Abstract
Epoxyeicosatrienoic acids (EETs), synthesized and released by astrocytes in response to glutamate, are known to play a pivotal role in neurovascular coupling. In vascular smooth muscle cells (VSMC), EETs activate large-conductance, Ca2+-activated K+ (BK) channels resulting in hyperpolarization and vasodilation. However, the functional role and mechanism of action for glial-derived EETs are still to be determined. In this study, we evaluated the effect of the synthetic EET analog 11-nonyloxy-undec-8(Z)-enoic acid (NUD-GA) on outward K+ currents mediated by calcium-activated K+ channels. Addition of NUD-GA significantly increased intracellular Ca2+ and outward K+ currents in perivascular astrocytes. NUD-GA-induced currents were significantly inhibited by BK channel blockers paxilline and tetraethylammonium (TEA) (23.4 ± 2.4%; P < 0.0005). Similarly, NUD-GA-induced currents were also significantly inhibited in the presence of the small-conductance Ca2+-activated K+ channel inhibitor apamin along with a combination of blockers against glutamate receptors (12.8 ± 2.70%; P < 0.05). No changes in outward currents were observed in the presence of the channel blocker for intermediate-conductance K+ channels TRAM-34. Blockade of the endogenous production of EETs with N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH) significantly blunted (dl)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (t-ACPD)-induced outward K+ currents (P < 0.05; n = 6). Both NUD-GA and t-ACPD significantly increased BK channel single open probability; the later was blocked following MS-PPOH incubation. Our data supports the idea that EETs are potent K+ channel modulators in cortical perivascular astrocytes and further suggest that these metabolites may participate in NVC by modulating the levels of K+ released at the gliovascular space.
Keywords: electrophysiology, calcium imaging, calcium-activated potassium channels
in recent years, significant progress has been made in the understanding of the signaling mechanisms underlying neurovascular coupling (NVC) in the brain (22, 26). Epoxyeicosatrienoic acids (EETs), metabolites of arachidonic acid (AA), are among the various proposed signaling candidates contributing to NVC (3, 42). In addition to their potent vasodilatory action (19, 24), EETs have been associated with a number of physiological functions such as angiogenesis (46, 53) and anti-inflammation (30, 43). Both in vitro and in vivo data have validated the importance of these metabolites in cerebral blood flow (CBF) regulation (32, 33, 39). In addition, changes in EET signaling have been observed in disease conditions such as hypertension and stroke (14, 38, 40, 51, 54, 55). It has been reported that as a compensatory mechanism, soluble epoxide hydrolase (sEH), the enzyme that converts EETs to their corresponding diols, is increased in hypertensive animals (1, 14, 52). For example, inhibition of sEH significantly lowered arterial blood pressure in angiotensin II-induced hypertensive animals (25). In spontaneously hypertensive stroke-prone rats, similar treatment significantly reduced cerebral infarct size following middle cerebral artery occlusion (12, 40). These findings highlight the importance of a detailed study addressing the mechanism of action for EETs at the neurovascular unit, the site where multiple target cells (neuronal terminals, astrocytes, and vascular cells) interact to optimize CBF to the brain.
The current hypothesis in the field suggests that EETs, released by activated astrocytes (2), diffuse to vascular smooth muscle cells (VSMC) where they induce vasodilation (23, 26). Specifically, glutamate-induced activation of metabotropic glutamate receptors (mGluR) in astrocytes results in an increase in intracellular Ca2+ triggering AA mobilization and CYP 2C11 activation, which leads to the formation and release of all four EETs regioisomers (5,6-, 8,9-, 11,12-, and 14,15-EETs) (2, 4, 9, 29).
The signaling mechanism by which astrocyte-derived EETs induced vasodilation is thought to occur through the activation of the large-conductance, Ca2+-activated K+ channel (BK) in VSMC (13, 27). In addition to the effects on VSMC, several lines of evidence support the idea that EETs may also act in an autocrine manner as seen by a significant increase in intracellular Ca2+ in cortical astrocytes (7) and the activation of BK channel currents in cultured hippocampal astrocytes (50). The rapid Ca2+ response, combined with the notion that astrocytic endfeet are enriched with BK channels (36), raises the possibility that EETs, in addition to their VSMC effects, may also contribute to K+ signaling in cortical astrocytes (17).
In a previous study, we showed that K+ signaling from astrocytic endfeet, via BK channel activation, induced vasodilation during NVC (17). Moreover, 11,12-EET-induced vasodilations were blocked in the presence of the BK channel blocker tetraethylammonium (TEA) (7). In cultured hippocampal astrocytes, Gebremedhin et al. (20) showed that inhibition of endogenous EETs formation with miconazole blunted the glutamate-induced increase in BK single channel open probability following mGluR activation (20). Based on these findings, we hypothesized that endogenously produced EETs may act in an autocrine manner, modulating BK channels expressed at the very same site where these metabolites are released, namely the gliovascular space. In this way, following neuronal activation astrocyte-derived EETs would sustain astrocytic K+ efflux, thus prolonging vasodilation.
Using patch-clamp electrophysiological recordings and calcium imaging from cortical perivascular astrocytes, we characterize the effects of the EETs analog 11-nonyloxy-undec-8(Z)-enoic acid (NUD-GA) on intracellular calcium responses and Ca2+-sensitive K+ currents.
METHODS
Brain slice preparation.
Cortical brain slices were prepared from juvenile (P23-31) Wistar rats following protocols approved by the Office of Animal Care Management at the Medical College of Georgia. The cortex was rapidly removed and cut into 300-μm thick coronal slices using a vibratome (Leica VT 1200S, Leica Microsystems, Wetzlar, Germany) in cold artificial cerebrospinal fluid (aCSF) (in mM): 3 KCl, 125 NaCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 2 CaCl2, and 400 μM l-ascorbic acid, with osmolarity at 300–305 mosM, equilibrated with 95% O2-5% CO2. Slices were then incubated at room temperature (RT) in aCSF equilibrated with 95% O2-5% CO2 (pH 7.4) until needed.
Electrophysiology.
Cortical astrocytes in close proximity (<50 μm) to a parenchymal arteriole were identified using a ×63 water-immersion objective (numerical aperature 1.0). Whole cell patch-current recordings were obtained using an Axopatch 200B amplifier (Axon instruments, Foster City, CA). Patch pipettes were made from thin-walled borosilicate glass (outer diameter 1.5 mm, internal diameter 0.86 mm; Sutter instrument BF150-86-7.5) and pulled (P-97 puller Sutter Instruments, Novato, CA) to resistances between 5 and 7 mΩ. The internal solution for whole cell recordings consisted of (in mM) 130 K+ gluconate, 10 HEPES, 0.2 EGTA, 10 KCl, 0.9 MgCl2, 4 Mg2ATP, 0.3 Na2GTP, and 20 phosphocreatine; pH adjusted to 7.2 with KOH. For intracellular labeling, 5% Lucifer yellow potassium salt (Invitrogen, Carlsbad, CA) was added to the pipette solution. The osmolarity of the intracellular solutions was 291–295 mosM. Current signals were filtered at 1 kHz low-pass filter and digitized with a Digidata 1320 board (Axon instrument). pClamp 9.2 (Axon instrument) was used for data acquisition and storage. Single-channel K+ currents were recorded using the cell-attached configuration from an astrocytic endfoot attached to a cerebral arteriole. The pipette solution for cell-attached recordings consisted of (in mM) 140 KCl, 1 MgCl2, and 10 HEPES; pH adjusted at 7.2 with KOH and osmolarity at 280 mosM. Single channel activity was measured at +50 mV. Mean open state probability (NPo) was determined as previously described (11, 49). Single channel conductance was calculated from a linear slope fitted regression of the current-voltage (I-V) relationship obtained from channel current amplitudes at potentials between −60 and +60 mV. Perforated patch recordings were used to measure astrocytic endfeet whole cell currents for N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH) experiments. The perforated pipette solution consisted of (in mM) 30 KCl, 110 K+ aspartate, 10 NaCl, 1 MgCl2, and 10 HEPES adjusted pH to 7.2 with NaOH. 500 μg/ml amphotericin-B was added to the solution. Lucifer yellow (5%) was added to assure membrane integrity as shown by the lack of intracellular staining (37). All experiments were conducted at 33–35°C. The liquid junctional potential (+6.5 mV) was previously determined experimentally and described in Sonner et al. (41).
Confocal immunohistochemical imaging.
For glial fibrillary acidic protein (GFAP) immunostaining of recorded cells, cortical slices containing Lucifer yellow (5%) filled cells were fixed in 4% paraformaldehyde overnight. For colocalization studies of astrocytic endfeet and BK channel expression, paraformaldehyde-fixed, frozen brain sections were permeabilized and blocked in PBS containing 0.3% Triton X-100 and 10% horse serum for 1 h. Slices were incubated overnight at 4°C with mouse anti-GFAP (1:10,000; Chemicon) and rabbit-anti-BK α-subunit (1:100; Chemicon) antibodies. GFAP signal was detected with donkey anti-mouse CY3 (Jackson ImmunoResearch). Anti-BK channel antibody was labeled with anti-Rb biotinylated antibody followed by Streptavidin-FITC (Jackson ImmunoResearch). Images of Lucifer yellow-filled astrocytes or GFAP and BK channel-stained astrocytes were captured by Zeiss LSM510 confocal microscope with ×40 and ×63 magnification objectives using the Zeiss LSM510 program (Carl Zeiss, Thornwood, NY).
Calcium imaging.
Ca2+ imaging was performed using the Andor Technology Revolution system (iXON EMCCD camera with the Yokogawa CSU 10, confocal scanning unit). This unit was attached to a Zeiss microscope (Axioscope 2FS). Briefly, cortical slices were incubated at room temperature in aCSF containing 10 μM Fluo-4 AM and pluronic acid (2.5 μg/ml). After a 2-h incubation period, slices were placed in aCSF at room temperature until needed. At the time of the experiment, a slice was transferred to a perfusion chamber on the microscope and held with a nylon grid and continuously superfused with aCSF at 33–35°C. Using this loading protocol, we are able to visualize Ca2+ transients in astrocytes. In accordance with previous reports (34), neurons do not load sufficiently under these conditions for Ca2+ detection. Parenchymal microvessels were visualized with a ×63 water-dipping objective (numerical aperature 1.0). Fluorescence images were obtained using a krypton-argon laser at 488 nm and emitted light at >495. Images were acquired at 2 image/s for 5–8 min.
Drugs and chemicals.
The synthetic EET analog, the glycine amide of NUD-GA, and 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) were synthesized by Dr. J. R. Falck. All chemicals were obtained from Sigma Chemicals (St. Louis MO) otherwise noted. mGluR agonist (dl)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (t-ACPD), metabotropic glutamate receptor5 (mGluR5) antagonists 2-methyl-6-phenylethynylpyridine hydrochloride (MPEP), mGluR1a receptor antagonist (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY-367385), CYP epoxygenase inhibitor MS-PPOH, and 1,2-bis(2-aminophenoxyl)ethane-N,N,N,N,-tetraacetic acid tetra (acetoxymethyl ester) (BAPTA-AM) were purchased from Tocris Bioscience (Ellisville, MS). Isolection-GS-IB4-Alexa Fluor-568 conjugate was purchased from Invitrogen (Carlsbad, CA). Tetrodotoxin (TTX) was obtained from Ascent Scientific LLC (Princeton, NJ).
Data analysis.
Analysis for electrophysiological recordings was performed using Clampfit 9.2, pClamp version 9.2 (Axon instrument) and GraphPad Prism software 4 (Graphpad software, La Jolla, CA). Wilcoxon paired t-test was used to analyze current changes (in pA/pF) between control and each drug treatment. A one-way analysis of variance was used to compare current changes (pA/pF) between control and drug-treated groups presented in Fig. 3E. Ca2+ imaging was analyzed using Andor IQ 1.6 software. Fractional fluorescence (F/Fo) was determined by dividing the fluorescence intensity (F) within a region of interest (ROI) by a baseline fluorescence value (Fo) determined from ∼50 images showing no activity. The frequency of Ca2+ oscillations was determined within a ROI (10 × 10 pixels or 2.5 × 2.5 μm) on a cell exhibiting Ca2+ oscillations. The number of peaks over a given time was automatically detected from oscillations crossing a set threshold value (>1.30 F/F0). All values are expressed as means ± SE. Statistical significance was tested at 95% (P < 0.05) confidence level and denoted with asterisks, when the P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***).
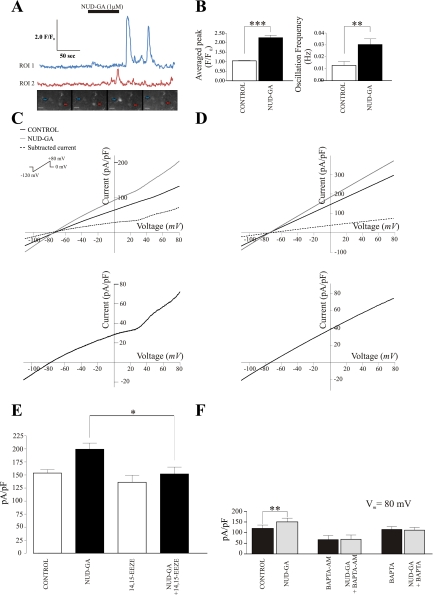
Fig. 3.
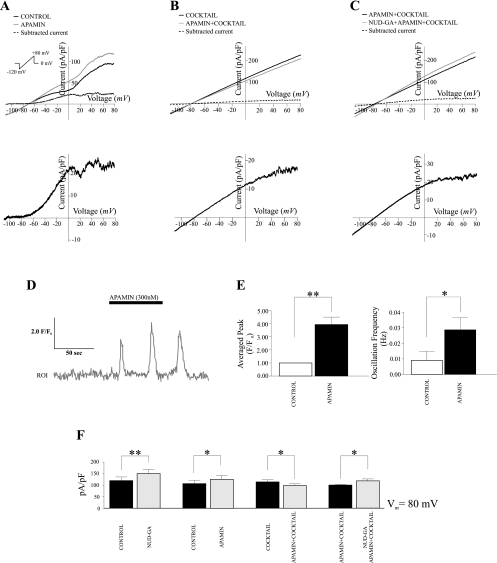
Calcium and electrophysiological responses of cortical astrocytes to the EET agonist 11-nonyloxy-undec-8(Z)-enoic acid (NUD-GA). A: NUD-GA-induced calcium transients in cortical astrocytes, representative traces and fluorescence images are shown. B: summary data of NUD-GA-induced changes in averaged peak F/F0 (***P < 0.0001, n = 11) and calcium oscillation frequency (**P < 0.01, n = 11). C and D: representative NUD-GA-induced outward voltage-dependent and linear membrane currents in response to a ramp protocol from −120 to +80 mV in perivascular astrocytes, respectively. The corresponding subtracted currents for C and D are shown below. E: summary data of peak currents (pA/pF) at +80 mV under control conditions, in the presence of NUD-GA, in the presence of 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) and in the presence of 14,15-EEZE and NUD-GA (*P < 0.005, n = 7). F: summary data of peak currents (pA/pF) at +80 mV in the presence and absence of the calcium chelator BAPTA (**P < 0.05, n = 5). Calibration bar, 10 μm.
RESULTS
Properties of cortical perivascular astrocytes.
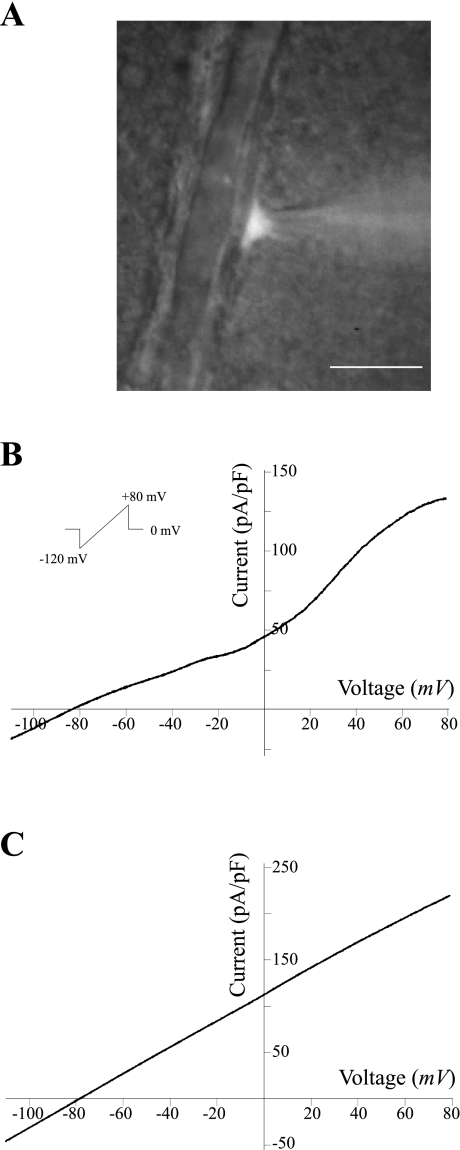
Only astrocytes in the vicinity of a cortical arteriole were studied; these cells were identified by their lack of action potential firing and their distinct morphology, namely small round somata and, in some cases, endfeet extending to the nearest arteriole. Figure 1A shows a differential interference contrast (DIC) image of a perivascular astrocyte loaded with Lucifer yellow. After a successful seal, astrocytes were subjected to a voltage ramp protocol (from −120 mV to +80 mV, in 400 ms, Fig. 1B, inset). In whole cell configuration, mean resting membrane potential (Vm) was −72.4 ± 1.9 mV and input resistance 74.6 ± 3.7 mΩ (n = 81). Figure 1, B and C, shows representative traces of the two I-V response patterns obtained from cortical perivascular astrocytes. Of 81 recorded astrocytes, 60.5% exhibited a voltage-dependent outwardly rectifying pattern (Fig. 1B), whereas 39.5% showed a linear I-V response (Fig. 1C). These response patterns are comparable to those previously reported (8, 10, 56). The averaged reversal potential for the overall whole cell current was −77.01 ± 0.52 mV, near the expected EK+.
Fig. 1.
Representative whole cell current profiles from cortical perivascular astrocytes. A: differential interference contrast (DIC) image of a Lucifer yellow-filled astrocyte in direct contact to an intraparenchymal arteriole. Calibration bar, 10 μm. Representative voltage-dependent (B) and linear membrane currents (C) in response to a ramp protocol from −120 to +80 mV.
Functional implication of CYP 2C11 activity in EETs-induced outward K+ currents in cortical astrocytes.
The vasodilatory action of EETs has been attributed to their hyperpolarizing effect on VSMC, resulting from the activation of BK channels in these cells (13, 19, 23, 26, 27). Likewise, EETs also induce BK channel activation in cultured hippocampal astrocytes (50). Since there is evidence that astrocytes synthesize EETs (3), and this synthesis is enhanced by glutamate (2), we hypothesized that endogenously produced EETs may act in an autocrine manner, modulating BK channels expressed at the very same site where these metabolites are released, namely, the gliovascular space. In this way, astrocyte-derived EETs would sustain astrocytic K+ efflux following neuronal activation, thus prolonging vasodilation.
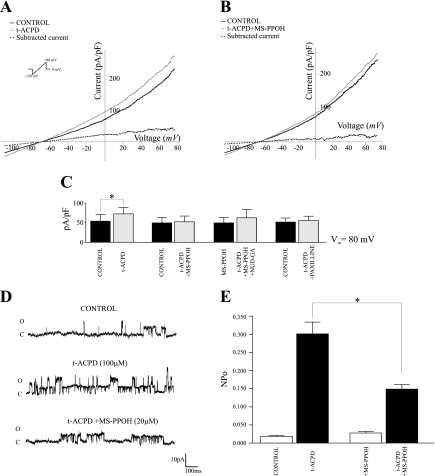
To test our hypothesis, brain slices were preincubated with MS-PPOH (20 μM for 30 min), a selective CYP epoxygenase substrate inhibitor (45), followed by stimulation of astrocytes with the mGluR agonist t-ACPD (100 μM). We speculated that following astrocytic mGluR activation, K+ currents would be substantially attenuated due to the lack of EETs-related autoregulatory effects. As expected, in the absence of MS-PPOH application, t-ACPD (100 μM) elicited a significant increase in outward currents (64 ± 29.8%; P < 0.05; n = 6), as measured at +80 mV (Fig. 2A). On the other hand, in slices preincubated with MS-PPOH, t-ACPD induced a nonsignificant increase of only 4.4 ± 2.6% in K+ outward currents (at +80 mV; n = 5; Fig. 2B). These data provide, for the first time, strong evidence that most of the K+ current (∼96%) elicited by mGluR activation in perivascular astrocytes in situ depends on endogenous EET synthesis.
Fig. 2.
Effect of endogenous epoxyeicosatrienoic acids (EETs) on outward currents induced by activation of metabotropic glutamate receptors (mGluR) in perivascular astrocytes. (dl)-1-Aminocyclopentane-trans-1,3-dicarboxylic acid (t-ACPD)-induced (100 μM) outward currents in the absence (A) and presence (B) of the CYP epoxygenase substrate inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH) (20 μM) (solid black line: control, solid gray line: t-ACPD, dashed line: subtracted current) are shown. C: summary data of averaged peak currents (pA/pF) at Vm = 80 mV induced by t-ACPD in the presence and absence of MS-PPOH and the large-conductance, Ca2+-activated K+ (BK) channel blocker paxilline. D: representative traces of single BK channel activation induced by t-ACPD in the presence and absence of MS-PPOH. E: changes in single channel open probability (NPo) induced by t-ACPD in the presence and absence of MS-PPOH (*P < 0.05, n = 4).
To assess the participation of BK channels in mGluR-induced outward currents, experiments were repeated in the presence of the BK channel blocker paxilline (2 μM). Under these conditions, t-ACPD-induced currents were significantly attenuated (by 87.6%) compared with control responses (+80 mV; P < 0.05; n = 5). Importantly, in slices preincubated with MS-PPOH, t-ACPD-induced outward currents were partially restored in the presence of NUD-GA (Fig. 2C). Finally, the activity of single BK channels at the astrocytic endfoot in response to t-ACPD was assessed using the cell attached configuration. After t-ACPD exposure, a ∼16-fold increase in single channel NPo (from 0.0185 ± 0.003 to 0.301 ± 0.033; P < 0.005, n = 4) was observed (Fig. 2, D and E). Similarly, BK single channel recordings showed that mGluR-mediated increases in NPo were significantly reduced following MS-PPOH incubation (from 0.0277 ± 0.004 to 0.149 ± 0.025). Altogether, these studies support BK channels as mediators of EETs-induced outward currents.
EET analog NUD-GA elicits calcium-dependent K+ currents in perivascular astrocytes.
Given the importance of K+ channels, we investigated in detail the effects of EETs on outward K+ currents. We previously showed that 11,12-EET triggers potent Ca2+ responses in cortical astrocytes (7). To verify that similar responses could be induced by the EET analog used in the present study, brain slices were perfused with NUD-GA (1 μM). As shown in Fig. 3, A and B (see online supplementary movie at the AJP-Cell Physiology website), NUD-GA significantly increased both the frequency of astrocytic Ca2+ oscillations, from 0.013 ± 0.003 to 0.030 ± 0.005 Hz (P < 0.005; n = 11 slices) as well as the peak averaged fluorescence (F/F0) from 1.05 ± 0.02 to 2.26 ± 0.13 (P < 0.0001; n = 11 slices).
Next, we determined whether the robust Ca2+ response induced by NUD-GA could lead to the activation of Ca2+-sensitive K+ channels. After a 5-min exposure, NUD-GA (1 μM) significantly increased astrocytic outward K+ currents by 37.5 ± 11.9% at +80 mV (P < 0.001, n = 10) (Fig. 3, C and D). The reversal potential under these conditions was not altered. No differences were observed when comparing the magnitude of the NUD-GA-induced response between astrocytes displaying voltage-dependent or linear ramp responses. Thus data from these two populations were pooled. In a subgroup of cells, we tested NUD-GA specificity by measuring astrocytic outward K+ currents in the presence or absence of the specific EETs antagonist 14,15-EEZE. In the presence of 14,15-EEZE (10 μM), NUD-GA-induced increases in outward currents at 80 mV were significantly decreased from 29.72 ± 4.5% to 12.54 ± 2.5% (n = 7, P < 0.005) (Fig. 3E).
To confirm the Ca2+ dependence of the observed responses, two sets of experiments were conducted. First, brain slices were incubated with the Ca2+ chelator BAPTA-AM (10 μM) for 30 min and the electrophysiological response to NUD-GA was determined. In a second set of experiments, BAPTA (10 mM) was added to the pipette solution and allowed to dialyze and equilibrate into the cell for 10 min before exposure to NUD-GA. Under both experimental conditions, the stimulatory effect of NUD-GA on K+ currents was abolished, indicating that a raise in intracellular Ca2+ mediates such effect (Fig. 3F). We next proceeded to study the identity of the K+ channels responsible for the outward currents elicited by NUD-GA.
NUD-GA-induced changes to BK channel currents in cortical perivascular astrocytes.
Examination of the I-V plots shown in Fig. 3, C and D, revealed a more complex pattern than that expected from the activation of a single channel type, suggesting that multiple K+ conductances may be activated in astrocytes upon exposure to the EET analog NUD-GA. Whereas the BK channel has been shown to be the effector of outward K+ currents induced by EETs in cultured hippocampal astrocytes and VSMCs (19, 50) and is thus a main candidate to mediate NUD-GA-induced currents in our preparation, other potential contributors to this current are the intermediate- (IK) and small-conductance (SK), Ca2+-sensitive K+ channels IK and SK, respectively. Therefore, we employed pharmacological inhibitors to evaluate the contribution of each of these channels to the NUD-GA-sensitive K+ current.
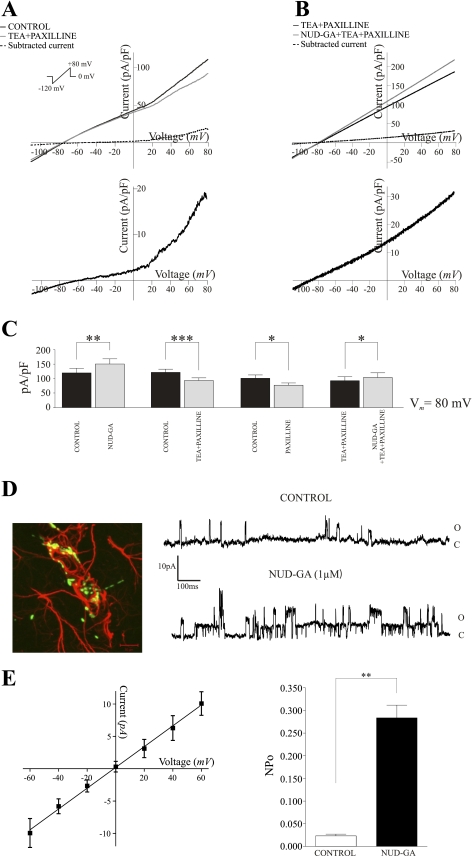
We first determined the contribution of BK channels to whole cell outward currents in the absence of the EETs agonist. To this end, ramp protocols were run in the presence and absence of BK channel blockers paxilline (2 μM) and TEA (1 mM). In the presence of both inhibitors, outward K+ currents were significantly reduced by 23.4 ± 2.4% at +80 mV (P < 0.0005, n = 11) (Fig. 4A). A similar attenuation was observed in the presence of the specific BK channel inhibitor paxilline alone (23.1 ± 2.6%; P < 0.05, n = 6) (Fig. 4C).
Fig. 4.
Contribution of BK channels to NUD-GA-induced outward currents. A: representative current profiles of perivascular astrocytes in the presence and absence of the BK channel blocker paxilline (2 μM) and tetraethylammonium (TEA, 1 mM) in response to a ramp protocol from −120 to +80 mV (P < 0.001, n = 11). Subtracted currents are shown below. B: representative NUD-GA-induced outward K+ currents from an astrocyte in the presence of the BK channel blocker paxilline and TEA. Corresponding subtract currents are shown below. C: summary data of peak currents at +80 mV in the presence (*P < 0.05, n = 7) and absence of BK channel blockers.**P < 0.01; ***P < 0.001 D: left, immunolabeling against BK channels (green) and the astrocyte marker glial fibrillary acidic protein (GFAP) (red) (scale bar = 10 μm); right, representative single channel activity from an astrocytic endfoot in the absence and presence of NUD-GA, O, open state and C, closed state. E: left, averaged current-voltage relationship for BK channels expressed in astrocytic endfeet; right, NUD-GA-induced increased in NPo (**P < 0.01, n = 4).
We next determined the contribution of BK channels to NUD-GA-induced K+ outward currents. In the presence of both TEA and paxilline, NUD-GA still elicited a significant increase in outward currents (11.5 ± 1.8%; P < 0.05; n = 7), which represents, however, a decrease of 69.3% compared with responses to NUD-GA alone (compare Fig. 3C with 4B). These data indicate that about 70% of the NUD-GA-sensitive current in perivascular astrocytes can be attributed to activation of BK channels.
To further confirm and to assess the effects of NUD-GA on the properties of single BK channels, we next performed cell-attached recordings on astrocytic endfeet, the site where these channels were previously shown to be highly concentrated (36). In agreement with previous studies, we found a robust BK channel immunoreactivity in clusters around parenchymal microvessels and which colocalized with the astrocyte-specific marker GFAP (Fig. 4D). The EETs analog NUD-GA caused a 12-fold increase in single channel NPo from 0.0231 ± 0.041 to 0.284 ± 0.028 (P < 0.05; n = 4) (Fig. 4, D and E). The calculated slope conductance for these channels was 171 pS. This value is consistent with the range of single channel conductance previously shown for BK channels in cultured astrocytes (16, 39) (Fig. 4E). These data further confirm the involvement of BK channels in NUD-GA-induced K+ currents in perivascular astrocytic endfeet, giving strength to the idea that EETs modulate astrocyte K+ efflux, being a likely mechanism affecting vascular tone in the mammalian brain (7, 15).
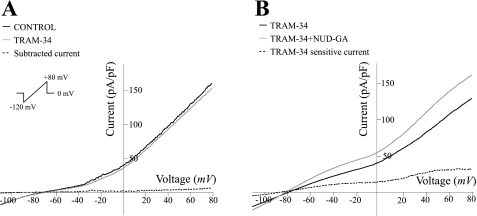
NUD-GA-induced changes to IK channel currents in cortical perivascular astrocytes.
The incomplete NUD-GA-induced current block observed upon BK channel inhibition suggests that other K+ channels are also activated upon exposure to this EET analog. To evaluate the potential contribution of IK channels on NUD-GA-induced K+ currents, whole cell recordings were performed in the presence of the specific IK channel blocker TRAM-34 (1 μM). TRAM-34 did not alter the I–V relationship of cortical astrocytes either in control conditions (no agonist; Fig. 5A) or upon combined exposure with NUD-GA (Fig. 5B; n = 6). In addition, in the presence of TRAM-34, NUD-GA induced similar changes in current amplitude at all different voltages at (+80 mV; 22.52 ± 3.7%) suggesting that cortical astrocytes do not possess functional IK channels or that these channels are not activated by the EET analog NUD-GA (Fig. 5B).
Fig. 5.
Lack of contribution of intermediate conductance (IK) channels to NUD-GA-induced outward currents. A: representative ramp profile of a perivascular astrocyte in the presence and absence of the IK channel blocker TRAM-34 (1 μM). B: representative ramp profile of a perivascular astrocyte in the presence and absence of NUD-GA in the presense of TRAM-34.
NUD-GA-induced changes to SK channel currents in cortical perivascular astrocytes.
Finally, we used the specific SK channel blocker apamin to determine whether SK channels, reportedly expressed in astrocytes from the supraoptic nucleus of the hypothalamus (5), contribute to the increase in outward K+ currents induced by NUD-GA. Unexpectedly, in the presence of the SK channel blocker apamin (300 nM), control currents were significantly increased (18.4 ± 5.6% at +80 mV; P < 0.05; n = 7) (Fig. 6A). We reasoned this response could be attributed to the inhibition of neuronal SK channels leading to the release of glutamate and subsequent astrocytic activation (31), as it would be possible in the slice preparation. Under these conditions, the concomitant rise in astrocytic Ca2+ expected to result from the activation of mGluR (35) may explain the rise in outward currents in the presence of apamin. To test this possibility, the intracellular Ca2+ response to apamin was measured in cortical astrocytes. As shown in Fig. 6, D and E, apamin (300 nM) significantly increased both the frequency (0.009 ± 0.006 to 0.03 ± 0.008; P < 0.05, n = 7 slices) and the amplitude (F/F0) (1 ± 0.002 to 3.66 ± 0.5; P < 0.01, n = 7 slices) of astrocytic intracellular Ca2+ oscillations. These data confirm that SK channel blockade in the slice preparation induces astrocytic Ca2+ elevations. Attesting to the involvement of neuronal activity in this response, the apamin-mediated increases in astrocytic Ca2+ oscillation frequency and amplitude were abolished upon combined inhibition of Na+ channels (0.5 μM TTX) and glutamate receptors: ionotropic (1 mM kynurenic acid) and group I mGluR (50 μM LY-367385 and 100 μM MPEP) (n = 9, data not shown).
Fig. 6.
Contribution of small conductance (SK) channels to NUD-GA-induced outward currents. A: representative ramp profiles of perivascular astrocytes in the presence and absence of the SK channel blocker apamin (300 nM) (P < 0.05, n = 7), subtracted currents are shown below. B: representative ramp profiles of perivascular astrocytes in the presence of tetradotoxin (TTX) (0.5 μM) and glutamate receptor blockers kyneurenic acid (1 mM), LY-367385 (50 μM), and 2-methyl-6-phenylethynylpyridine hydrochloride (MPEP, 100 μM) with or without apamin. C: NUD-GA-induced outward currents in the presence of apamin and combined inhibitors against ionotropic and metabotropic GluR. D: representative calcium trace showing apamin-induced calcium oscillations in astrocytes. E: summary data of apamin-induced changes in averaged peak F/F0 and calcium oscillation frequency from cortical astrocytes (**P < 0.01, n = 7). F: summary data of averaged peak currents at +80 mV in the presence and absence of NUD-GA, combined inhibitors against ionotropic and metabotropic GluR and the SK channel blocker apamin. *P < 0.05.
Thus we applied the above combination of inhibitors to elucidate the effects of apamin on both control (no agonist) and NUD-GA-sensitive K+ currents in astrocytes. After application of voltage-gated Na+ channel and glutamate blockers, baseline I-V ramp responses were unaffected. In the presence of apamin, outward currents were 12.8 ± 2.7% smaller than those measured in the presence of the blockers alone (Fig. 6B; +80 mV; P < 0.05; n = 7). When both apamin and the blocker cocktail were present, NUD-GA exposure induced an increase in outward currents of 17.6 ± 4.7% (+80 mV; P < 0.05; n = 6) (Fig. 6C), which represents a ∼53% reduction with respect to the response to NUD-GA alone. These data suggest that SK channels also participate in NUD-GA-induced K+ currents.
DISCUSSION
Although a contribution of EETs to the regulation of vascular reactivity and CBF (32, 33, 39) has been supported by several studies, the cellular mediators, the specific mechanisms involved and overall physiological relevance remain incompletely understood. Among the questions that deserve further attention are the endogenous site(s) of EET production, the signals that mediate its production and/or release, and the molecular mechanisms by which changes in vascular tone are induced.
A physiologically relevant pathway leading to cerebrovascular vasodilation during NVC comprises the release of glutamate by neurons and subsequent activation of mGluR in astrocytes (16, 57). The rise in intracellular Ca2+ following mGluR activation has been linked to a number of vasoactive compounds, potential mediators of the hyperemic response (22, 26, 39). To this end, we showed that a BK channel-dependent K+ efflux from astrocytic endfeet contributes to the vasodilatory response associated with neuronal stimulation (17). Moreover, we also showed that exogenously applied 11,12-EET elicits a rapid increase in intracellular Ca2+ in cortical astrocytes in situ (7). These findings raise the possibility that EETs, rather than, or in addition to, diffusing to VSMC, may act in an autocrine manner to potentiate K+ efflux from astrocytic endfeet, thus contributing to astrocyte-induced vascular responses (17). In this study, we tested the hypothesis that endogenous EET production in astrocytes contributes to the increase in K+ efflux that follows activation of mGluR in astrocytes. In support of our hypothesis, our results show that inhibition of endogenous production of EETs with MS-PPOH abolished whole cell mGluR-induced outward K+ currents in astrocytes and attenuated the open probability of single BK channels at their endfeet. Concurrent with our previous observations that BK channel inhibition blocked EETs-mediated vasodilatory responses of parenchymal arterioles (7), the present data supports a role for EETs and K+ signaling in glutamate-mediated NVC.
Since astrocytes synthesize (3) and respond to EETs with an increase in K+ conductance (50), we employed the synthetic EET analog NUD-GA in the present study to identify specific K+ channels activated by EETs in perivascular astrocytes in situ and potentially involved with NVC. We show that perivascular astrocytes respond to the EET analog NUD-GA with a raise in intracellular Ca2+, which leads to a significant increase in Ca2+-dependent outward K+ currents mainly through the activation of BK and SK channels. The Ca2+ dependence of NUD-GA-induced K+ currents was confirmed by the complete current inhibition observed upon either bath application or cell dialysis with the Ca2+ chelator BAPTA. Of note, the increase in Ca2+ oscillatory frequency and amplitude elicited by NUD-GA was comparable to that observed previously by us using a similar dose of 11,12-EET (5). Calcium responses induced by EETs have been attributed to the putative activation of TRPV4 channels of the vanilloid family (13), which are targets for these metabolites (47). In astrocytes, TRPV4 channels are preferentially expressed on perivascular processes (6), which is consistent with a preferential action of EETs on glial endfeet.
The present data show that BK channel activation, assessed upon its blockade with TEA or paxilline, predictably accounts for the largest portion of the NUD-GA-induced K+ current (∼70%). To further assess this effect, single BK channel activity was measured in native astrocytic endfeet, the site where these channels are densely expressed (36). In response to NUD-GA, we observed a ∼12-fold increase in BK channel open probability, with a concurrent single channel conductance of 171 pS, similar to that previously reported (50). Hence, the strategic expression of BK channels in astrocytic endfeet (36) and their prominent activation by EETs (18) support the idea that EET-induced cell Ca2+ increase and BK channel activation are coordinated events that contribute importantly to the control of vascular tone in the brain (7, 13, 17).
Altogether the data suggest that EETs are potent modulators of BK and SK channel activity in astrocytes and likely function as key contributors of the glutamate-mGluR-K+ signaling pathway during NVC. In this respect, it is conceivable that after the increase in Ca2+ resulting from mGluR activation, EETs production secondary to intracellular AA release may serve to amplify or prolong the raise in Ca2+, leading to a more robust or sustained change in vascular tone. As mentioned before, an open question pertains to the possible release of astrocytic EETs into the gliovascular space (23, 26), where they may directly exert an increase in Ca2+ in VSMCs leading to BK channel activation and vasodilation (13, 19).
We propose EETs contribute to NVC by increasing intracellular Ca2+ in astrocytes and altering the activity of K+ channels. Through these mechanisms, EETs may modulate the levels of K+ being released at endfeet processess and may further affect the type of vascular response elicited (vasodilation vs. vasoconstrictions). In this sense, we previously showed that vascular responses of penetrating arterioles in brain slices to exogenously applied EETs were dependent on the level of tone of the arteriole (7). Moreover, although commonly deemed as a vasodilator, the amount of K+ released by astrocytes (which correlated with the amount of intracellular Ca2+ increased in astrocytes) was recently shown to differentially induce vasodilatory or vasoconstricting responses, regardless of the level of tone of the arteriole, thus highlighting the importance and complexity of K+ signaling in NVC (21).
While not directly related to the data presented in this study, in a recent study, Marowsky et al. (28) showed expression of sEH (the enzyme that converts EETs to their corresponding dihydroxyeicosatrienoic acids) primarily in astrocytes and particularly in processes outlining the vasculature (endfeet). Thus the strategic expression of sEH in astrocytic endfeet (28) may help modulate K+ levels at the gliovascular space.
In summary, in this study we showed that the EET analog NUD-GA increased intracellular calcium in perivascular astrocytes and significantly increased BK and SK channel currents. In addition, blockade of the endogenous formation of EETs blunted outward K+ currents induced by the activation of mGluR receptors. Thus, through modulation of astrocyte K+ channels, EETs stand as critical players in glutamate-related NVC. A current hypothesis about NVC states that the rise in calcium in astrocytes initiates the metabolism of AA, which leads to the formation of metabolites such as prostaglandins (44, 57) and EETs (3), and that these metabolites diffuse to VSMCs inducing vasodilation (22, 23). Our results expand on previous studies in hippocampal cultured astrocytes (50) and suggest an additional pathway by which EETs may also participate in NVC by acting directly on astrocytic endfeet and in turn modulating Ca2+ levels and K+ currents in an autocrine manner. Given the limitation of the brain slice preparation, lack of intraluminal pressure/flow, the present study focused primarily on signaling events in astrocytes with the exclusion of potential factors arising from the vasculature itself. Future studies on EETs-mediated mechanisms arising from the circulation as it occurs during changes in shear stress should not be ruled out since they may also significantly influence K+ signaling in astrocytes. This form of communication may be the basis for bidirectional flow of information at the neurovascular unit (18).
GRANTS
This work was funded by grants from National Heart, Lung, and Blood Institute (R01HL-089067) and National Institutes of Health (GM-32178) and by the Robert A. Welch Foundation (GL-625910).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Javier Stern at Medical College of Georgia (MCG) for input on the manuscript, as well as Dr. Richard E. White at the MCG for help in the single channel recordings and analysis.
REFERENCES
- 1.Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA 104: 9018–9023, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28: 1066–1072, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27: 971–979, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem 58: 503–510, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Armstrong WE, Rubrum A, Teruyama R, Bond CT, Adelman JP. Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J Comp Neurol 491: 175–185, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148: 876–892, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol 294: H2855–H2863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia 30: 27–38, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Capdevila JH, Karara A, Waxman DJ, Martin MV, Falck JR, Guenguerich FP. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem 265: 10865–10871, 1990 [PubMed] [Google Scholar]
- 10.D'Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, 2nd, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci 18: 4425–4438, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitropoulou C, West L, Field MB, White RE, Reddy LM, Falck JR, Imig JD. Protein phosphatase 2A and Ca2+-activated K+ channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial relaxation. Prostaglandins Other Lipid Mediat 83: 50–61, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 46: 842–848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fang X. Soluble epoxide hydrolase: a novel target for the treatment of hypertension. Recent Pat Cardiovasc Drug Discov 1: 67–72, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Filosa JA, Blanco VM. Neurovascular coupling in the mammalian brain. Exp Physiol 92: 641–646, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res 95: e73–81, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Filosa JA. Vascular tone and neurovascular coupling: considerations towards an improved in vitro model. Front Neuroendocrinol 2:16 doi:10.3389/fnene.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol 263: H519–H525, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Gebremedhin D, Yamaura K, Zhang C, Bylund J, Koehler RC, Harder DR. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J Neurosci 23: 1678–1687, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 107: 3811–3816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia 55: 1214–1221, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke 29: 229–234, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Iliff JJ, Wang R, Zeldin DC, Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol 296: H1352–H1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39: 690–694, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32: 160–169, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res 80: 877–884, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience 163: 646–661, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Nithipatikom K, Grall AJ, Holmes BB, Harder DR, Falck JR, Campbell WB. Liquid chromatographic-electrospray ionization-mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal Biochem 298: 327–336, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem 276: 9762–9769, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 283: H2029–H2037, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab 24: 509–517, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci 23: 9888–9896, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci 16: 5073–5081, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res 956: 183–193, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods 37: 15–26, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Sellers KW, Sun C, Diez-Freire C, Waki H, Morisseau C, Falck JR, Hammock BD, Paton JF, Raizada MK. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J 19: 626–628, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab 28: 111–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol 174: 2086–2095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol 582: 1219–1238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292: C996–C1012, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys 433: 413–420, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther 284: 966–973, 1998 [PubMed] [Google Scholar]
- 46.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, Wang DW, Zeldin DC. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther 314: 522–532, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol 145: 775–784, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res 77: 936–942, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Yamaura K, Gebremedhin D, Zhang C, Narayanan J, Hoefert K, Jacobs ER, Koehler RC, Harder DR. Contribution of epoxyeicosatrienoic acids to the hypoxia-induced activation of Ca2+ -activated K+ channel current in cultured rat hippocampal astrocytes. Neuroscience 143: 703–716, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Yu Z, Huse LM, Adler P, Graham L, Ma J, Zeldin DC, Kroetz DL. Increased CYP2J expression and epoxyeicosatrienoic acid formation in spontaneously hypertensive rat kidney. Mol Pharmacol 57: 1011–1020, 2000 [PubMed] [Google Scholar]
- 52.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke 33: 2957–2964, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27: 1931–1940, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 39: 2073–2078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol 95: 134–143, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003 [DOI] [PubMed] [Google Scholar]