Abstract
Previous studies in mouse pulmonary arterial smooth muscle cells (PASMCs) showed that cannonical transient receptor potential channel TRPC1 and stromal interaction molecule 1 (STIM1) mediate the sustained component of capacitative Ca2+ entry (CCE), but the molecular candidate(s) that mediate the transient component of CCE remain unknown. The aim of the present study was to examine whether Orai1 mediates the transient component of CCE through activation of STIM1 in mouse PASMCs. In primary cultured mouse PASMCs loaded with fura-2, cyclopiazonic acid (CPA) caused a transient followed by a sustained rise in intracellular Ca2+ concentration ([Ca2+]i). The transient but not the sustained rise in [Ca2+]i was partially inhibited by nifedipine. The nifedipine-insensitive transient rise in [Ca2+]i and the increase in Mn2+ quench of fura-2 fluorescence caused by CPA were both reduced in cells treated with Orai1 siRNA. These responses to CPA were further reduced in cells treated with Orai1 and STIM1 small interfering (si)RNA. Moreover, overexpression of STIM1 enhanced the rise in [Ca2+]i and the increase in Mn2+ quench of fura-2 fluorescence caused by CPA, and these responses were reduced in cells treated with Orai1 siRNA. RT-PCR revealed Orai1 and STIM1 mRNAs, and Western blot analysis identified Orai1 and STIM1 proteins in mouse PASMCs. Furthermore, Orai1 was found to coimmunoprecipitate with STIM1, and the precipitation level of Orai1 was increased in cells subjected to store-depletion. Immunostaining revealed colocalization of Orai1 and STIM1 proteins, and the colocalization of these proteins was more apparent after store-depletion. These data provide direct evidence that the transient component of CCE is mediated by Orai1 channel as a result of STIM1 activation in mouse PASMCs.
Keywords: stromal interaction molecule 1, store-depletion, intracellular Ca2+ concentration
over the past 10 years, Ca2+ entry through store-operated channels [SOCs; so-called capacitative Ca2+ entry, CCE] was shown to play an important role in the regulation of vascular smooth muscle tone, cell proliferation, and migration (9, 10, 18, 30, 37). CCE is activated in response to a decrease in intracellular sarcoplasmic reticulum (SR) Ca2+ concentration. This can be achieved by depletion of Ca2+ from the SR induced by agonists activating receptors coupled to the inositol 1,4,5-trisphosphate (IP3) signaling pathway or by agents that inhibit the SR Ca2+-ATPase (SERCA), such as cyclopiazonic acid (CPA) or thapsigargin (2, 17, 35). So far, the best-characterized SOCs are the Ca2+ release-activated Ca2+ (CRAC) channels, which are the predominant Ca2+ channels in nonexcitable cells. This channel is highly selective to Ca2+, and the channel has a very low unitary conductance of <1 pS (see Ref. 35 for review). In contrast, SOCs in vascular smooth muscle cells appear to be nonselective cation channels and have an unitary conductance of 2–5 pS, suggesting a heterogeneity in SOCs in different cell types (1, 10, 30, 40, 41, 49). Nevertheless, the molecular composition of SOCs and the molecular mechanism underlying the activation of these channels in vascular smooth muscle remain unclear.
The identification of seven mammalian canonical transient receptor potential (TRPC) subunits, which form cation channels, has led to a better understanding of the molecular makeup of SOCs (3, 35, 36). Accumulating studies have confirmed the existence of TRPC channels in various vascular preparations (3, 17). Among all TRPC members, TRPC1 has been extensively studied owing to its relatively abundant expression in vascular smooth muscle, including pulmonary artery smooth muscle cells (PASMCs) (24, 30, 51, 52). There is increasing evidence that TRPC1 functions as SOC in pulmonary arteries. In human PASMCs, CCE is enhanced in proliferative cells (9) and this is associated with an increase in TRPC1 expression in these cells (10). In addition, TRPC1 gene expression and CCE were significantly reduced in human PASMCs treated with TRPC1 antisense (46). Overexpression of human TRPC1 in rat pulmonary arteries enhanced the contractile responses to CPA (16). Knockdown of TRPC1 protein with small interfering (si)RNA inhibited cation influx caused by thapsigargin in rat PASMCs (22), providing further support that TRPC1 is an important molecular candidate for SOCs in PASMCs. More recently, our study in mouse PASMCs also suggests that TRPC1 is an essential component of SOCs, but TRPC1 was found to only partially mediate CCE (32). Thus, it is possible that TRPC1 may form homotetramer or heterotetramer with other TRPC channels, or interact with other transmembrane protein(s) to form SOCs.
The recent discovery of two transmembrane proteins, Orai1 and stromal interaction molecule 1 (STIM1) in nonexcitable cells, has opened a new direction toward the search for the molecular makeup of SOCs and a molecular intermediate that activates these channels. Orai1 was shown to be a pore subunit of CRAC channels (8, 38), whereas STIM1 was found to act as a sensor within the stores (39, 59) and may also play a role in the plasma membrane (45, 59) to activate CRAC current. In addition, coexpression of STIM1 and Orai1 results in a significant gain in CRAC channel function, suggesting that STIM1 interacts with Orai1 to cause CCE (29, 43, 44). To date, there is very little evidence for the roles of Orai1 and STIM1 in vascular smooth muscle cells. STIM1 was shown to express in cultured coronary artery smooth muscle cells (47), saphenous vein cells (18), mesenteric artery smooth muscle cells (5), aorta smooth muscle cells (5, 7, 37), and PASMCs (24, 32). siRNA targeting STIM1 resulted in reduction of Ca2+ entry and whole cell current activated by CPA or thapsigargin (18, 25, 32, 37, 47). On the other hand, Orai1 was found to express in cultured mesenteric artery smooth muscle cells (5) and cultured aorta smooth muscle cells (4, 5, 37). In addition, siRNA knockdown of Orai1 causes inhibition of Ca2+ entry and whole cell current activated by store-depletion in cultured aorta smooth muscle cells (4, 37). These findings suggest an important role of Orai1 and STIM1 in mediating CCE in vascular smooth muscle cells. However, there is no evidence for the existence of Orai1 in PASMCs, and the functional role of Orai1 in PASMCs has not been reported.
In vascular smooth muscle cells, STIM1 and TRPC1 are found to associate with one another in the activation of SOCs in cultured human coronary smooth muscle cells (48) and cultured human saphenous vein cells (18). On the other hand, STIM1 and Orai1 were recently found to mediate store-operated currents in cultured rat aorta smooth muscle cells (37). Our recent study in cultured mouse PASMCs reveals two Ca2+ components of CCE, a transient followed by a sustained Ca2+ component, and TRPC1 was shown to associate with STIM1 to mediate the sustained component (32). However, the molecular mechanism underlying the transient Ca2+ component has not been identified. The aims of the present study were to investigate whether Orai1 plays a role in mouse PASMCs and to determine whether Orai1 mediates the transient Ca2+ component of CCE as a result of STIM1 activation in these cells.
MATERIALS AND METHODS
PASMCs isolation and cell culture.
Male C57BL/6 mice were killed by inhalation of 5% isoflurane in oxygen followed by cervical dislocation, as approved by the University of Nevada Reno Institutional Animal Care and Use Committee. The heart and lungs were removed, and second and third branches of the intrapulmonary artery were dissected in a low-Ca2+ physiological salt solution (PSS) composed of the following (in mM): 125 NaCl, 5.36 KCl, 0.34 Na2HPO4, 0.44 K2HPO4, 1.2 MgCl2, 11 HEPES, 10 glucose, and 0.05 CaCl2 (pH 7.4 adjusted with Tris). To disperse cells, pulmonary arterial tissue was incubated with the low-Ca2+ PSS containing (in mg/ml) 1 collagenase type XI, 2 trypsin inhibitor, 0.45 protease, 1.3 taurine, and 2 bovine serum albumin (fat free) for 30 min at 5°C followed by 8 min at 33°C. The tissue was then transferred to an enzyme-free, low-Ca2+ PSS and triturated with a fire-polished Pasteur pipette. The resulting dispersed PASMCs were subjected to cell culture as previously described (32). Freshly dispersed PASMCs were plated onto a 60-mm cell culture dish and incubated with DMEM cell culture medium containing 10% newborn calf serum (NCS), penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were incubated in a humidified atmosphere of 5% CO2 in air at 37°C and grown to 90–95% confluence. These primary cultured cells were then trypsinized and passaged onto a coverslip and grown to 70–80% confluence. Confluent cells were then growth arrested in 0.1% NCS medium for 24 h before experimental use.
Measurement of intracellular Ca2+.
The cytosolic Ca2+ concentration was estimated in PASMCs loaded with fura-2 acetoxymethyl ester (fura-2 AM) (Molecular Probes, Eugene, OR) using a dual excitation digital Ca2+ imaging system (IonOptix, Milton, MA) equipped with an intensified charge-coupled device (CCD) camera as previously described (31, 32). PASMCs were loaded with 10 μM fura-2 AM for 1 h in the dark at room temperature and placed on the coverslip of a 0.2-ml perfusion chamber mounted on an inverted epifluorescence microscope (Nikon) outfitted with a ×40 oil immersion objective (numerical aperture 1.3, Nikon). Cells were washed several times at 1 ml/min to remove extracellular fura-2 AM with 2 mM Ca2+-PSS composed of the following (in mM): 126 NaCl, 5 KCl, 0.3 NaH2PO4, 10 HEPES, 1 MgCl2, 2 CaCl2, and 10 glucose (pH 7.4 with NaOH). Cells were illuminated with xenon arc lamp at 340 ± 15 and 380 ± 12 nm (Omega Optical, Brattleboro, VT), and emitted light was collected from regions that encompassed single cells with a CCD camera at 510 nm (Nikon). Images were acquired at 1 Hz and stored on the compact disk for later analysis. Background fluorescence was collected automatically and subtracted from the acquired fluorescence video images during each experiment. The ratio of fluorescence (R) excited at the two excitation wavelengths was used to estimate intracellular Ca2+ concentration ([Ca2+]i) as described by Grynkiewicz et al. (11):
The values for Sf2 (fluorescence measured at 380 nm in Ca2+-free solution), Sb2 (fluorescence measured at 380 nm in Ca2+-saturating conditions), Rmin (minimum ratio), and Rmax (maximum ratio) were determined from in situ calibrations of fura-2 for each cell. The dissociation constant for Ca2+ binding, Kd, was assumed to be 224 nM (11). To determine Rmin, cells were dialyzed with 4 μM ionomycin in Ca2+-free PSS containing 10 mM EGTA at the end of each experiment. Rmax was determined from cells dialyzed with 4 μM ionomycin in PSS containing 10 mM CaCl2. ΔR is the change in fluorescence ratio by subtracting the fluorescence ratio from the basal fluorescence ratio. Δ[Ca2+]i is the change in [Ca2+]i by subtracting the estimated [Ca2+]i from the basal [Ca2+]i.
In experiments where the effects of store-depletion were investigated, CPA was used to deplete the SR Ca2+ stores in Ca2+-free PSS followed by reexposure of cells with 2 mM Ca2+-PSS as previously described (31, 32). An elevation in [Ca2+]i above basal levels during 2 mM Ca2+ readdition was used as a marker of CCE-mediated extracellular Ca2+ entry. In experiments where the Ca2+ influx through SOCs was studied, the rate of Mn2+-induced quenching of fura-2 fluorescence was recorded during excitation at 360 nm in nominally Ca2+-free PSS containing nifedipine (31, 32).
Total RNA isolation and RT-PCR.
Total RNA was isolated from cultured mouse PASMCs using TRIzol reagent (Invitrogen, Carlsbad, CA) as previously described (32). First-strand cDNA was prepared from the RNA preparations by using Superscript III Reverse Transcriptase (Invitrogen). The resulting cDNA was then amplified by PCR with primers specific for mouse Orai1 (sense, 5′-GGCCAGAGTTACTCCGAGGTGATG-3′; antisense, 5′-GGCAGGATGCAGGTGCTGATC-3′) and STIM1 (sense, 5′-CAATGGTGATGTGGATGTGGAAGA-3′; antisense, 5′-AGTAACGGTTCTGGATATAGGCAAACC-3′). Primers for mouse β-actin (sense, 5′-TGGCTACAGCTTCACCACC-3′; antisense, 5′-ACTCCTGCTTGCTGATCCAC-3′) were used as an internal control. The amplification cycle parameters were 95°C for 10 min, 35 cycles at 95°C for 30 s (denaturation), 58°C for 30 s (annealing), and 72°C for 45 s (extension). Sample was then heated at 72°C for 7 min to ensure complete product extension. For reverse transcription (RT) controls, reverse transcriptase was omitted from cDNA reaction. Amplified products were resolved by gel electrophoresis, purified, and verified by sequencing.
Transfection of PASMCs with siRNAs.
PASMCs were transiently transfected with Orai1 siRNA (ID: s99511, Silencer Select Pre-designed siRNA, Ambion, Austin, TX) and/or STIM1 siRNA (ID: s74488, Silencer Select Pre-designed siRNA) using siPORT Amine transfection reagent (Ambion) as previously described (32). For every 35-mm culture dish of cells, 10 μl of STIM1 siRNA (50 μM) was diluted in 90 μl of OPTIMEM I (Invitrogen). Then, 10 μl of siPORT Amine was diluted in 90 μl of OPTIMEM I and mixed with the diluted siRNA. The mixture (200 μl) was incubated at room temperature for 20 min to allow formation of transfection complexes. Primary cultured PASMCs were then trypsinized and incubated in DMEM cell culture medium containing 10% NCS and antibiotics, and the cells were subsequently passaged onto three 35-mm cell culture dishes. To each culture dish, the transfection complexes were added onto the cells to give a final volume of 2.5 ml in growth medium and a final concentration of 200 nM siRNA. The cells were incubated with the transfection complexes at 37°C for 24 h and grown to 70–80% confluence. The cells were then washed with fresh medium containing 10% NCS for 24 h and then growth arrested in medium containing 0.1% NCS at 37°C for another 24 h before experimental use. For negative control, the cells were transfected with a scrambled siRNA (Silencer Negative Control no. 1 siRNA, Ambion) using the same transfection method.
Generation of recombinant STIM1 adenovirus.
STIM1 adenoviruses were generated as previously described (32). STIM1 cDNA was isolated from mouse brain and cloned into pcDNA3.1, and the STIM1 construct was confirmed using terminator cycle sequencing. Recombinant adenoviruses for STIM1 were then produced in a pAdTrack-CMV/pAdEasy recombinant containing green fluorescent protein (Ad-GFP-STIM1), purified, and amplified by using the AdEasy adenoviral vector system (Stratagene, La Jolla, CA). To produce adenoviruses, the STIM1 adenovirus recombinants were transfected into a viral packaging cell line using the MBS mammalian transfection kit (Stratagene). Adenoviruses were then harvested, plaque-purified, and titered by an agarose overlay plaque assay. The same procedure was used to generate a control adenovirus containing GFP (Ad-GFP) with no insertion of STIM1 gene. For infection, cultured PASMCs were incubated with adenovirus in DMEM containing 0.1% NCS for 24 h. The cells were then washed with fresh 0.1% NCS medium for another 24 h. Infected cells were monitored by observing the number of green cells under fluorescence microscope and were subsequently used for calcium imaging study or Western blot analysis.
Western blot analysis, coimmunoprecipitation, and colocalization studies.
Total protein was obtained from cultured mouse PASMCs by using RIPA extraction buffer containing protease and phosphatase inhibitors as previously described (32). For Western blot analysis, equal amounts of total protein (40 μg) were resolved by 12% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes for 90 min at 24 V (Genie blotter, Idea Scientific, Minneapolis, MN). The membranes were then blocked for 1 h with LI-COR blocking solution (LI-COR, Lincoln, NE) and probed with a rabbit polyclonal Orai1 antibody (1:100, ProSci, Poway, CA) or mouse monoclonal STIM1 antibody (1:100, BD Biosciences, San Jose, CA). The membranes were simultaneously probed with goat polyclonal GAPDH antibody (1:20,000, Santa Cruz Biotechnology, CA, USA) as an internal control. The primary antibodies were incubated overnight at 4°C and after washout, membranes were incubated with two secondary antibodies in LI-COR solution for 45 min at room temperature: one coupled to an infrared fluorescence marker with emission wavelength of 800 nm (1:100,000; IR 800, Rockland Immunochemicals, Gilbertsville, PA), and the other coupled to an infrared fluorescence marker with emission wavelength of 680 nm (1:100,000; Alexa Fluor 680, Molecular Probes, Eugene, OR). Immunoblots were then scanned to obtain double-color fluorescent images with an Odyssey scanner (LI-COR).
For coimmunoprecipitation of STIM1 and Orai1, 0.40 mg of total protein was first diluted with an equal volume of PSS (with protease inhibitors) and mixed with 10 μg of Stim1 antibody (EXBIO, Czech Republic), and incubated with agitation at 4°C for 2 h. Then, 100 μl of slurry of agarose beads conjugated to goat-anti-mouse antibodies (Sigma) was washed with 1 ml PSS and incubated overnight with the protein/antibody complex at 4°C on an end-over-end mixer. The beads-protein-antibody complex was then washed three times with 1 ml of PSS. The protein was released from the beads by adding 35 μl of 4× SDS loading buffer and incubated for 20 min at room temperature before loading on a 12% SDS gel. After gel electrophoresis, the separated protein was transferred onto nitrocellulose membrane. To demonstrate immunoprecipitation of STIM1, the blot was probed with Stim1 antibody (1:100; BD Biosciences). To demonstrate coimmunoprecipitation of STIM1 and Orai1, the blot was subsequently probed with Orai1 antibody (1:100, ProSci).
For colocalization study, dual labeling of endogenous Orai1 and STIM1 proteins was performed using immunostaining method as previously described (31). The cultured mouse PASMCs were fixed in 4% paraformaldehyde and stained with a rabbit polyclonal Orai1 antibody (1:100, ProSci) and mouse monoclonal STIM1 antibody (1:100, EXBIO) using Vector M.O.M. immunodetection kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instruction. A secondary antibody conjugated with Alexa Flour 488 (1:200, Molecular Probes) was used to display Orai1 fluorescence image (excited at 495 nm and emitted at 519 nm), and secondary antibody conjugated with Alexa Flour 546 (1:200, Molecular Probes) was used to display STIM1 fluorescence image (excited at 556 nm and emitted at 573 nm). For control experiments, the cells were treated similarly in the absence of primary antibodies. The cells were then mounted in Vectashield mounting medium containing propidium iodide (Vector). Propidium iodide was used to stain cell nuclei and was excited at 535 nm and emitted at 615 nm to visualize the cell nuclei. The cells were examined under a Bio-Rad Radiance 2100 inverted laser-scanning confocal microscope with a Nikon Plan-Fluor ×60 oil immersion objective.
Drug solutions and data analysis.
CPA, nifedipine, and MnCl2 were obtained from Sigma-Aldrich (St. Louis, MO). Ionomycin was obtained from Calbiochem (San Diego, CA). Orai1 antibody was obtained from ProSci. STIM1 antibodies were obtained from BD Biosciences and EXBIO. Orai1 siRNA, STIM1 siRNA, and negative control siRNA were obtained from Ambion. CPA, nifedipine, and ionomycin were dissolved in dimethyl sulfoxide. Data are expressed as means ± SE of n cells from at least five cell culture dishes passaged from three primary cultured dishes of separate seedings. Statistical comparisons employed Student's unpaired t-tests or one-way analysis of variance as appropriate. A value of P < 0.05 was considered significant.
RESULTS
Orai1 and STIM1 expression in mouse PASMCs.
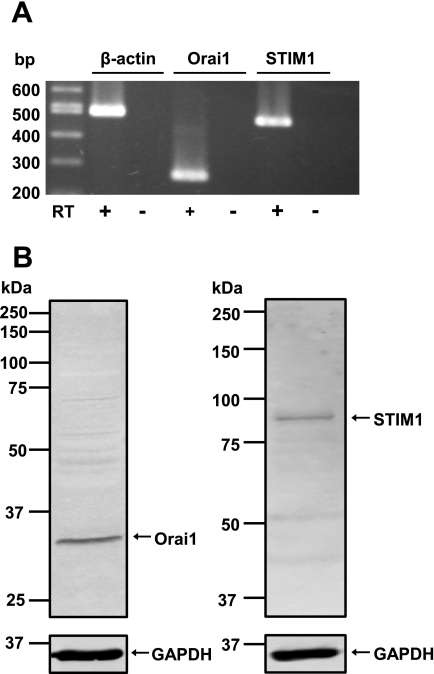
To determine whether endogenous Orai1 and STIM1 express in mouse PASMCs, mRNA and protein expression were detected using RT-PCR and Western blot analysis, respectively. Figure 1A shows that Orai1 and STIM1 mRNA express in cultured mouse PASMCs with a predicted size of 269 bp and 473 bp, respectively, and were confirmed to have the correct nucleotide sequence. Endogenous Orai1 and STIM1 proteins were also detected in cultured mouse PASMCs (Fig. 1B).
Fig. 1.
Orai1 and stromal-interacting molecule 1 (STIM1) expression in mouse pulmonary arterial smooth muscle cells (PASMCs). A: RT-PCR products from cultured mouse PASMCs amplified using primers for mouse Orai1 (269 bp), STIM1 (473 bp), and β-actin (498 bp). Three separate RT-PCR reactions were performed in the presence (+) and absence (−) of reverse transcriptase (RT). B: Orai1, STIM1, and GAPDH proteins were detected in cultured mouse PASMCs using Western blot analysis. Experiments were performed in 5 separate Western blot analyses.
Orai1 mediates CCE in mouse PASMCs.
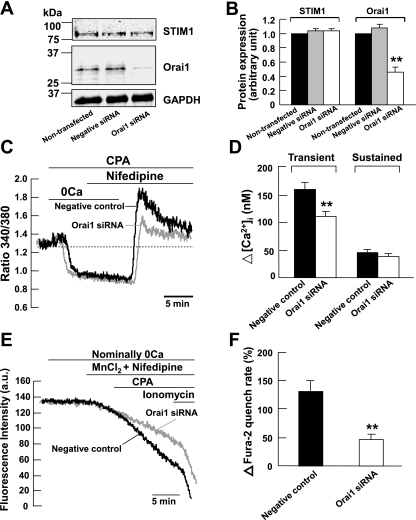
We previously found in cultured mouse PASMCs that depletion of intracellular Ca2+ stores caused two distinct CCE components: a dihydropyridine-insensitive transient increase in [Ca2+]i followed by a sustained increased in [Ca2+]i that was mediated by TRPC1 channels (32). To determine whether Orai1 channels are responsible for the transient component of CCE in mouse PASMCs, the effects of Orai1 knockdown were investigated in cells subjected to store-depletion in the presence of 10 μM nifedipine. We first verified whether siRNA knockdown of Orai1 mRNA reduced the expression level of Orai1 protein in cultured mouse PASMCs. Figure 2, A and B, shows that endogenous Orai1 protein was detected at similar levels in nontransfected cells and in cells transfected with 200 nM scrambled siRNA (negative control). The protein level was significantly reduced by ∼54% in cells transfected with 200 nM Orai1 siRNA compared with nontransfected cells and cells transfected with scrambled siRNA (P < 0.01). We then examined the effect of Orai1 siRNA on CPA-induced rise in [Ca2+]i in the presence of 10 μM nifedipine. Figure 2C shows that CPA caused an increase in nifedipine-insensitive transient and sustained rise in [Ca2+]i in cells transfected with 200 nM scrambled siRNA (negative control). When cells were superfused with Ca2+-free PSS containing 10 μM CPA, an early transient increase in [Ca2+]i was observed indicative of Ca2+ release from the intracellular stores. This early transient rise in [Ca2+]i decayed slowly to a mean level below baseline. Subsequent addition of 2 mM Ca2+ in the presence of CPA elicited a significant transient rise in [Ca2+]i of 160 ± 12 nM (ΔR = 0.67 ± 0.02) followed by a sustained rise in [Ca2+]i of 45 ± 6 nM (ΔR = 0.35 ± 0.01) above basal levels (Fig. 2, C and D; n = 89). The transient increase in [Ca2+]i was significantly reduced in Orai1 siRNA-transfected cells to 111 ± 9 nM (ΔR = 0.47 ± 0.02, n = 86, P < 0.01) (Fig. 2, C and D). The sustained increase in [Ca2+]i was not affected in Orai1 siRNA-transfected cells (39 ± 5 nM, ΔR = 0.35 ± 0.01, n = 86, P > 0.05) (Fig. 2, C and D).
Fig. 2.
Orai1 mediates capacitative Ca2+ entry (CCE) in mouse PASMCs. A and B: Orai1 protein and GAPDH were detected in nontransfected mouse PASMCs and in PASMCs transfected with 200 nM scrambled small interfering RNA (siRNA; negative control). The expression of Orai1 but not GAPDH was reduced significantly in cells transfected with 200 nM Orai1 siRNA. Experiments were performed in 3 separate Western blot analyses (**P < 0.01, ANOVA). C: siRNA knockdown of Orai1 reduced the cyclopiazonic acid (CPA)-induced transient but not the sustained increase in fura-2 fluorescence ratio in the presence of 10 μM nifedipine. 0Ca, Ca2+-free solution. D: bar graph showing mean changes in transient and sustained increase in intracellular Ca2+ concentration ([Ca2+]i) caused by 10 μM CPA after readdition of 2 mM Ca2+ in the presence of 10 μM nifedipine, in negative control cells (filled bars, n = 89), and in Orai1 siRNA-transfected cells (open bars, n = 86). **P < 0.01 (unpaired t-test). E: siRNA knockdown of Orai1 reduced the increase in Mn2+ quench of fura-2 fluorescence caused by 10 μM CPA in the presence of 10 μM nifedipine. AU, arbitrary units. F: bar graph showing percentage change in fura-2 quench rate after store-depletion in the presence of 10 μM nifedipine, in negative control cells (filled bar, n = 79), and in Orai1 siRNA-transfected cells (open bar, n = 86). **P < 0.01 (unpaired t-test).
We previously found in mouse PASMCs that store-depletion caused divalent cation entry through activation of CCE (32). To confirm that endogenous Orai1 mediates CCE in mouse PASMCs, we compared the effects of CPA on Mn2+ quench of fura-2 fluorescence between control cells transfected with scrambled siRNA and cells transfected with Orai1 siRNA. Figure 2E shows the fluorescence intensity recorded at an excitation wavelength of 360 nm in a single PASMC. Removal of extracellular Ca2+ did not cause any decline in fluorescence intensity. The addition of 30 μM MnCl2 in the presence of nifedipine caused the fluorescence to decline slightly. Subsequent depletion of the SR Ca2+ stores by 10 μM CPA resulted in a 132 ± 19% (Fig. 2F, n = 79) increase in the rate of decline of fluorescence in negative control cells, corresponding to enhanced Mn2+ quench of fura-2 indicative of store-depletion activated Ca2+ entry (32). This increase in Mn2+ quench rate was significantly reduced to 46 ± 10% (Fig. 2, E and F, n = 86, P < 0.01) in cells transfected with 200 nM Orai1 siRNA.
STIM1 functionally associates with Orai1 to mediate CCE in mouse PASMCs.
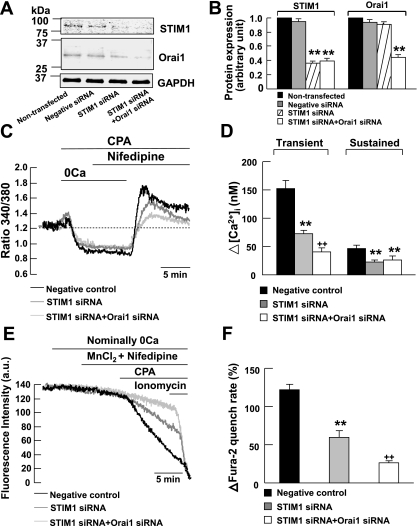
To examine whether endogenous STIM1 protein functionally associates with Orai1 and contributes to the transient component of CCE, we compared the effects of 10 μM CPA in cells transfected with STIM1 and Orai1 siRNAs to cells transfected with scrambled siRNA (negative control) and cells transfected with STIM1 siRNA. We first verified whether siRNA knockdown of STIM1 and Orai1 mRNAs reduced the expression level of STIM1 and Orai1 proteins in cultured mouse PASMCs. Figure 3, A and B, shows that endogenous STIM1 and Orai1 proteins were detected in nontransfected cells and their expression levels were not affected in cells transfected with 200 nM scrambled siRNA (negative control). In cells transfected with 200 nM STIM1 siRNA, STIM1 but not Orai1 protein level was significantly reduced by ∼64% compared with nontransfected cells and negative control cells (P < 0.01). Both STIM1 and Orai1 protein level was significantly reduced by 61% and 56%, respectively, in cells transfected with 200 nM STIM1 siRNA and 200 nM Orai1 siRNA compared with nontransfected cells and negative control cells (P < 0.01).
Fig. 3.
STIM1 is associated with Orai1 to mediate CCE in mouse PASMCs. A and B: STIM1, Orai1, and GAPDH proteins were detected in nontransfected mouse PASMCs and in PASMCs transfected with 200 nM scrambled siRNA (negative control). The expression of STIM1 but not Orai1 or GAPDH was reduced significantly in cells transfected with 200 nM STIM1 siRNA. The expressions of STIM1 and Orai1 but not GAPDH were reduced significantly in cells transfected with both 200 nM STIM1 siRNA and 200 nM Orai1 siRNA. Experiments were performed in 3 separate Western blot analyses (**P < 0.01, ANOVA). C: siRNA knockdown of STIM1 reduced the CPA-induced transient and sustained increase in fura-2 fluorescence ratio in the presence of 10 μM nifedipine. siRNA knockdown of STIM1 and Orai1 further reduced the CPA-induced transient but not sustained increase in fura-2 fluorescence ratio in the presence of nifedipine. D: bar graph showing mean changes in transient and sustained increase in [Ca2+]i caused by 10 μM CPA after readdition of 2 mM Ca2+ in the presence of 10 μM nifedipine, in negative control cells (filled bars, n = 103), in STIM1 siRNA-transfected cells (shaded bars, n = 148), and in STIM1 siRNA and Orai1 siRNA-transfected cells (open bars, n = 70). **P < 0.01, compared with negative control cells (ANOVA); ++P < 0.01, compared with negative control cells and STIM1 siRNA-transfected cells (ANOVA). E: siRNA knockdown of STIM1 reduced the increase in Mn2+ quench of fura-2 fluorescence caused by 10 μM CPA in the presence of 10 μM nifedipine. siRNA knockdown of STIM1 and Orai1 further reduced the increase in Mn2+ quench of fura-2 fluorescence caused by CPA in the presence of nifedipine. F: bar graph showing percentage change in fura-2 quench rate after store-depletion in the presence of 10 μM nifedipine, in negative control cells (filled bar, n = 125), in STIM1 siRNA-transfected cells (shaded bar, n = 151), and in STIM1 siRNA and Orai1 siRNA-transfected cells (open bar, n = 137). **P < 0.01, compared with negative control cells (ANOVA); ++P < 0.01, compared with the negative control cells and STIM1 siRNA-transfected cells (ANOVA).
In mouse PASMCs, endogenous STIM1 mediates the transient and sustained components of CCE (32). To confirm our previous finding, we examined the effect of STIM1 siRNA on CPA-induced rise in [Ca2+]i in the presence of 10 μM nifedipine. Figure 3C shows that 10 μM CPA caused an increase in nifedipine-insensitive transient and sustained rise in [Ca2+]i in cells transfected with 200 nM scrambled siRNA (negative control). Both transient and sustained increase in [Ca2+]i were significantly reduced in STIM1 siRNA-transfected cells from 152 ± 15 nM (ΔR = 0.40 ± 0.05, n = 103) to 73 ± 6 nM (ΔR = 0.22 ± 0.02, n = 148, P < 0.01) and 46 ± 6 nM (ΔR = 0.18 ± 0.02, n = 103) to 23 ± 3 nM (ΔR = 0.08 ± 0.01, n = 148, P < 0.01), respectively (Fig. 3, C and D). Interestingly, the transient but not sustained increase in [Ca2+]i was further reduced to 41 ± 7 nM (ΔR = 0.15 ± 0.03, n = 70, P < 0.01) in cells transfected with STIM1 and Orai1 siRNAs (Fig. 3, C and D).
To confirm that endogenous STIM1 functionally associated with Orai1 to mediate the transient component of CCE in mouse PASMCs, we compared the effects of CPA on Mn2+ quench of fura-2 fluorescence among control cells transfected with scrambled siRNA, cells transfected with STIM1 siRNA, and cells transfected with STIM1 and Orai1 siRNAs. Figure 3E shows that 10 μM CPA caused a 122 ± 7% (n = 125) increase in Mn2+ quench of fura-2 in the presence of 10 μM nifedipine in negative control cells. The increase in Mn2+ quench rate was significantly reduced to 60 ± 8% (Fig. 3, E and F, n = 151, P < 0.01) in cells transfected with 200 nM STIM1 siRNA. This increase in Mn2+ quench rate was further reduced to 26 ± 3% (Fig. 3, E and F, n = 137, P < 0.01) in cells transfected with 200 nM STIM1 siRNA and 200 nM Orai1 siRNA.
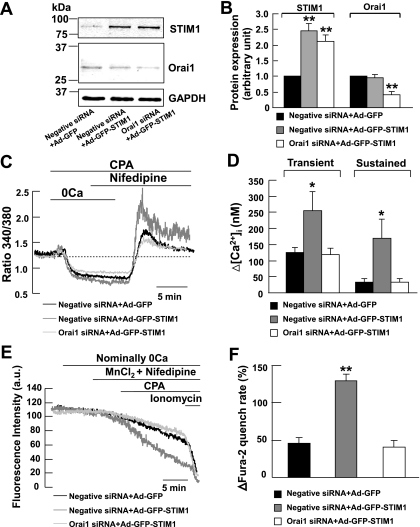
Overexpression of STIM1 in mouse PASMCs significantly increased the transient and sustained components of CCE (32). To further confirm the functional interaction between STIM1 and Orai1 in the contribution of CCE, we studied the effects of 10 μM CPA in the presence of 10 μM nifedipine in STIM1-overexpressing cells transfected with Orai1 siRNA. Figure 4, A and B, shows that endogenous STIM1 and Orai1 proteins were detected in scrambled siRNA transfected cells (negative siRNA) infected with adenovirus containing GFP (Ad-GFP). The protein level of STIM1 but not Orai1 was significantly increased by about 2- to 2.5-fold in cells infected with STIM1-GFP adenovirus (Ad-GFP-STIM1) as compared with Ad-GFP cells (P < 0.01). In Ad-GFP-STIM1 cells transfected with 200 nM Orai1 siRNA, the Orai1 expression level was reduced by ∼59% compared with Ad-GFP-STIM1 cells or Ad-GFP cells transfected with 200 nM scrambled siRNA (P < 0.01).
Fig. 4.
knockdown of Orai1 reduced CCE in STIM1-overexpressing mouse PASMCs. A and B: STIM1, Orai1, and GAPDH proteins were detected in cells infected with adenovirus containing green fluorescent protein (Ad-GFP) transfected with 200 nM scrambled siRNA. The expression of STIM1 but not Orai1 or GAPDH increased markedly in cells infected with STIM1-GFP-adenovirus (Ad-GFP-STIM1) transfected with scrambled siRNA. The expression of Orai1 but not STIM1 or GAPDH was reduced significantly in STIM1-overexpressing cells transfected with 200 nM Orai1 siRNA. Experiments were performed in 3 separate Western blot analyses (**P < 0.01, ANOVA). C: overexpression of STIM1 in scrambled siRNA-transfected cells caused an increase in CPA-induced transient and sustained rise in fura-2 fluorescence ratio in the presence of 10 μM nifedipine. The increases in fluorescence ratio were reduced in Orai1 siRNA-transfected cells overexpressed with STIM1. D: bar graph showing mean changes in transient and sustained increase in [Ca2+]i caused by 10 μM CPA after readdition of 2 mM Ca2+ in the presence of 10 μM nifedipine, in GFP-infected cells transfected with scrambled siRNA (filled bars, n = 33), in STIM1-overexpressing cells transfected with scrambled siRNA (shaded bars, n = 65), and in STIM1-overexpressing cells transfected with Orai1 siRNA (open bars, n = 50). *P < 0.05, compared with GFP-infected cells transfected with scrambled siRNA and STIM1-overexpressing cells transfected with Orai1 siRNA (ANOVA). E: overexpression of STIM1 in scrambled siRNA-transfected cells caused a CPA-induced increase in Mn2+ quench of fura-2 fluorescence in the presence of 10 μM nifedipine. The increases in Mn2+ quench of fura-2 fluorescence was reduced in Orai1 siRNA-transfected cells overexpressed with STIM1. F: bar graph showing percentage change in fura-2 quench rate after store-depletion in the presence of 10 μM nifedipine, in GFP-infected cells transfected with scrambled siRNA (filled bars, n = 40), in STIM1-overexpressing cells transfected with scrambled siRNA (shaded bars, n = 95), and in STIM1-overexpressing cells transfected with Orai1 siRNA (open bars, n = 57). **P < 0.01, compared with GFP-infected cells transfected with scrambled siRNA and STIM1-overexpressing cells transfected with Orai1 siRNA (ANOVA).
Figure 4, C and D, shows that 10 μM CPA caused an increase in nifedipine-insensitive transient (125 ± 15 nM, ΔR = 0.40 ± 0.06, n = 33) and sustained rise in [Ca2+]i (32 ± 12 nM, ΔR = 0.11 ± 0.06, n = 33) in Ad-GFP cells transfected with 200 nM scrambled siRNA. Both the transient and sustained rise in [Ca2+]i was significantly increased to 256 ± 58 nM (ΔR = 0.87 ± 0.12, n = 65, P < 0.05) and 169 ± 59 nM (ΔR = 0.45 ± 0.13, n = 65, P < 0.05), respectively, in Ad-GFP-STIM1 cells transfected with 200 nM scrambled siRNA. These transient and sustained increases in [Ca2+]i were respectively reduced to 119 ± 20 nM (ΔR = 0.38 ± 0.09, n = 50, P < 0.05) and 34 ± 11 nM (ΔR = 0.12 ± 0.05, n = 50, P < 0.05) in Ad-GFP-STIM1 cells transfected with 200 nM Orai1 siRNA. To further confirm that STIM1 is associated with Orai1 in mediating CCE in mouse PASMCs, we compared the effects of 10 μM CPA on Mn2+ quench of fura-2 fluorescence among Ad-GFP cells transfected with scrambled siRNA, Ad-GFP-STIM1 cells transfected with scrambled siRNA, and Ad-GFP-STIM1 cells transfected with Orai1 siRNA. In Ad-GFP cells transfected with 200 nM scrambled siRNA, CPA caused a 46 ± 7% (n = 40) increase in Mn2+ quench of fura-2 in the presence of 10 μM nifedipine (Fig. 4, E and F). This increase in Mn2+ quench rate was significantly increased to 130 ± 8% (n = 95, P < 0.01) in Ad-GFP-STIM1 cells transfected with scrambled siRNA (Fig. 4, E and F). Knockdown of Orai1 protein in Ad-GFP-STIM1 cells significantly reduced the Mn2+ quench rate to 41 ± 8% (Fig. 4, E and F; n = 57, P < 0.01).
It is noteworthy that transfection of cells with various siRNAs or infection of cells with adenoviruses did not significantly affect the basal Mn2+ quench rate. In cells transfected with scrambled siRNA (negative control), the basal Mn2+ quench rate was found to be 0.030 ± 0.001 arbitrary units per second (AU/s; n = 204). In cells transfected with Orai1 siRNA, STIM1 siRNA, and both STIM1 siRNA and Orai1 siRNA, the basal Mn2+ quench rates were found to be 0.034 ± 0.002 AU/s (n = 86), 0.039 ± 0.002 AU/s (n = 151), and 0.036 ± 0.001 AU/s (n = 137), respectively. In Ad-GFP cells transfected with scrambled siRNA, the basal Mn2+ quench rate was found to be 0.37 ± 0.004 AU/s (n = 40). In Ad-GFP-STIM1 cells transfected with scrambled siRNA and Ad-GFP-STIM1 cells transfected with Orai1 siRNA, the basal Mn2+ quench rate was found to be 0.041 ± 0.003 AU/s (n = 95) and 0.035 ± 0.004 AU/s (n = 57), respectively. These data show that the basal Mn2+ quench rates were very similar in cells under control conditions and in cells subjected to various gene manipulations and therefore did not affect the comparison and interpretation of the data. However, we noticed a reduction in CPA-induced increase in Mn2+ quench rate in adenovirus-infected cells transfected with scrambled siRNA (Fig. 4F) as compared with noninfected cells transfected with scrambled siRNA (Figs. 2F and 3F). This is likely due to the perturbation of cell function caused by adenovirus. Therefore, to avoid misinterpretation of our data, Ad-GFP cells were used as a control in experiments for comparison of CCE in Ad-GFP-STIM1 cells in the present study because they were both treated under the same infection conditions.
Orai1 and STIM1 form a molecular complex to mediate CCE in mouse PASMCs.
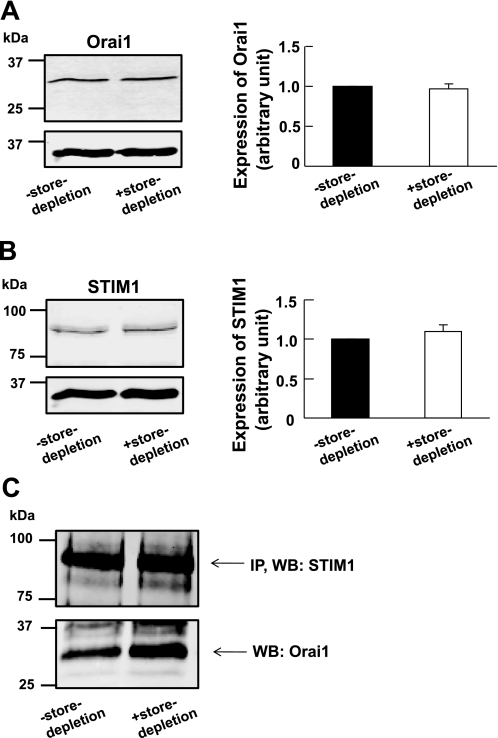
To investigate whether store-depletion affects the expression levels of Orai1 and STIM1, we compared the expression levels of Orai1 and STIM1 between control cells and cells subjected to store-depletion. In cells subjected to store-depletion, the cells were incubated with Ca2+-free PSS containing 10 μM CPA for 10 min. We found that store-depletion did not affect the expression levels of Orai1 (Fig. 5A) or STIM1 (Fig. 5B) as compared with the control cells. To determine whether Stim1 is associated with Orai1 channel in mouse PASMCs, a coimmunoprecipitation study was performed. Figure 5C shows that STIM1 coimmunoprecipitates Orai1, indicating a molecular complex formed between STIM1 proteins and Orai1 channels in mouse PASMCs. Interestingly, more Orai1 was coimmunoprecipitated with STIM1 in cells subjected to store-depletion as compared with the control cells (Fig. 5C). These data suggest that during store-depletion, the association of STIM1 with Orai1 is enhanced in mouse PASMCs.
Fig. 5.
Orai1 coimmunoprecipitates with STIM1 in mouse PASMCs. A, left: Orai1 was detected in cultured mouse PASMCs in the absence and presence of store-depletion. Right: bar graph showing expression levels of Orai1 measured relative to GAPDH in control cells (denoted as 1, filled bar) and in cells subjected to store-depletion (open bar). Data are means ± SE of 7 separate Western blot analyses. B, left: STIM1 was detected in cultured mouse PASMCs in the absence and presence of store-depletion. Right: bar graph showing expression levels of STIM1 measured relative to GAPDH in control cells (denoted as 1, filled bar) and in cells subjected to store-depletion (open bar). Data are means ± SE of 7 separate Western blot analyses. C: STIM1 coimmunoprecipitated Orai1 in cultured mouse PASMCs in the absence and presence of store-depletion. STIM1 was first immunoprecipitated (IP) with EXBIO STIM1 antibody (10 μg), and the blot was subsequently probed with BD Biosciences STIM1 antibody (WB, 1:100). The blot was then probed for coimmunoprecipitation (co-IP) of Orai1 expression using Orai1 antibody (WB, 1:100, ProSci). Experiments were performed in 3 separate co-IP procedures and Western blot analyses.
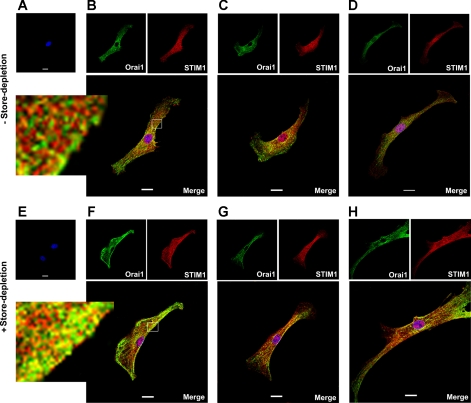
To confirm the physical interaction of Orai1 and STIM1 in the contribution of CCE, we examined colocalization of these proteins in cultured mouse PASMCs. Figure 6A shows no staining of Orai1 or STIM1 in the absence of primary antibodies under control conditions. Figure 6, B–D, shows three single permeabilized PASMCs labeled with Orai1 antibody (green) and STIM1 antibody (red). Partial colocalization of Orai1 and STIM1 (yellow/orange) was found in mouse PASMCs, but independent expression of Orai1 and STIM1 was also apparent. In cells subjected to store-depletion, no staining of Orai1 or STIM1 was found in the absence of primary antibodies (Fig. 6E). Depletion of intracellular Ca2+ stores with CPA appeared to cause redistribution of Orai1 and STIM1 from a diffuse pattern to a more visible aggregated or “punctate” appearance at the cell periphery (Fig. 6, F–H). Colocalization of Orai1 and STIM1 was more apparent (yellow/orange) after store-depletion (Fig. 6, F–H).
Fig. 6.
Colocalization of Orai1 and STIM1 in mouse PASMCs. A–D: staining of mouse cultured PASMCs after exposure of live cells with normal bath physiological salt solution (PSS). A: omission of Orai1 and STIM1 antibody resulted in no Orai1 or STIM1 staining. B–D: three representative cells dual-labeled with anti-Orai1 antibody (green) and STIM1 antibody (red). Orai1 and STIM1 colocalization (yellow/orange) is shown in the merged images. E–H: staining of mouse cultured PASMCs after exposure of live cells with Ca2+-free PSS containing 10 μM CPA. E: omission of Orai1 and STIM1 antibody resulted in no Orai1 or STIM1 staining. F–H: three representative cells dual-labeled with anti-Orai1 antibody (green) and STIM1 antibody (red). Colocalization of Orai1 and STIM1 is more apparent (yellow/orange) after store-depletion as shown in the merged images. Nuclei were stained with DAPI (blue). Experiments were performed in 3 separate immunostaining procedure, each with duplicate coverslips. Scale bars, 20 μm.
DISCUSSION
Earlier work in mouse PASMCs showed that CCE is composed of two distinct Ca2+ components: a dihydropyridine-insensitive transient rise in [Ca2+]i followed by a dihydropyridine-insensitive sustained rise in [Ca2+]i (32). We also found that the sustained CCE component is mediated by TRPC1 channels (32). In the present study we provide the first direct evidence that Orai1 mediates the transient component of CCE as a result of STIM1 activation in mouse PASMCs. This was supported by our functional experiments showing that knockdown of Orai1 proteins using Orai1 siRNA significantly inhibited the transient but not sustained rise in [Ca2+]i, and the increased Mn2+ quench rate caused by CPA was also reduced in these cells (Fig. 2). So far, there is very little evidence that Orai1 functions as SOC in vascular smooth muscle cells. The only evidence that Orai1 mediates ICRAC-like currents and CCE was recently reported in cultured aortic smooth muscle cells (4, 37). However, the existence of Orai1 and functional role of Orai1 have not been studied in pulmonary artery, a unique low-pressure and low- resistance vascular system. The present study provides the first evidence for the existence of endogenous Orai1 proteins in mouse PASMCs and its functional role in mediating CCE in mouse PASMCs.
We also found that STIM1 functionally interacts with Orai1 to mediate CCE in mouse PASMCs. This is indicated by a reduction in the transient rise in [Ca2+]i and Mn2+ quench rate caused by CPA in cells where STIM1 and Orai1 proteins were knocked down (Figs. 2 and 3). In addition, overexpression of STIM1 enhanced the increase in [Ca2+]i and Mn2+ quench rate caused by CPA, and these responses were reduced in cells treated with Orai1 siRNA (Fig. 4). Although STIM1 was previously shown to mediate CCE in coronary arterial smooth muscle cells (47), saphenous vein cells (18), aorta smooth muscle cells (7, 37), and PASMCs (25, 32), our present findings provide the first functional evidence for an interaction between STIM1 and Orai1 in mediating CCE in vascular smooth muscle cells. Another interesting observation in the present study is that the inhibition of CCE in cells treated with both STIM1 siRNA and Orai1 siRNA was additive as compared with cells treated with STIM1 siRNA alone (see Fig. 3). This suggests that Orai1 channel can be activated by other intracellular messengers such as STIM2 besides STIM1. Although it is not our present aim to study the functional role of STIM2, this can be supported by the recent study in cultured rat PASMCs that siRNA knockdown of STIM2 caused a smaller, but significant reduction in CCE as compared with cell knockdown of STIM1 (25). Therefore, knockdown of STIM1 alone may not be sufficient to fully inhibit Orai1 function, but subsequent knockdown of Orai1 proteins further reduces CCE (Fig. 3) because STIM2 may also play a role in activating Orai1 proteins independent of STIM1 in mouse PASMCs.
Recently, two models have been proposed to explain the function of STIM1 in the activation of SOC: an “insertional” model and an “interactional” model (12). The “insertional” model suggests that STIM1 in the endoplasmic reticulum (ER) is translocated to the plasma membrane during store-depletion, which in turn activates the SOCs. This model is supported by the findings that a significant amount of STIM1 was found in the plasma membrane (28, 53, 54) and store-depletion causes increased surface expression of STIM1, suggesting that STIM1 is translocated to the plasma membrane (59). In addition, application of anti-STIM1 antibody raised against the extracellular NH2-terminal STIM1 causes inhibition of store-operated currents, further supporting the role of plasma membrane STIM1 in the activation of SOCs (18, 45). However, many other studies supported the interactional model, which proposes that during store-depletion, STIM1 aggregates and form clusters or so-called “puncta” in junctional ER located 10–25 nm from the plasma membrane so that STIM1 may interact directly with the SOCs in the plasma membrane (23, 26, 27, 50, 56). Indeed, a recent study showed that STIM1 in the ER can activate SOC without being inserted into the membrane but was required for interaction with the STIM1 in the plasma membrane, suggesting a regulatory role of plasma membrane STIM1 in the activation of SOC (13).
One of the most important findings in the present study is that STIM1 physically associated with Orai1 in mouse PASMCs. We found that STIM1 coimmunoprecipitates Orai1 and the precipitation level of Orai1 increased during store-depletion (Fig. 5C). In addition, STIM1 and Orai1 colocalize in mouse PASMCs, and colocalization of these proteins was more apparent after store-depletion (Fig. 6). Interestingly, STIM1 and Orai1 appear to be redistributed and changed to puncta-like structures at the cell periphery after store-depletion (Fig. 6, F–H). Thus, it is very likely that store-depletion causes clustering of STIM1 in the SR and translocates into a localized area of the SR close to the plasma membrane. The puncta-like localization of Orai1 could be due to clustering of Orai1 by STIM1 binding at the SR-plasma membrane junction where the interaction between STIM1 and Orai1 causes the activation of CCE (14, 34, 50, 55, 56). Our present finding that STIM1 and Orai1 form a molecular complex has not been described in vascular smooth muscle cells but it may support the interactional model described in nonexcitable cells.
It is noteworthy that STIM1 coimmunoprecipitates Orai1 even before store-depletion (Fig. 5C). This suggests that STIM1 and Orai1 form a molecular complex under resting conditions, possibly through the interaction of STIM1 and Orai1 in the plasma membrane. This STIM1-Orai1 complex may be constitutively active or stimulated by factors that do not require store-depletion (18). It is also possible that this STIM1-Orai1 complex is not active until the SR Ca2+ stores are depleted leading to the activation of STIM1 in the SR, which interacts with this complex in the plasma membrane and functions as SOC. A similar finding in STIM1 and TRPC1 has also been observed in mouse PASMCs (see Ref. 32, Fig. 9C) and human saphenous vein cells (18) that endogenous STIM1 coimmunoprecipitates TRPC1 in the absence of store-depletion, suggesting that STIM1 and TRPC1 form a molecular complex under resting conditions.
Although Orai1 has been shown to interact with STIM1 and mediate CRAC currents in many nonexcitable cells (e.g., 29, 43), it is unlikely that Orai1 alone could account for the molecular composition of SOCs. Orai1 is a tetra-spanning membrane protein, and it was found to be a pore subunit of the CRAC channel in nonexcitable cells (8, 38, 57). Recently, Orai1 was shown to form a ternary complex with STIM1 and TRPC1 to activate SOCs in human salivary gland cells (6, 33). In human embryonic kidney (HEK) 293 cells, STIM1 was found to bind to TRPC1, TRPC4, and TRPC5 and directly regulate these channels, whereas the regulation of TRPC3 and TRPC6 by STIM1 was mediated by STIM1-dependent heteromultimerization of TRPC3 with TRPC1 and TRPC6 with TRPC4 (58). Furthermore, Orai1 was found to interact with TRPC1 to mediate SOCs as a result of STIM1 activation in HEK 293 cells (15) but Orai1 was also found to interact with the store-depletion-insensitive channels TRPC3, TRPC6, and TRPC7 and confer store-depletion sensitivity to these channels in these cells (19, 20, 21). In mouse PASMCs, we reported a sustained CCE component that was recently shown to be mediated by TRPC1 and STIM1 (32), whereas in this study we show a physical and functional interaction of Orai1 and STIM1 that contributes to the transient component of CCE. Thus, CCE is mediated by STIM1, TRPC1, and Orai1 in PASMCs. However, how the two potentially distinct pore-forming proteins (i.e., TRPC1 and Orai1) contribute to SOC activity is presently unclear. Several possible mechanisms have been proposed: 1) TRPC1/STIM1 and Orai1/STIM1 could function as distinct channels and contribute independently to CCE (42); 2) Orai1 and TRPC1 form distinct channels, whereby Orai1 regulates the function of TRPC1 (6); and 3) TRPC1 and Orai1 form the same channel (6, 15, 33). Although we have no direct evidence to support these possibilities, we would like to propose two possible models for SOC complex in mouse PASMCs, under normal conditions and under conditions in which the PASMCs are overexpressed with STIM1 based on the data in our present study and SOC studies reported by other groups in nonexcitable cells. The first model is that TRPC1 and Orai1 serve as two distinct channels and mediate CCE in cultured mouse PASMCs under normal conditions. When the SR Ca2+ stores are depleted of Ca2+, STIM1 proteins form aggregates in the SR and translocate into a localized area of the SR close to the plasma membrane and interact with Orai1 and TRPC1 independently in the plasma membrane. This may lead to the clustering of Orai1 and TRPC1 to form STIM1-Orai1 and STIM1-TRPC1 complexes, which are recruited in the SR-plasma membrane junction where STIM1-Orai1 and STIM1-TRPC1 function as two distinct channels and contribute independently to CCE (42). This model can be supported by our present study that knockdown of Orai1 protein with Orai1 siRNA reduced the transient but not the sustained component of CCE (Fig. 2) and TRPC1 antibody did not affect the transient but reduced the sustained component of CCE (see Ref. 32, Fig. 4). Another possibility for this model is that Orai1-STIM1 and TRPC1-STIM1 form two distinct channels that interact. How they interact with each other remains unclear, but the pores of the two channels may communicate and sense the function of each other (15). This can be due to activation of these channels by the ions flowing through each channel or that only the open channel conformations can positively affect each other (15). We currently have no evidence to support this idea, but this possibility should be investigated in the future.
The second model applies to cells overexpressing STIM1, in which introduction of exogenous STIM1 to the native system may result in the formation of TRPC1-Orai1-STIM1 complex after store-depletion. The formation of TRPC1-Orai1-STIM1 complex can be due to the increased amount of STIM1 proteins in the STIM1-overexpressing cells, which interact with TRPC1 and Orai1 in the plasma membrane and recruits these proteins from outside to inside of the lipid rafts to form a ternary complex and functions as SOC as proposed by Birnbaumer's group in HEK cells (20, 21) and Ambudkar's group in human salivary gland cells (6, 33). How Orai1 heteromerizes with TRPC1 is unclear, but recruitment of this complex into the lipid rafts depends on STIM1, and without overexpression of STIM1, the assembly of the Orai1-TRPC1-STIM1 complex to form SOC is not favored (21). This model can be supported by our present study that knockdown of Orai1 protein with Orai1 siRNA (Fig. 4) or inhibition of TRPC1 channel function by using TRPC1 antibody (see Ref. 32, Fig. 8) reduced both transient and sustained component of CCE in cells overexpressed with STIM1. Future studies are required to precisely determine the functional and physical association between Orai1 and TRPC1 in PASMCs.
In conclusion, our data provide the first direct evidence in PASMCs that Orai1 interacts with STIM1 and mediates CCE. This molecular complex may contribute significantly to the molecular mechanisms of hypoxic pulmonary vasoconstriction and thus may represent useful targets for the development of new drugs to treat pulmonary hypertension.
GRANTS
This work was supported by National Institutes of Health Grants HL-49254 and P20-RR-15581 from National Center for Research Resources (to J. R. Hume), and an American Heart Association Scientist Development Grant (to L.-C. Ng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Albert AP, Large WA. Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J Physiol 544: 113–125, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium 33: 345–356, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol 583: 25–36, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 295: C779–C790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem 283: 12935–12940, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich A, Kalwa H, Storch U, Mederos YSM, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455: 465–477, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Golovina VA. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am J Physiol Cell Physiol 277: C343–C349, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 12.Hawavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 42: 173–182, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hewavitharana T, Deng X, Wang Y, Ritchie MF, Girish GV, Soboloff J, Gill DL. Location and function of STIM1 in the activation of Ca2+ entry signals. J Biol Chem 283: 26252–26262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klejman ME, Gruszczynska-Biegala J, Skibinska-Kijek A, Wisniewska MB, Misztal K, Blazejczyk M, Bojarski L, Kuznicki J. Expression of STIM1 in brain and puncta-like co-localization of STIM1 and ORAI1 upon depletion of Ca2+ store in neurons. Neurochem Int 54: 49–55, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem 284: 9733–9741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPCs enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 287: L962–L969, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Leung FP, Yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol 153: 846–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res 103: e97–e104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA 104: 4682–4687, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA 105: 2895–2900, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci USA 106: 3202–3206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L17–L25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol 174: 815–825, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454: 538–542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta 1481: 147–155, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem 281: 24979–24990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res 89: 923–929, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Ng LC, Kyle BD, Lennox AR, Shen XM, Hatton WJ, Hume JR. Cell culture alters Ca2+ entry pathways activated by store-depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 294: C313–C323, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Ng LC, McCormack M, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587: 2429–2442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem 282: 9105–9116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium 38: 233–252, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J 23: 2425–2437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 577: 479–495, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA. Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol 586: 2463–2476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW., Jr Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 1763: 1147–1160, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem 281: 20661–20665, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Soboloff J, Spassova MA, Dziadek MA, Gill DL. Calcium signals mediated by STIM and Orai proteins-A new paradigm in inter-organelle communication. Biochim Biophys Acta 1763: 1161–1168, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA 103: 4040–4045, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Takahashi Y, Watanabe H, Murakami M, Ono K, Munehisa Y, Koyama T, Nobori K, Iijima T, Ito H. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun 361: 934–940, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis 195: 287–296, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM. The properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem 276: 7782–7790, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Várnai P, Tóth B, Tóth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem 282: 29678–29690, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol 280: C1184–C1192, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J 357: 673–685, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams RT, Senior PV, Van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim Biophys Acta 1596: 131–137, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Wu MM, Luik RM, Lewis RS. Some assembly required: constructing the elementary units of store-operated Ca2+ entry. Cell Calcium 42: 163–172, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun 350: 969–976, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443: 226–229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]