–544, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Singh AK, Devor DC, Gerlach AC, Gondor M, Pilewski JM, Bridges RJ. Stimulation of Cl(−) secretion by chlorzoxazone. J Pharmacol Exp Ther 292: 778–787, 2000 [PubMed] [Google Scholar]
- 58.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther 296: 600–611, 2001 [PubMed] [Google Scholar]
- 59.Spinosa MR, Progida C, De Luca A, Colucci AM, Alifano P, Bucci C. Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. J Neurosci 28: 1640–1648, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staudacher I, Schweizer PA, Katus HA, Thomas D. hERG: protein trafficking and potential for therapy and drug side effects. Curr Opin Drug Discov Devel 13: 23–30, 2010 [PubMed] [Google Scholar]
- 61.Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10: 925–937, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 278: C570–C581, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Syme CA, Hamilton KL, Jones HM, Gerlach AC, Giltinan L, Papworth GD, Watkins SC, Bradbury NA, Devor DC. Trafficking of the Ca2+-activated K+ channel, hIK1, is dependent upon a C-terminal leucine zipper. J Biol Chem 278: 8476–8486, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93: 124–131, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196: 193–222, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Vanlandingham PA, Ceresa BP. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J Biol Chem 284: 12110–12124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, Scott A, Krausslich HG, Kaplan J, Morham SG, Sundquist WI. The protein network of HIV budding. Cell 114: 701–713, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Walker SD, Dora KA, Ings NT, Crane GJ, Garland CJ. Activation of endothelial cell IK(Ca) with 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery. Br J Pharmacol 134: 1548–1554, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature 458: 172–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem 14: 1437–1457, 2007 [DOI] [PubMed] [Google Scholar]