Abstract
The human breast cancer resistance protein (BCRP/ABCG2) mediates efflux of drugs and xenobiotics. In this study, we investigated the role of polar residues within or near the predicted transmembrane α-helices 1 and 6 of BCRP in drug transport. We substituted Asn387, Gln398, Asn629, and Thr642 with Ala, Thr402 with Ala and Arg, and Tyr645 with Phe, and the mutants were stably expressed in human embryonic kidney-293 or Flp-In-293 cells. Immunoblotting and confocal microscopy analysis revealed that all of the mutants were well expressed and predominantly targeted to the plasma membrane. While T402A and T402R showed a significant global reduction in the efflux of mitoxantrone, Hoechst 33342, and BODIPY-prazosin, N629A exhibited significantly increased efflux activities for all of the substrates. N387A and Q398A displayed significantly impaired efflux for mitoxantrone and Hoechst 33342, but not for BODIPY-prazosin. In contrast, T642A and Y645F showed a moderate reduction in Hoechst 33342 efflux only. Drug resistance profiles of human embryonic kidney-293 cells expressing the mutants generally correlated with the efflux data. Furthermore, N629A was associated with a marked increase, and N387A and T402A with a significant reduction, in BCRP ATPase activity. Mutations of some of the polar residues may cause conformational changes, as manifested by the altered binding of the 5D3 antibody to BCRP in the presence of prazosin. The inward-facing homology model of BCRP indicated that Thr402 within transmembrane 1 may be important for helical interactions, and Asn629 may be involved in BCRP-substrate interaction. In conclusion, we have demonstrated the functional importance of some of these polar residues in BCRP activity.
Keywords: adenosine 5′-triphosphate-binding cassette efflux transporter, multidrug resistance, site-directed mutagenesis, structure-function of adenosine 5′-triphosphate-binding cassette transporter
multidrug resistance of cancer cells is often associated with overexpression of ATP-binding cassette (ABC) transporters, which can efflux a broad spectrum of drugs using the energy of ATP hydrolysis. Human breast cancer resistance protein (BCRP, gene symbol ABCG2) is one such transporter that confers resistance to chemotherapeutic agents, such as mitoxantrone, flavopiridol, and topotecan (14, 24). BCRP also transports nonchemotherapy substances, such as glyburide (34) and prazosin (8). BCRP is highly expressed in stem cells (35), the apical membrane of the small intestine and colon epithelium (12), the liver canalicular membrane (12), the brain microvessel endothelium (4), and the apical membrane of placental syncytiotrophoblasts (12). Hence, BCRP is also important in disposition and tissue exposure of drugs and xenobiotics (24).
BCRP comprises only one nucleotide binding domain (NBD) at the NH2 terminus followed by one membrane spanning domain (MSD) at the COOH terminus (24). Such a domain organization (NBD-MSD) differs from those of most of the other ABC transporters, such as P-glycoprotein (P-gp) and multidrug resistance protein 1, which consist of two repeated halves in a reverse domain organization (MSD-NBD-MSD-NBD) (26). The MSD of BCRP has been shown to contain six transmembrane (TM) α-helices (30). Three putative N-linked glycosylation sites, of which only Asn596 was shown to be glycosylated, locate in an extracellular loop connecting TM5 and TM6 and do not appear to be important in trafficking and function of the protein (7). Since BCRP is a half ABC transporter, it is widely believed that BCRP functions as a homodimer or a higher order oligomer. To date, little is known about the structure of BCRP. A three-dimensional structure at ∼18 Å resolution revealed by electron microscopy analysis suggests that BCRP may exist as a tetrameric complex with four BCRP homodimers (15). Most recently, we have obtained projection structures of BCRP at moderate resolutions (5–7 Å), which suggest conformational changes upon substrate binding (25). How exactly BCRP binds and transports a large number of structurally unrelated drug substrates remains unknown. Mutagenesis studies have demonstrated functional importance of several individual amino acid residues, particularly residues in TMs of BCRP, in drug transport. Notably, Arg482 within TM3 is critical for substrate selectivity and overall transport activity (16, 22). Mutations of Glu446, Asn557, and His630 within or near TMs have been shown to alter drug resistance phenotype (16).
Polar residues are generally underrepresented in TMs of membrane proteins, and mutations of these residues often cause adverse effects on protein stability, changes in substrate specificity, and/or changes in the structure, suggesting conserved structural and functional roles (6). Polar residues within or near TMs have been shown to be important determinants for substrate specificity and transport capability of P-gp and multidrug resistance protein 1 (10, 31–33). There are only a few polar residues within or near TM1 and TM6 of BCRP. This promoted us to ask the question whether these polar residues are functionally important. Hence, in this study, Asn387, Gln398, and Thr402 within or near TM1, and Asn629 and Thr642 within TM6, were replaced individually with Ala. Tyr645 near TM6 was substituted with Phe. The BCRP mutants were expressed in human embryonic kidney (HEK)-293 or Flp-In-293 cells by stable transfection and characterized with respect to their ability to transport drugs, drug resistance, basal ATPase activities, and their binding affinity for the 5D3 antibody in the presence of a substrate. Homology model of BCRP was used to interpret the experimental data.
MATERIALS AND METHODS
Materials.
Mitoxantrone hydrochloride (MX), doxorubicin hydrochloride (Dox), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). Fumitremorgin C (FTC) was obtained from National Cancer Institute (Bethesda, MD). BODIPY FL-prazosin was from Molecular Probes (Eugene, OR). Rhodamine 123 (Rho-123) was from MP Biomedicals (Irvine, CA). SN-38 was from TOCRIS Bioscience (Ellisville, MO). High-performance liquid chromatography-grade DMSO was from Fisher Scientific (Waltham, MA). Fetal bovine serum (FBS) was purchased from HyClone (Logan, UT). Phosphate-buffered saline (PBS), Eagle's minimal essential medium (MEM), 4′,6-diamidino-2-phenylindole (DAPI), Hoechst 33342, trypsin-EDTA solution, geneticin (G418), and Alexa Fluor 488-conjugated goat anti-mouse IgG (H + L) (Fab′)2 fragment were from Invitrogen (Carlsbad, CA). FuGENE HD transfection reagent and protease inhibitor cocktail tablets were from Roche Applied Science (Indianapolis, IN). All restriction enzymes were from New England Biolaboratories (Beverly, MA). Primers used for PCR mutagenesis were synthesized by Operon (Huntsville, AL). The mouse monoclonal antibody (MAb) BXP-21 specifically against BCRP was from Kamiya Biomedical (Seattle, WA). The monoclonal goat anti-mouse horseradish peroxidase-conjugated antibody was from Bio-Rad (Hercules, CA). Phycoerythrin-conjugated anti-BCRP MAb 5D3 and the phycoerythrin-conjugated negative control antibody IgG2b were obtained from eBioscience (San Diego, CA). Fluoromount G was from Southern Biotech (Birmingham, AL). The pcDNA3.1 plasmid containing full-length, wild-type human BCRP cDNA was kindly provided by Dr. Susan E. Bates (National Cancer Institute, Bethesda, MD). Flp-In-293 cells, the pcDNA5/FRT and pOG44 plasmids, zeocin, and hygromycin B were from Invitrogen. All other reagents and chemicals were of the highest purity available commercially.
Site-directed mutagenesis.
The pcDNA3.1 plasmid containing full-length, wild-type human BCRP cDNA was used as a template to generate all of the BCRP mutants using PCR mutagenesis. The forward primers used for mutagenesis were as follows: N387A (5′-AAG CGT TCA TTC AAA GCC TTG CTG GGT AAT CCC-3′), Q398A (5′-CAG GCC TCT ATA GCT GCG ATC ATT GTC ACA GTC-3′), T402A (5′-GCT CAG ATC ATT GTC GCA GTC GTA CTG GGA CTG-3′), N629A (5′-CTG GGG CTT GTG GAA GGC TCA CGT GGC CTT GGC TTG-3′), T642A (5′-GAT TGT TAT TTT CCT CGC AAT TGC CTA CCT GAA ATT G-3′), and Y645F (5′-TTC CTC ACA ATT GCC TTC CTG AAA TTG TTA TTT C-3′). The reverse primers were complementary to the respective forward primers. All of the mutants were confirmed by DNA sequencing, and the entire BCRP cDNA in each mutant construct was also sequenced to make sure that no additional mutations were introduced during PCR reactions.
Cell culture and establishment of stable transfectants of mutant BCRP.
HEK cells (HEK-293) obtained from American Type Culture Collection (Manassas, VA) were grown and maintained in MEM containing 10% FBS at 37°C in a humidified incubator with 5% CO2. For stable transfection, 2 μg of plasmid DNA in 1 ml of serum-free MEM medium were used to transfect 4.5 × 105 cells with 5 μl of the FuGENE HD transfection reagent in each 35-mm dish, according to the manufacturer's instructions. After 12 h, cells were incubated in fresh medium containing 10% FBS without G418 for an additional 48 h. Cells were then cultured in 1 mg/ml G418 for 3 days. Subsequently, cells were pooled and subcultured in 1 mg/ml G418 by frequently removing dead cells and changing medium until G418-resistant colonies appeared. Approximately 3 wk after transfection, individual cell colonies were isolated with limited dilution and expanded in the presence of 0.5 mg/ml G418. BCRP protein levels in G418-resistant cell clones were examined by immunoblotting. All of the G418-resistant cell lines were cultured and maintained in MEM supplemented with 10% FBS in the presence of 0.5 mg/ml G418 at 37°C in a 5% CO2 incubator. Cells were grown to 80–90% confluence and harvested with 0.25% trypsin-EDTA for subculturing, immunoblotting, confocal microscopy analysis, flow cytometry assays, or other functional studies. Only cells within 10 passages were used in these experiments.
Generation of Flp-In-293 cells stably expressing wild-type BCRP and the mutants T402A and T402R.
The full-length human BCRP cDNA in the pcDNA3.1 plasmid was amplified using PCR with the following primer pair (5′-ACG ACC AAG CTT ACC ATG TCT TCC AGT AAT GTC GAA GTT TTT ATC-3′ and 5′-CTT CGG CTC GAG TTA AGA ATA TTT TTT AAG AAA TAA CAA TTT C-3′). The PCR product was digested with Hind III and Xho I and subcloned into the pcDNA5/FRT expression vector, which was digested with the same restriction enzymes. The resulting pcDNA5/FRT/BCRP plasmid containing full-length human BCRP cDNA was used as a template to generate T402A and T402R, as described above. The forward primer used to generate T402R was 5′-GCT CAG ATC ATT GTC AGA GTC GTA CTG GGA CTG-3′. The forward primer used to generate T402A was the same as described. One microgram of the pcDNA5/FRT plasmid containing wild-type BCRP, T402A, or T402R cDNA or the empty vector were used to transfect Flp-In-293 cells, in combination with 2 μg of pOG44 using the FuGENE HD transfection reagent, according to the manufacturer's instruction. After recovery, cells were selected in 125 μg/ml of hygromycin for ∼3–4 wk. The cells expressing wild-type BCRP, T402A, or T402R were then maintained in complete MEM medium containing 10% FBS, l-glutamine, and 125 μg/ml of hygromycin for subsequent experiments.
Plasma membrane preparation.
Plasma membranes were prepared essentially the same as previously described (9, 29) with minor modifications. Briefly, HEK-293 cells stably expressing wild-type and mutant BCRP and the vector control cells were suspended in the homogenization buffer (250 mM sucrose, 50 mM Tris·HCl, pH 7.4, 0.25 mM CaCl2, and the protease inhibitor cocktail and benzamidine) and disrupted by N2 cavitation. Broken cells were centrifuged. The supernatant was layered over 35% (wt/wt) sucrose and centrifuged at 4°C. The interface layer was collected, washed twice by centrifugation, and resuspended in the buffer containing 50 mM Tris·HCl, pH 7.5, and 250 mM sucrose. Relative BCRP protein levels in the plasma membranes were determined by immunoblotting using MAb BXP-21 and densitometric analysis, as described below.
Immunoblotting.
Protein concentrations of whole cell lysates or plasma membranes were measured by the Pierce BCA protein assay using bovine serum albumin as standard. Whole cell lysates (20 μg each lane) or plasma membrane samples (5 μg each lane) were subjected to 10% SDS-polyacrylamide gel electrophoresis. Blots were probed with the MAb BXP-21, as previously described (30). Human β-actin in whole cell lysates was detected as an internal control. Relative BCRP protein levels were determined by densitometric analysis of the immunoblots using the National Institutes of Health Scion Image software (Scion, Frederick, MD).
Confocal microscopy.
Wild-type and mutant BCRP-expressing HEK-293 or Flp-In-293 cells and the vector control cells were plated in four-chamber polysterene vessels on glass slides (Falcon; BD Biosciences, Bedford, MA) at ∼2 × 105 cells/well. Cells were grown to 80% confluence and washed twice with PBS at room temperature. Cells were then fixed with 4% paraformaldehyde in PBS at room temperature for 30 min, washed twice with PBS, and incubated in PBS containing 0.1% Triton X-100 and 2% FBS at room temperature for 60 min. Cells were then incubated with BXP-21 (1:500 dilution in PBS containing 0.1% Triton X-100 and 2% FBS) for 60 min, followed by incubation in the dark with the second antibody Alexa Fluor 488-conjugated goat anti-mouse IgG (H+L) (Fab′)2 fragment (1:1,000 dilution in PBS containing 0.1% Triton X-100 and 2% horse serum) for 1 h. Cell nuclei were stained with 300 nM DAPI. Finally, cells were washed twice with PBS and mounted in Fluoromount G and observed using a Zeiss 510 META confocal microscope with a 420- to 488-nm band-pass filter for DAPI (excited at 405 nm) or a 505-nm long-pass filter for Alexa Fluor 488 (excited at 488 nm).
Cell surface expression of wild-type and mutant BCRP.
The 5D3 antibody recognizes a conformation-sensitive extracellular epitope in BCRP (17). Binding of 5D3 to HEK-293 cells expressing wild-type and mutant BCRP were examined using flow cytometry, as described (29). Briefly, 5 × 105 cells were incubated with phycoerythrin-conjugated 5D3 (20 μl) or the phycoerythrin-conjugated IgG2b control (20 μl) in 0.75 ml of PBS containing 2% bovine serum albumin for 30 min at room temperature. Cells were then washed once with PBS and analyzed on a BD FACScan flow cytometer equipped with a 488-nm argon laser and a 585/42-nm band-pass filter. The differences in phycoerythrin fluorescence between cells incubated with 5D3 and the IgG2b control were used to express the relative cell surface expression of BCRP.
Flow cytometric efflux assay.
Flow cytometric efflux assays were performed essentially the same as previously described (29), with minor modifications. Briefly, cells were first incubated with 10 μM MX, 500 nM BODIPY-prazosin, or 0.05 μg/ml Hoechst 33342, in the presence and absence of 10 μM FTC for 30 min at 37°C. Cells were then washed once with ice-cold PBS and resuspended in 1 ml of fluorescent compound-free incubation buffer, with or without 10 μM FTC, and the incubation was continued for 1 h at 37°C. Cells were washed, and intracellular fluorescence was measured with a 488-nm argon laser and a 650-nm long-pass filter for MX, and a 488-nm argon laser and a 530-nm band-pass filter for BODIPY-prazosin in a BD FACScan flow cytometer. A FACSVantage flow cytometer equipped with a 360-nm UV laser was used to detect Hoechst 33342 fluorescence. Cells in medium containing a fluorescent compound alone or in medium containing the fluorescent compound and FTC generated the efflux and FTC/efflux histograms, respectively. The difference in median fluorescence (ΔF) between the FTC/efflux histogram and the respective efflux histogram was used as a measure of FTC-inhibitable efflux activity of wild-type and mutant BCRP. The efflux activities were then normalized to the BCRP protein levels to take into account the differences in BCRP expression. Statistical significance of the differences in efflux activities between wild-type and mutant BCRP was analyzed using the Student's t-test. A difference with a P value of <0.05 was considered statistically significant.
Cytotoxicity assay.
Drug-resistance profiles of HEK-293 cells expressing wild-type and mutant BCRP and the vector control cells were determined using the MTT microtiter plate assay, as previously described (29). Briefly, cells were seeded in 96-well collogen-coated plates at a density of 4,000–5,000 cells/well with 200 μl of MEM supplemented with 10% FBS and 0.5 mg/ml G418. Cells were incubated with MX, SN-38, Dox, or Rho-123 at various concentrations for 72 h. The cell survival rate was determined by the MTT assay. Untreated cells were used as control (100% cell survival). The drug concentrations leading to 50% cell survival (IC50 values) were calculated by fitting the equation, as previously described (29), to the data points using nonlinear regression (GraphPad Prism, version 3.03, GraphPad Software, San Diego, CA). Relative resistance factors were calculated as ratios of the IC50 values of cells expressing wild-type and mutant BCRP to the IC50 values of the vector control cells. The relative resistance levels were normalized to the BCRP protein levels to take into account the differences in BCRP expression. Differences in resistance factors between wild-type and mutant BCRP were analyzed using the Student's t-test. A difference with a P value of <0.05 was considered statistically significant.
Vanadate-sensitive ATPase activity.
Vanadate-sensitive ATPase activities of wild-type and mutant BCRP were determined by measuring inorganic phosphate liberation from ATP, as previously described (3, 13). Reactions were initiated by mixing plasma membranes (5 μg of protein per reaction) with 100 μl of the reaction buffer containing 50 mM HEPES, pH 7.0, 5 mM MgCl2, 10 mM NaN3, 2 mM EGTA, 2 mM ouabain, and varying concentrations of MgATP up to 10 mM in the presence or absence of 1 mM sodium vanadate. Reactions were maintained at 37°C for 30 min and terminated by adding 6% SDS. The liberation of inorganic phosphate was measured immediately by determining the optical density at 650 nm. The differences between the ATPase activities determined in the absence and in the presence of vanadate were used to express vanadate-sensitive ATPase activities. The vanadate-sensitive ATPase activities of wild-type and mutant BCRP were then calculated by subtracting the background activities of the vector control samples. The Km and Vmax values of ATP hydrolysis were determined by fitting the Michaelis-Menten equation to the data points using nonlinear regression (GraphPad Prism, version 3.03).
Effects of prazosin on the binding of 5D3 to wild-type and mutant BCRP.
The effects of prazosin, a substrate of BCRP, on the binding of 5D3 to wild-type and mutant BCRP were determined essentially the same as for determining cell surface expression. Briefly, ∼5 × 105 HEK-293 cells expressing wild-type and mutant BCRP were incubated in the presence or absence of various concentrations (0–40 μM) of prazosin for 5 min at 37°C, followed by the addition of the 5D3 or IgG2b antibody (20 μl each), and incubation was continued for 30 min at 37°C. Cells were washed once with PBS and analyzed using flow cytometry, as described. In preliminary experiments, we incubated cells with 5D3 and prazosin for up to 2 h, and the fluorescence did not change compared with incubation for 30 min. The final concentration of DMSO used to dissolve prazosin in the incubations was less than 1% (vol/vol), and no effects of the vehicle on fluorescence detection were observed at this concentration. The differences (ΔF/F0) in phycoerythrin fluorescence between the cells incubated with 5D3 in the presence of prazosin and 5D3 alone (F0) were used to express the concentration-dependent effects of prazosin on 5D3 binding to BCRP. Differences in the 5D3 binding between wild-type and mutant BCRP were analyzed using the Student's t-test. A difference with a P value of < 0.05 was considered statistically significant.
Homology model of BCRP.
Our laboratory has previously generated the homology model of BCRP based on the recently published crystal structure of mouse P-gp (1), which represents the substrate-bound and nucleotide-free, inward-facing form of BCRP (25). This model was used to analyze the possible roles of the polar residues investigated in this study.
RESULTS
Stable expression of wild-type and mutant BCRP in HEK-293 cells.
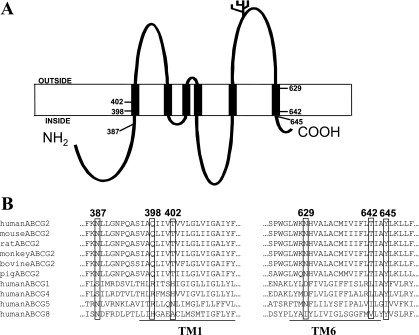
According to the recent topology model (30), TM1 and TM6 of BCRP comprise approximately amino acid residues 395–414 and 625–644, respectively. Polar residues examined in this study, Asn387, Gln398, and Thr402 within or near TM1, as well as Asn629, Thr642, and Tyr645 within or near TM6 (Fig. 1A), are strictly conserved in the human, mouse, rat, monkey, bovine, and porcine orthologs of BCRP. Although not fully conserved, most of these polar residues share similarity in polarity with the corresponding residues in members of the human ABCG subfamily (Fig. 1B). To evaluate functional importance of these polar residues in substrate specificity and overall transport activity of BCRP, we generated six mutants in which Asn387, Gln398, Thr402, Asn629, and Thr642 were replaced with Ala. Tyr645 was replaced with Phe to assess the role of potential hydrogen bond of this residue in drug transport, but with the minimal probability of introducing structural changes.
Fig. 1.
Human breast cancer resistance protein (BCRP) topology and sequence alignment. A: the topology structure of human BCRP with 6 transmembrane (TM) α-helices deduced from experimental determination (30). B: human BCRP amino acid sequences in the predicted TM1 and TM6 (underlined) were aligned with the corresponding sequences of BCRP homologs and orthologs using Clustal W.
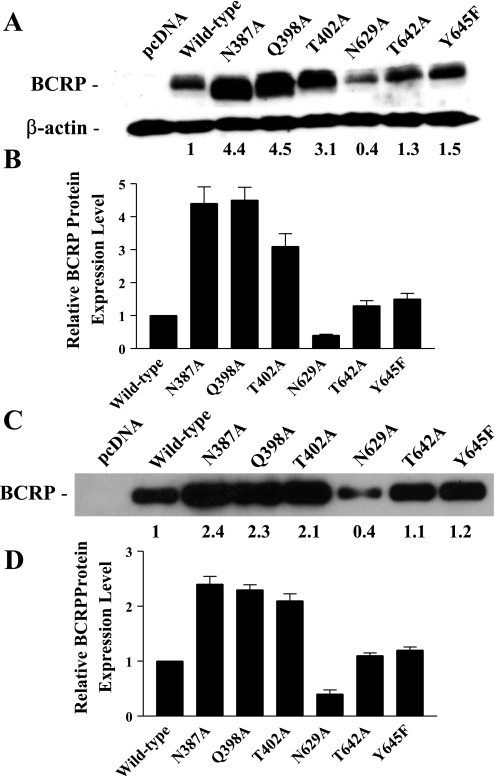
Stable transfectants were generated by transfection of pcDNA3.1 vectors containing full-length cDNAs of mutant BCRP into HEK-293 cells and selection with G418. The resultant single stable cell clones for each mutant expressed substantially variable levels of BCRP, and only the clones expressing the highest levels of BCRP were selected for subsequent studies. The expression levels of mutant BCRP relative to wild-type protein were determined by immunoblotting and densitometry. The levels of N387A, Q398A, T402A, N629A, T642A, and Y645F, determined by immunoblotting of whole cell lysates using β-actin as an internal standard, were ∼4.4-, 4.5-, 3.1-, 0.4-, 1.3-, and 1.5-fold that of wild-type BCRP (Fig. 2, A and B). The expression levels of wild-type and mutant BCRP determined in whole cell lysates and the corresponding plasma membranes were generally comparable (Fig. 2, C and D).
Fig. 2.
Immunoblotting analysis of the expression levels of wild-type and mutant BCRP. The protein levels of wild-type and mutant BCRP in stably transfected human embryonic kidney-293 (HEK-293) cells were determined by immunoblotting and densitometric analysis. A: representative immunoblot of whole cell lysates for wild-type and mutant BCRP. The numbers below the blot refer to the average protein levels of the mutants relative to wild-type BCRP after normalization to β-actin. B: the relative protein levels of wild-type and mutant BCRP determined by immunoblotting of whole cell lysates are presented as means ± SD of 5 experiments. C: a representative immunoblot of plasma membrane preparations for wild-type and mutant BCRP. The numbers below the blot refer to the average protein levels of the mutants relative to wild-type BCRP. D: the relative protein levels of wild-type and mutant BCRP determined by immunoblotting of plasma membrane preparations are presented as means ± SD of 3 experiments.
Plasma membrane localization and cell surface expression of wild-type and mutant BCRP in HEK-293 cells.
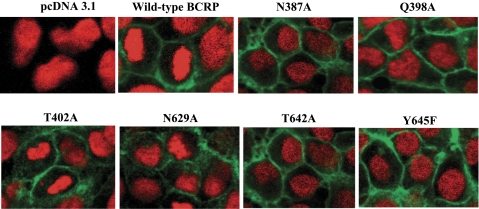
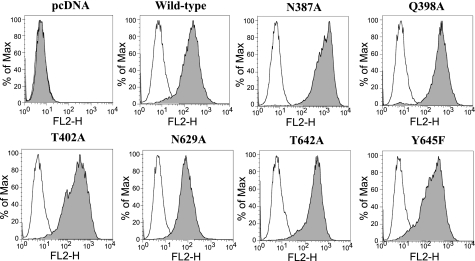
To examine whether mutations might affect maturation and targeting of BCRP to the plasma membrane, HEK-293 cells stably expressing wild-type and mutant BCRP were analyzed by immunofluorescent confocal microscopy. Plasma membrane localization of wild-type and mutant BCRP was evaluated by immunoreactivity with the BCRP-specific MAb BXP-21. Wild-type BCRP and the six mutants were predominantly localized on the plasma membrane with little or no intracellular signal (Fig. 3), suggesting that protein folding and trafficking of BCRP remained largely unaffected by mutations of these polar residues. To further demonstrate cell surface expression of these mutants, cells were probed with the phycoerythrin-conjugated BCRP-specific MAb 5D3 or the IgG2b negative control. The differences in the cell-associated phycoerythrin fluorescence between the incubation of 5D3 and the IgG2b control represent approximately the relative expression levels of BCRP on cell surface. As expected, the cells transfected with the empty vector did not display any cell surface expression of BCRP (Fig. 4). Wild-type and mutant BCRP all showed significant cell surface expression, at levels comparable to those obtained by immunoblotting (Fig. 2).
Fig. 3.
Confocal microscopy of HEK-293 cells stably expressing wild-type and mutant BCRP. The cellular localization of wild-type and mutant BCRP in HEK-293 cells (shown in green) was determined by immunofluorescence detection using the BCRP-specific antibody BXP-21, as described. Cell nuclei were stained with DAPI and are shown in red. No green fluorescence was detected in the vector control cells. Representative areas of HEK-293 cells expressing wild-type BCRP and the mutants N387A, Q398A, T402A, N629A, T642A, and Y645F are shown. Images have been enhanced for maximal contrast between the black background and green fluorescence and were not intended for quantitative determination of BCRP expression.
Fig. 4.
Cell surface expression of wild-type and mutant BCRP. Expression of wild-type and mutant BCRP on cell surface of stably transfected HEK-293 cells was detected using the monoclonal antibody 5D3, as described. Representative flow cytometry histograms showing cell surface expression of wild-type and mutant BCRP are presented. The open and solid peaks represent the phycoerythrin fluorescence associated with cells treated with the IgG2b negative control and the 5D3 antibodies, respectively. No surface expression of BCRP was detected in the pcDNA vector control cells. The experiments were repeated three times, and similar results were obtained. FL2-H, histogram of the FL2 peak fluorescence emission values.
FTC-inhibitable efflux activities of wild-type and mutant BCRP.
We next examined the effects of these mutations on the efflux activity of BCRP using a flow cytometric efflux assay with three fluorescent substrates: MX, BODIPY-prazosin, and Hoechst 33342. This assay has been widely used to assess BCRP efflux activity (23, 29). MX, BODIPY-prazosin, and Hoechst 33342 have been widely used and well characterized as BCRP substrates (2, 17, 19, 23, 29). They do not seem to share any similarity in chemical structures, and, therefore, the data obtained may shed light on the roles of the studied polar residues in determining substrate specificity. Results are shown in Table 1. As expected, no significant increase in intracellular accumulation of the three substrates by the FTC treatment was observed in the vector control HEK-293 cells (data not shown). However, HEK-293 cells expressing wild-type and mutant BCRP showed substantial efflux activities for all of the three substrates tested, with the exception of T402A for BODIPY-prazosin, suggesting that mutation of Thr402 drastically impaired the ability of BCRP to transport BODIPY-prazosin. After normalization to the BCRP protein levels, neither T642A nor Y645F seem to affect the efflux of MX and BODIPY-prazosin compared with wild-type protein, except for a moderate decrease in the efflux of Hoechst 33342. However, T402A showed significantly decreased efflux activities for all the three substrates by 60–90%, with particularly lower efflux activities for BODIPY-prazosin and Hoechst 3342. N387A and Q398A also showed significantly reduced efflux activities for MX and Hoechst 33342 by 40–80%, but were fully active in transporting BODIPY-prazosin. In contrast, the efflux activities of N629A for all of the three substrates were increased up to fourfold. These results indicate that mutations of Asn387 and Gln398 influenced substrate specificity of BCRP, whereas mutations of Thr402 and Asn629 affected the ability of BCRP to transport the substrates. We used the BCRP protein levels of whole cell lysates, but not those of plasma membranes, in the calculations for three reasons. First, all of the mutants were predominantly expressed on the plasma membrane. Second, unlike whole cell lysates, plasma membranes were prepared through cell breakage and multiple centrifugation steps, which could introduce more variations. Third, the BCRP protein levels of whole cell lysates were corrected by normalization to the internal standard β-actin.
Table 1.
FTC-inhibitable efflux activities of HEK-293 cells stably expressing wild-type and mutant BCRP
| Mitoxantrone |
BODIPY-Prazosin |
Hoechst 33342 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔF | ΔF′ | Ratio | ΔF | ΔF′ | Ratio | ΔF | ΔF′ | Ratio | |
| Wild-type BCRP | 10.6 ± 0.6 | 10.6 ± 0.6 | 1.0 | 12.5 ± 4.3 | 12.5 ± 4.3 | 1.0 | 2150.0 ± 25.5 | 2150.0 ± 25.5 | 1.0 |
| N387A | 11.3 ± 0.5 | 2.5 ± 0.1* | 0.2 | 54.5 ± 5.8 | 12.3 ± 1.3 | 1.0 | 5,024.7 ± 570.5 | 1,131.7 ± 128.5* | 0.5 |
| Q398A | 28.1 ± 5.2 | 6.3 ± 1.2* | 0.6 | 69.3 ± 10.6 | 15.5 ± 2.4 | 1.2 | 4,206.7 ± 252.1 | 941.1 ± 56.4* | 0.4 |
| T402A | 12.3 ± 2.4 | 4.0 ± 0.8* | 0.4 | 4.5 ± 1.8 | 1.5 ± 0.6* | 0.1 | 999.3 ± 352.9 | 322.4 ± 113.8* | 0.1 |
| N629A | 19.3 ± 3.7 | 46.0 ± 8.8* | 4.3 | 12.8 ± 1.5 | 30.5 ± 3.6* | 2.4 | 2,681.0 ± 370.5 | 6,383.3 ± 882.1* | 3.0 |
| T642A | 17.0 ± 0.3 | 12.9 ± 0.2 | 1.2 | 15.4 ± 1.3 | 11.8 ± 1.0 | 0.9 | 1,860.7 ± 462.3 | 1,420.4 ± 352.9* | 0.7 |
| Y645F | 16.8 ± 2.3 | 11.6 ± 1.6 | 1.1 | 15.1 ± 4.6 | 10.4 ± 3.2 | 0.8 | 1,358.7 ± 35.5 | 937.0 ± 24.5* | 0.4 |
Values are means ± SD of 3 independent experiments. Fumitremorgin C (FTC)-inhibitable efflux activities of human embryonic kidney-293 (HEK-293) cells expressing wild-type and mutant breast cancer resistance protein (BCRP) for mitoxantrone, BODIPY-prazosin, and Hoechst 33342 are expressed as the differences in median fluorescence (ΔF) between the FTC/efflux and efflux histograms. ΔF′ represents the ΔF value after normalization for differences in BCRP protein levels. The relative efflux activities of the mutants compared with wild-type BCRP after normalization to the BCRP protein levels are presented as ratios on which the activities of wild-type BCRP are set as 1. The relative BCRP protein levels determined by immunoblotting of whole cell lysates shown in Fig. 2 were used for the calculations. Differences between efflux activities of wild-type and mutant BCRP after normalization to the BCRP protein levels are statistically significant:
P < 0.05 by the Student's t-test.
Drug resistance conferred by wild-type and mutant BCRP.
To further explore whether the mutations affect drug resistance profiles of BCRP, we determined resistance of HEK-293 cells expressing wild-type and mutant BCRP to MX, SN-38, Dox, and Rho-123. Results are summarized in Table 2. Compared with the vector control cells, cells expressing wild-type and mutant BCRP all conferred resistance to MX and SN-38, but not to Rho-123 and Dox, which are consistent with previous observations that Rho-123 and Dox are not BCRP substrates (29). After normalization to the BCRP protein levels, the effects of mutations on resistance to MX were generally consistent with the efflux data. Thus N387A, Q398A, and T402A exhibited a significantly lower resistance to MX than wild-type BCRP, whereas N629A displayed an approximately threefold increase in resistance to MX. Similarly, resistance to SN-38 conferred by N629A was increased approximately threefold, and resistance to SN-38 conferred by other mutants was decreased by 60–90%. We did not calculate the normalized resistance factors of all the mutants for Dox and Rho-123, as they did not confer any resistance to the two drugs.
Table 2.
Relative drug resistance of HEK-293 cells expressing wild-type and mutant BCRP
| MX |
SN-38 |
Dox |
Rho-123 |
|||||
|---|---|---|---|---|---|---|---|---|
| IC50, nM | RR (ratio) | IC50, nM | RR (ratio) | IC50, nM | RR | IC50, nM | RR | |
| pcDNA control | 8.5 ± 0.6 | 2.3 ± 0.28 | 125.6 ± 32.0 | 1,881 ± 168.2 | ||||
| Wild-type BCRP | 64.8 ± 3.9 | 7.6 (1.0) | 89.7 ± 5.4 | 39.0 (1.0) | 168.0 ± 24.5 | 1.3 | 3,341 ± 267.4 | 1.8 |
| N387A | 54.8 ± 6.3 | 6.4 (0.2)* | 44.8 ± 6.3 | 19.5 (0.1)* | 120.4 ± 31.6 | 1.0 | 3,279 ± 436.8 | 1.7 |
| Q398A | 49.9 ± 4.6 | 5.9 (0.2)* | 71.6 ± 4.3 | 31.1 (0.2)* | 173.4 ± 36.5 | 1.4 | 2,534 ± 376.5 | 1.3 |
| T402A | 43.3 ± 7.6 | 5.1 (0.2)* | 63.0 ± 5.4 | 27.4 (0.2)* | 129.4 ± 31.4 | 1.0 | 2,140 ± 210.7 | 1.1 |
| N629A | 76.5 ± 12.3 | 9.0 (2.8)* | 108.3 ± 12.3 | 47.1 (2.9)* | 164.6 ± 14.8 | 1.3 | 3,051 ± 286.5 | 1.6 |
| T642A | 50.0 ± 9.5 | 5.9 (0.6) | 49.9 ± 9.9 | 21.7 (0.4)* | 156.1 ± 20.0 | 1.2 | 1,393 ± 221.6 | 0.7 |
| Y645F | 42.8 ± 6.8 | 5.0 (0.5)* | 44.8 ± 6.3 | 19.5 (0.3)* | 132.4 ± 18.4 | 1.1 | 1,846 ± 206.2 | 1.0 |
The IC50 values shown are means ± SD of 3 independent experiments. RR is the relative resistance factor. The ratios represent the relative levels of resistance of the mutants compared with wild-type BCRP after normalization for differences in BCRP protein levels. Relative BCRP protein levels of whole cell lysates shown in Fig. 2 were used for the calculations. Since wild-type and mutant BCRP did not confer resistance to doxorubicin hydrochloride (Dox) and rhodamine 123 (Rho-123); ratios for the two compounds were not calculated. MX, mitoxantrone hydrochloride. Differences between the IC50 values of wild-type and mutant BCRP after normalization to the BCRP protein levels are statistically significant:
P< 0.05 by the Student's t-test.
ATPase activities of wild-type and mutant BCRP.
To test if the changes in efflux activity can be explained by alterations in ATP hydrolysis activity, we measured vanadate-sensitive ATPase activities of plasma membranes isolated from stable transfectants expressing wild-type and mutant BCRP. Vanadate-sensitive ATPase activities attributable to wild-type and mutant BCRP were calculated by subtracting the background vanadate-sensitive ATPase activities of plasma membranes from the vector control. Km and Vmax values for ATP hydrolysis were estimated by fitting the Michaelis-Menten equation to the data points and shown in Table 3. The Km values of N387A, T402A, T642A, and Y645F were comparable to that of wild-type protein; however, the Km values of Q398A and N629A were decreased by ∼50–60%. This suggests that the ATP binding affinity to Q398A and N629A is increased. After normalization to the BCRP protein levels, the Vmax values of N387A, Q398A, and T402A were approximately one-half of that of wild-type BCRP, whereas the Vmax value of N629A was increased by ∼90%. Importantly, the Vmax-to-Km ratio of N629A was increased approximately fourfold compared with that of wild-type protein, suggesting that this mutant possesses a more efficient intrinsic ATP hydrolysis capability than wild-type protein. In contrast, the Vmax/Km values of N387A and T402A were decreased by ∼50%. The Vmax/Km values of Q398A, T642A, and Y645F were comparable to that of wild-type protein.
Table 3.
Kinetic parameters of ATP hydrolysis by wild-type and mutant BCRP
| Wild-type BCRP | N387A | Q398A | T402A | N629A | T642A | Y645F | |
|---|---|---|---|---|---|---|---|
| Vmax, nmol Pi·min−1·mg protein−1 | 14.5 ± 1.9 | 16.9 ± 1.5 | 14.9 ± 0.93 | 17.4 ± 1.7 | 11.5 ± 1.1 | 17.9 ± 1.9 | 14.5 ± 1.9 |
| Vmax normalized to BCRP protein level, nmol Pi·min−1·mg protein−1 | 14.5 ± 1.9 | 7.0 ± 0.63 | 6.5 ± 0.41 | 8.3 ± 0.81 | 28.0 ± 2.7 | 16.3 ± 1.7 | 12.0 ± 1.6 |
| Km for ATP, mM | 0.89 ± 0.40 | 0.90 ± 0.27 | 0.48 ± 0.12 | 1.1 ± 0.35 | 0.40 ± 0.15 | 0.93 ± 0.34 | 0.89 ± 0.43 |
| Vmax/Km, nmol Pi·min−1·mg protein−1·mM−1 | 16.3 | 7.8 | 13.5 | 7.5 | 70.0 | 17.5 | 13.5 |
Values are means ± SD of 3 independent determinations. Kinetic parameters for ATP hydrolysis were determined using plasma membrane preparations of HEK-293 cells expressing wild-type and mutant BCRP and were calculated by fitting the Michaelis-Menten equation to the data points by nonlinear regression using the GraphPad software. The Vmax values were normalized to the BCRP protein levels determined by immunoblotting of plasma membranes shown in Fig. 2.
Concentration-dependent effects of prazosin on the binding of the 5D3 antibody to wild-type and mutant BCRP.
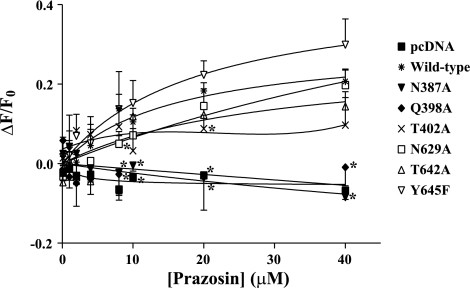
It has been shown that the 5D3 antibody recognizes conformational changes in BCRP induced by substrates or inhibitors (17, 28). Such substrate or inhibitor binding-induced conformational changes may be affected by the mutations, thereby also contributing to alterations in transport activity. Binding of the 5D3 antibody to wild-type and mutant BCRP was measured in the presence and absence of varying concentrations of prazosin (0.1–40 μM), a BCRP substrate. When HEK-293 cells expressing wild-type BCRP were incubated with the control IgG2b-phycoerythrin antibody, prazosin at concentrations up to 40 μM had no effect on phycoerythrin fluorescence (data not shown), suggesting that prazosin itself had no contribution to fluorescence detection. Only a slight decrease in 5D3-phycoerythrin fluorescence was observed for the vector control cells (Fig. 5); however, the 5D3-phycoerythrin fluorescence was differentially increased in a prazosin concentration-dependent manner for wild-type BCRP, N629A, T642A, and Y645F. In contrast, N387A and Q398A were associated with a slight decrease in 5D3-phycoerythrin fluorescence. The pattern of 5D3 binding to T402A fell between the above two groups. These data suggest that at least some of the mutations differentially affect the prazosin binding-induced conformational changes in BCRP. In particular, significant differences in 5D3 binding were observed between wild-type BCRP and the mutants N387A, Q398A, T402A, and N629A at certain prazosin concentrations (Fig. 5).
Fig. 5.
The effects of prazosin on the binding of 5D3 to wild-type and mutant BCRP. The effects of prazosin on the binding of 5D3 to wild-type and mutant BCRP expressed in HEK-293 cells over a concentration range of 0–40 μM were determined using flow cytometry, as described. ΔF/F0, differences in phycoerythrin fluorescence between the cells incubated with 5D3 in the presence of prazosin and 5D3 alone (F0) divided by F0. Shown are means and SD of 3 independent experiments. Significant differences (P < 0.05) in 5D3 binding analyzed by the Student's t-test were observed between wild-type BCRP and the mutant N387A, Q398A, T402A, or N629A at certain prazosin concentrations and are marked (*) on the right of respective data points.
Efflux activities of the mutants T402A and T402R expressed in Flp-In-293 cells.
In the experiments described above, the protein expression level of T402A in HEK-293 cells was approximately three times greater than that of wild-type BCRP (Fig. 2). To investigate whether the decreased efflux activity of T402A was caused by altered pattern of oligomerization resulting from the higher level of protein expression that could subsequently affect BCRP activity, we generated Flp-In-293 cells stably expressing wild-type BCRP, T402A, or T402R. T402R was also analyzed, because a recent study revealed that this mutant could possibly affect BCRP activity (19). By transfection into Flp-In-293 host cells, one copy of BCRP or the mutant cDNA can be integrated into the same genomic locus in each of the host cells after selection with hygromycin. Therefore, all of the cells are theoretically identical, leading to comparable levels of expression for BCRP and its mutants. Indeed, as shown in Fig. 6A, wild-type BCRP, T402A, and T402R were expressed at comparable protein levels. Immunofluorescent confocal microscopy analysis indicated that T402A and T402R were predominantly localized on the plasma membrane of Flp-In-293 cells as was wild-type BCRP (Fig. 6B). Consistent with the efflux data of T402A expressed in regular HEK-293 cells, the mutant expressed in Flp-In-293 cells also showed significantly decreased efflux activities for MX, BODIPY-prazosin, and Hoechst 33342 (Fig. 6C). Likewise, T402R also exhibited significantly lower activities compared with wild-type BCRP. Interestingly, efflux activities of T402R were further decreased compared with T402A, suggesting that Arg substitution of Thr402 caused a greater effect on BCRP function than Ala substitution, possibly due to more drastic changes in both size and polarity at position 402. These data further illustrated the important role of Thr402 in BCRP activity.
Fig. 6.
Expression and efflux activities of T402A and T402R in Flp-In-293 cells. A: representative immunoblot of whole cell lysates for wild-type BCRP and its mutants T402A and T402R expressed in Flp-In-293 cells. The same experimental conditions as described in Fig. 2 were used. B: confocal microscopy analysis of Flp-In-293 cells expressing wild-type BCRP and its mutants T402A and T402R. The same experimental conditions as described in Fig. 3 were used. C: fumitremorgin C (FTC)-inhibitable efflux activities of Flp-In-293 cells expressing wild-type BCRP and its mutants T402A and T402R for mitoxantrone, BODIPY-prazosin, and Hoechst 33342. The same experimental conditions as described in Table 1 were used. The activities were expressed as the differences in median fluorescence (ΔF) between the FTC/efflux and efflux histograms. Shown are means ± SD of 3 independent experiments. Differences between efflux activities of wild-type and mutant BCRP are statistically significant: *P < 0.05 by the Student's t-test.
Locations of the polar residues in the three-dimensional model of BCRP.
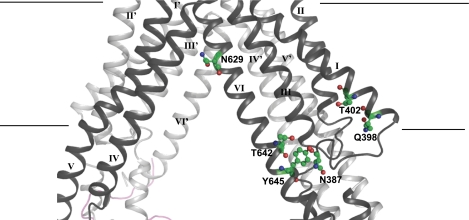
Based on the homology model of BCRP, there is a large central binding cavity in which all of the TMs appear to participate (Fig. 7). Of the six polar residues analyzed, only Asn629 was predicted to be located in the putative drug-binding cavity and, therefore, may be directly involved in interaction with drug substrates, presumably via polar interactions. Asn387, Thr642, and Tyr645 are clustered at the bottom of the drug-binding pocket. Gln398 and Thr402 are situated within TM1 in the periphery of the drug-binding cavity.
Fig. 7.
Three-dimensional model of BCRP. A side view of a three-dimensional model of BCRP in the substrate-bound and nucleotide-free inward-facing form is shown. Shown is a close-up of the membrane-spanning domain (MSD). The putative boundaries of the MSD are indicated with straight lines. TM segments are labeled with roman numerals. I, II, III, IV, V, and VI, as well as I′, II′, III′, IV′, V′, and VI′ indicate TM segments from different BCRP monomers in the BCRP dimer. The polar residues Asn387, Gln398, Thr402, Asn629, Thr642, and Tyr645 in one BCRP monomer are shown in a stick format and labeled with N387, Q398, T402, N629, T642, and Y645, respectively.
DISCUSSION
In the present study, we have analyzed the functional importance of various polar residues within or near TM1 and TM6 of BCRP (Fig. 1) for drug transport. All of the mutants were well expressed and properly routed to the plasma membrane (Figs. 2 and 3), suggesting that none of the mutations had a significant impact on proper folding, plasma membrane targeting, and biogenesis of BCRP.
Of particular interest of this study was the finding that substitution of Thr402 within TM1 with Ala caused a significant reduction in efflux of MX, BODIPY-prazosin, and Hoechst 33342 and the ability to confer resistance to MX and SN-38 (Tables 1 and 2). When this paper was in preparation, another study came out and also demonstrated that mutations of Thr402 affected BCRP activity (19). Two mutants of Thr402, T402L and T402R, were generated in that study. Although activities of the mutants were not quantitatively analyzed, the flow cytometric histograms indicated that the efflux activities of both mutants for mitoxantrone, BODIPY-prazosin, and pheophorbide were decreased, and similarly, the extent of reduction in efflux activity was dependent on substrates. For example, a particularly strong reduction in efflux of BODIPY-prazosin was associated with T402R. We verified that T402A and T402R, which were expressed in Flp-In-293 cells at comparable protein levels to wild-type BCRP, also exhibited significantly decreased efflux activities (Fig. 6). Thus both studies support the functional importance of Thr402. According to the homology model of BCRP based on the Sav1866 structure, these authors suggested that Thr402 interacts with residues in TM5 and TM6 from the opposite BCRP monomer (19), and, therefore, Thr402 is involved in interhelical interactions, possibly promoting dimer formation. This appears to be consistent with the fact that Thr402 is adjacent to the GXXXG dimerization motif in TM1 (20). In the present study, the homology model of BCRP based on the mouse P-gp structure suggests that Thr402 is located outside the putative drug-binding cavity and hence not directly involved in substrate binding (Fig. 7). However, Thr402 in TM1 could interact with residues in TM2 and/or TM3 of the same BCRP monomer, which likely form part of the drug translocation pathway. Therefore, mutations of Thr402 could disturb interhelical interactions between TM1 and TM2 or TM3, causing an indirect effect on BCRP-substrate interaction. Since it is well known that Thr residues are frequently involved in interhelical interactions (6, 27), the two studies could be consistent in the sense of the involvement of Thr402 in interhelical interactions that are functionally important. The exact interaction partners of Thr402 in TM1 remain to be determined.
Replacement of Asn387 and Gln398 with Ala significantly but selectively impaired the efflux of MX and Hoechst 33342, but not of BODIPY-prazosin, and showed lower resistance to MX and SN-38 (Tables 1 and 2), suggesting that mutations of the two polar residues influenced substrate specificity. Similarly, our laboratory has recently shown that insertion of an hemagglutinin tag at position 387 also causes a drastic decrease in transporting MX (30). The reason for such substrate-specific effects is not clear. According to the topology structure of BCRP that our laboratory recently determined (30), Asn387 is located in a cytosolic region near TM1, which is likely part of the linker region connecting the NBD to the MSD. Mutation of Arg383 in the same region with Ala dramatically affected biogenesis of BCRP (18). However, we showed that mutation of Asn387 with Ala did not impair BCRP expression, folding, or trafficking to the plasma membrane to any significant extent. Gln398, located within TM1 at its NH2-terminal end, might help to stabilize TMs by interacting with head groups of membrane phospholipids. However, this cannot explain why the functional perturbation by mutation of Gln398 was restricted to MX and Hoechst 33342, but not to BODIPY-prazosin. A possible explanation would be that the communication between MSD and NBD could be influenced by mutations of Asn387 and Gln398, leading to altered transport activity, as Asn387 and Gln398 are predicted to be located in or near the linker region and appear to have little potential of directly participating in substrate binding (Fig. 7). Such substrate-specific effects might be related to substrate-dependent alterations in the conformational coupling between MSD and NBD. Similar cases have been reported for P-gp. For example, Gly989 in TM12 of P-gp, a residue that may not directly participate in substrate binding, was suggested to be involved in communicating nicardipine, but not vinblatstine, binding to the NBDs, probably due to distinct binding sites of the two drugs (5). BODIPY-prazosin has been shown to interact with BCRP at a pharmacologically distinct site from those of MX and Hoechst 33342 (11). Therefore, by analogy, it is also possible that mutations of Asn387 and Gln398 may interrupt communication of MX and Hoechst 33342, but not BODIPY-prazosin binding to the NBD, due to different binding sites of these substrates.
Replacement of Asn629 with Ala significantly increased transport activity of BCRP for all of the substrates tested (Tables 1 and 2). The P-gp-based homology model suggests that Asn629 is located in the large central drug-binding cavity (Fig. 7) and possibly participates in BCRP-substrate interaction. This is consistent with the results of docking calculations of various BCRP substrates to the homology model (2). Hence, substitution of Asn629 may directly alter the interaction mode of substrate with BCRP. Notably, N629A was associated with an ∼60% decrease in Km and an approximately fourfold increase in Vmax/Km for ATP hydrolysis (Table 3). This suggests that ATP binding affinity and the efficiency of ATP hydrolysis are increased for N629A, which could be linked to the increased transport activity of the mutant. Likewise, R482T with a similar “gain-of-function” also showed decreased Km and increased Vmax/Km values for ATP hydrolysis (21). A conservative mutation of Tyr645 to Phe and Ala substitution of Thr642 caused only moderate changes in drug efflux and resistance that were restricted to Hoechst 33342 and SN-38 (Tables 1 and 2). This suggests that Thr642 and Tyr645 do not appear to play a critical role in BCRP activity, which is consistent with the findings that mutations of the two residues did not cause major alterations in ATPase activity and 5D3 binding (Table 3 and Fig. 5). Homology modeling also suggests that Thr642 and Tyr645 are not directly involved in BCRP-substrate interaction (Fig. 7).
N387A, Q398A, and T402A also showed changes in Km and/or Vmax for ATP hydrolysis (Table 3). Consequently, these mutants displayed similar changes in efflux activities (Tables 1 and 2), which highlights the importance of the precise cooperativity between MSD and NBD, i.e., any effect on conformational changes in the MSD possibly induced by mutations in TMs could impact the behavior of the NBD and, thereby, ATP hydrolysis. Similar observations have been reported for other ABC transporters. A980C in TM12 of P-gp demonstrated increased rate of ATP hydrolysis, whereas L976C and F978C in TM12 of P-gp showed reduced ATP binding affinity (5). The exact mechanism by which mutations of residues within TMs affect ATPase activity remains elusive. We have previously shown the substrate binding-induced conformational changes in BCRP by analyzing two-dimensional crystals in the presence and absence of a substrate (25). We, therefore, postulate that mutations of the polar residues within or near TM1 and TM6 could affect BCRP-substrate interaction by perturbing the substrate binding-induced conformational changes, thus leading to altered drug transport and/or ATP hydrolysis. To support this hypothesis, we analyzed 5D3 binding to wild-type and mutant BCRP in the presence and absence of a substrate. Prazosin differentially increased 5D3 binding to wild-type BCRP, N629A, T642A, and Y645F, whereas 5D3 binding to N387A and Q398A was slightly decreased (Fig. 5). Prazosin only moderately increased 5D3 binding to T402A (Fig. 5). Since 5D3 is a highly conformation-sensitive antibody, these data may indicate that some of the mutations, such as substitutions of Asn387, Gln398, and Thr402, affect the substrate binding-induced conformational changes in BCRP. The exact conformational changes caused by these mutations are currently not known. Nevertheless, these results are consistent with the possible roles of Asn387 and Gln398 in the conformational coupling between MSD and NBD, as well as the role of Thr402 in interhelical interactions.
In summary, we have identified several polar residues within or near TM1 and TM6 that play an important role in substrate specificity and/or overall transport activity of BCRP. These results provide new insight into the transport mechanism of BCRP. Whether our finding has physiological relevance is not known, as we did not find any single nucleotide polymorphisms of these polar residues in BCRP. The P-gp-based inward-facing model of BCRP is valuable to help explain the specific roles of individual amino acid residues; however, owing to the relatively low sequence identity between BCRP and P-gp, more studies are required to validate the predicted features.
GRANTS
This work was supported by the NIH National Institute of General Medical Sciences Grant GM073715.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Susan E. Bates (National Cancer Institute, Bethesda, MD) for providing the pcDNA3.1 expression vector containing full-length human wild-type BCRP cDNA. We also acknowledge the Drug Synthesis & Chemistry Branch, National Cancer Institute, National Institutes of Health (NIH) (Bethesda, MD) for providing FTC. We greatly acknowledge Drs. Haichuan Duan and Joanne Wang (Department of Pharmaceutics, University of Washington) for providing the pcDNA5/FRT expression vector.
REFERENCES
- 1.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323: 1718–1722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai X, Bikadi Z, Ni Z, Lee EW, Wang H, Rosenberg MF, Mao Q. Role of basic residues within or near the predicted transmembrane helix 2 of the human breast cancer resistance protein (BCRP/ABCG2) in drug transport. J Pharmacol Exp Ther 336: 670–681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chifflet S, Torriglia A, Chiesa R, Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem 168: 1–4, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 13: 2059–2063, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Crowley E, O'Mara ML, Reynolds C, Tieleman DP, Storm J, Kerr ID, Callaghan R. Transmembrane helix 12 modulates progression of the ATP catalytic cycle in ABCB1. Biochemistry 48: 6249–6258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran AR, Engelman DM. Sequence motifs, polar interactions and conformational changes in helical membrane proteins. Curr Opin Struct Biol 13: 412–417, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Diop NK, Hrycyna CA. N-linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry 44: 5420–5429, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2). J Cell Sci 113: 2011–2021, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Loe DW, Almquist KC, Deeley RG, Cole SP. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem 271: 9675–9682, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Loo TW, Bartlett MC, Clarke DM. Identification of residues in the drug translocation pathway of the human multidrug resistance P-glycoprotein by arginine mutagenesis. J Biol Chem 284: 24074–24087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loo TW, Bartlett MC, Clarke DM. Transmembrane segment 1 of human P-glycoprotein contributes to the drug-binding pocket. Biochem J 396: 537–545, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 61: 3458–3464, 2001 [PubMed] [Google Scholar]
- 13.Mao Q, Conseil G, Gupta A, Cole SP, Unadkat JD. Functional expression of the human breast cancer resistance protein in Pichia pastoris. Biochem Biophys Res Commun 320: 730–737, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J 7: E118–E133, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure 14: 1623–1632, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Miwa M, Tsukahara S, Ishikawa E, Asada S, Imai Y, Sugimoto Y. Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants. Int J Cancer 107: 757–763, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ozvegy-Laczka C, Varady G, Koblos G, Ujhelly O, Cervenak J, Schuetz JD, Sorrentino BP, Koomen GJ, Varadi A, Nemet K, Sarkadi B. Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface. J Biol Chem 280: 4219–4227, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Polgar O, Ediriwickrema LS, Robey RW, Sharma A, Hegde RS, Li Y, Xia D, Ward Y, Dean M, Ozvegy-Laczka C, Sarkadi B, Bates SE. Arginine 383 is a crucial residue in ABCG2 biogenesis. Biochim Biophys Acta 1788: 1434–1443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polgar O, Ierano C, Tamaki A, Stanley B, Ward Y, Xia D, Tarasova N, Robey RW, Bates SE. Mutational analysis of threonine 402 adjacent to the GXXXG dimerization motif in transmembrane segment 1 of ABCG2. Biochemistry 49: 2235–2245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polgar O, Robey RW, Morisaki K, Dean M, Michejda C, Sauna ZE, Ambudkar SV, Tarasova N, Bates SE. Mutational analysis of ABCG2: role of the GXXXG motif. Biochemistry 43: 9448–9456, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Pozza A, Perez-Victoria JM, Sardo A, Ahmed-Belkacem A, Di Pietro A. Purification of breast cancer resistance protein ABCG2 and role of arginine-482. Cell Mol Life Sci 63: 1912–1922, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer 89: 1971–1978, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res 7: 145–152, 2001 [PubMed] [Google Scholar]
- 24.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev 61: 3–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg MF, Bikadi Z, Chan J, Liu X, Ni Z, Cai X, Ford RC, Mao Q. The human breast cancer resistance protein (BCRP/ABCG2) shows conformational changes with mitoxantrone. Structure 18: 482–493, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 86: 1179–1236, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Senes A, Ubarretxena-Belandia I, Engelman DM. The C alpha-H O hydrogen bond: a determinant of stability and specificity in transmembrane helix interactions. Proc Natl Acad Sci U S A 98: 9056–9061, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos 37: 359–365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vethanayagam RR, Wang H, Gupta A, Zhang Y, Lewis F, Unadkat JD, Mao Q. Functional analysis of the human variants of breast cancer resistance protein: I206L, N590Y, and D620N. Drug Metab Dispos 33: 697–705, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Lee EW, Cai X, Ni Z, Zhou L, Mao Q. Membrane topology of the human breast cancer resistance protein (BCRP/ABCG2) determined by epitope insertion and immunofluorescence. Biochemistry 47: 13778–13787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang DW, Cole SP, Deeley RG. Determinants of the substrate specificity of multidrug resistance protein 1: role of amino acid residues with hydrogen bonding potential in predicted transmembrane helix 17. J Biol Chem 277: 20934–20941, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Zhang DW, Gu HM, Situ D, Haimeur A, Cole SP, Deeley RG. Functional importance of polar and charged amino acid residues in transmembrane helix 14 of multidrug resistance protein 1 (MRP1/ABCC1): identification of an aspartate residue critical for conversion from a high to low affinity substrate binding state. J Biol Chem 278: 46052–46063, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Zhang DW, Nunoya K, Vasa M, Gu HM, Theis A, Cole SP, Deeley RG. Transmembrane helix 11 of multidrug resistance protein 1 (MRP1/ABCC1): identification of polar amino acids important for substrate specificity and binding of ATP at nucleotide binding domain 1. Biochemistry 43: 9413–9425, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol 73: 949–959, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A 99: 12339–12344, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]